Abstract
Human Papilloma Virus related epithelial cancers have been speculated to derive from virus-infected tissue stem cells. Stem cells also are thought to provide a reservoir of latently infected cells that can persist for long periods. In this study we have examined the effects of HPV16 E6 and E7 oncogenes on multipotent epithelial stem cells, using in vivo systems. Our results show that expression of HPV16 oncogenes reduces the number of bulge label-retaining cells within hair follicles at telogen suggesting aberrant mobilization, a result supported by increased mobilization upon acute anagen induction. Importantly the loss of relative quiescence, a hallmark feature of stem cells, occurs in the absence of a reduction in other stem cell markers. This points to an atypical stem cell compartment in the context of E6 and E7 expression. We hypothesize that this aberrant compartment may have important roles in the viral life cycle and/or ensuing carcinogenesis.
Keywords: HPV, E6, E7, Stem cells
Introduction
A growing body of literature supports the notion that infectious agents perturb stem cell homeostasis in target tissues (Pitsouli et al., 2009). For pathogens that complete their life cycle inside the cell, such as viruses, changes in tissue stem cell homeostasis could have profound effects on infection outcome. This host–pathogen interaction is particularly interesting in cases where infection is associated with carcinogenesis, where aberrant stem cell homeostasis could be related to ensuing carcinogenesis. Cervical carcinomas have long been hypothesized to arise from cervical stem cells, but there is a scarcity of publications addressing the interplay of the viral oncogenes with tissue stem cells.
Human papillomaviral infections specifically arise in stratified squamous epithelia such as those lining the skin, the anogenital tract and oral epithelia and, in the context of high risk HPV infections, leads to the development of a number of cancers including cervical, anal, as well as head and neck cancers. The two most important papillomaviral oncogenes, E6 and E7, not only are necessary for the viral life cycle but also for the development as well as the persistence of HPV-associated cancers (Goodwin and DiMaio, 2000; Jabbar et al., 2009). Papilloma virus infections are thought to arise through infection of cells within the basal layer of stratified squamous epithelia, which is the layer of cells that makes direct contact with the basement membrane and constitutes the proliferative compartment of these epithelia (Kines et al., 2009). A limited body of evidence supports the notion that papillomaviruses infect cells with stem-like characteristics (Schmitt et al., 1996) but the consequences of viral oncogene expression in tissue stem cells in vivo are largely unknown. Furthermore, it has been proposed that tissue stem cells are the sites of persistent infection by the virus (Maglennon et al., 2011). In the cervix, the most common site of HPV16 infection, cancers are thought to arise from the transition zone between glandular and squamous epithelia where stem-like cells are thought to reside. It is therefore evident that stem cells are a potential direct target of infectious agents and, in accordance with studies indicating that some cancers derive from tissue stem cells, this may have an important role in carcinogenesis. However, it is still not clear whether HPV, or other similar cancer causing viruses, directly affects the development, function, or plasticity of tissue stem cells.
A clear challenge in assessing the consequences of viral oncogene expression in cervical tissue stem cells is the lack of well-characterized markers that would enable their successful detection as well as functional assays for tissue stem cells. An alternative model, which has been extensively used to study HPV infection and biology, is the skin (Auewarakul et al., 1994; Lambert et al., 1993; Merrick et al., 1992). The stem cell compartments of the mouse skin have been extensively characterized and various populations with “stemness characteristics” have now been described. The best understood stem cell population lies in the skin hair follicle, in a niche dubbed the “bulge”. This is a population that, like many other tissue stem cells, is relatively quiescent and the identification of its slow-cycling nature and label retaining ability dates back to 1990 (Cotsarelis et al., 1990). The nucleotide pulse-chase technique has been extensively used for the detection of the bulge population and led to its identification as a stem cell population and its contribution not only to hair follicle regeneration in vivo, but also to interfollicular epidermal skin healing after wounding (i.e. this is a multipotent tissue stem cell compartment) (Ito et al., 2005; Morris et al., 2004; Tumbar et al., 2004; Zhang et al., 2009). In addition to the pulse-chase technique, a marker used to genetically target bulge cells for lineage-tracing analysis is cytokeratin 15 (K15), which allows for labeling of the bulge and secondary hair germ regions (Liu et al., 2003; Lyle et al., 1998; Morris et al., 2004).
Contributing to the choice of using the skin tissue as a model is also the fact that it undergoes homeostasis resulting in the highly ordered process of hair follicle growth, which consists of cycles of growth and regression. In these cycles, a subset of bulge stem cells have been shown to mobilize out of their niche during the growth phase, known as anagen or stem cell mobilization, migrate to lower regions of the hair bulb where they proliferate and differentiate contributing to the formation of new hair (Blanpain and Fuchs, 2006). This stem cell model has been widely used to determine the implication of stem cells in carcinogenesis and it should be noted that several lines of evidence implicate the bulge stem cells specifically, as the cells of origin in squamous cell carcinomas (Lapouge et al., 2011).
To elucidate the role of viral oncogenes in modulating the behavior of quiescent tissue stem cells, we used transgenic animals expressing the viral oncogenes E6 and E7 of HPV16 in stratified squamous epithelia. Focusing specifically in the hair follicle bulge stem cells we describe here that, while the overall numbers of bulge stem cells do not appear to be reduced upon E6 and E7 expression, their ability to remain quiescent and thus retain label, is compromised, and their ability to proliferate upon anagenic stimuli is enhanced. A potential modulation of tissue stem cell mobilization may have important implications on infection outcome, including eventual carcinogenesis.
Results
Expression of the HPV16 oncogenes reduces the number of relatively quiescent cells detected at telogen
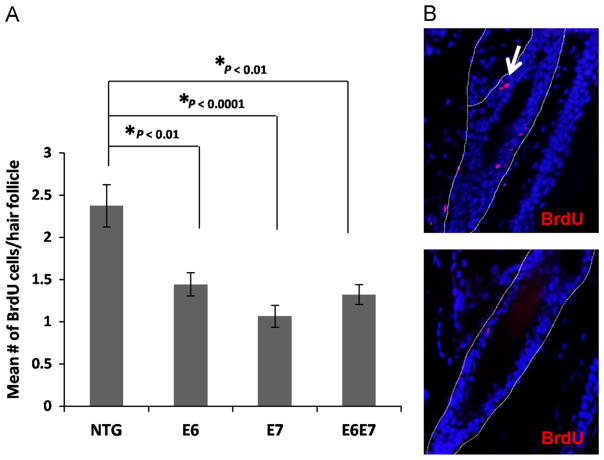
K14E6 and K14E7 mice have been previously generated and extensively characterized (Herber et al., 1996; Song et al., 1999). The keratin 14 promoter directs expression of the HPV16 oncogenes to the basal layer of stratified epithelia, including the bulge niche (Arbeit et al., 1994). In order to assess the effects of E6 and E7 expression in quiescent bulge stem cells, BrdU pulse-chase assays were performed as previously described (Cotsarelis et al., 1990; Bickenbach et al., 1986; Morris and Potten, 1999), and the numbers of label-retaining cells (LRCs) at second telogen (resting phase of hair cycle) were compared in wild type animals and animals transgenic for either one or both of the oncogenes (Fig. 1A). The numbers of LRCs were significantly reduced in mice expressing either one of the two, or both viral oncogenes suggesting either an overall reduction in the numbers of this particular stem cell type or enhanced proliferation, which could lead to a more rapid label loss.
Fig. 1. Expression of the HPV16 oncogenes leads to reduced detection of LRCs in hair follicle bulge at telogen.
(A) LRCs were labeled using a BrdU pulse administered shortly after birth and chased until second telogen. ~50 Hair follicles were selected from at least 3 mice of each genotype, NTG, E6, E7 and E6E7 mice. The mean number of BrdUrd positive cells per hair follicle bulge was quantified and plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. (B) Representative immunofluorescent figures of hair follicles showing BrdU positive cells (red–white arrow). Counterstaining was done with DAPI (blue).
Stem cells expressing the HPV16 oncogenes have increased mobilization upon acute anagen induction
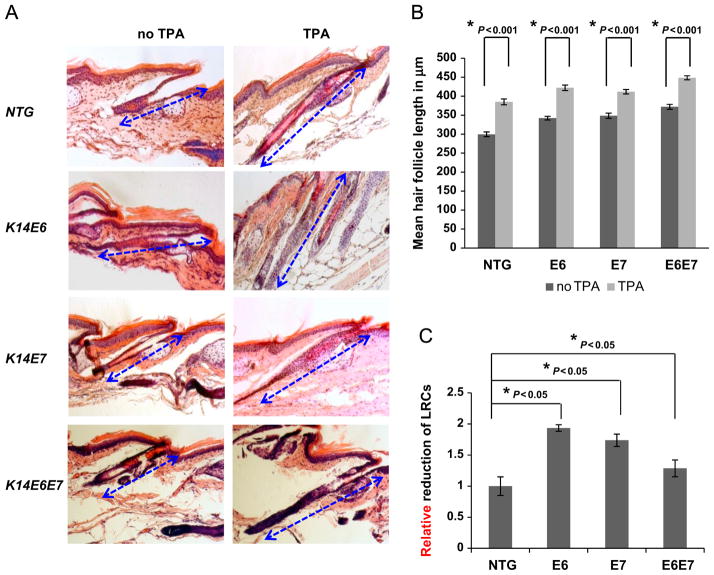
In order to further investigate the proliferation potential of stem cells expressing viral oncogenes, anagen was acutely induced. A pulse-chase protocol was performed as above and anagen was induced by repeated TPA administration prior to harvesting. Successful anagen induction was validated by the characteristic hair follicle elongation in all genotypes examined (Fig. 2A and B). Hair elongation was also observed in follicles expressing the HPV16 oncogenes under resting conditions. LRC mobilization was monitored as a function of BrdU label loss by means of BrdU-specific immunohistochemistry. In animals expressing E6, E7 or both oncogenes mobilization of stem cells was more pronounced compared to that seen in wild type animals (Fig. 2C) suggesting that HPV oncogene expression can lead to a higher level of basal proliferation, or even precocious anagen even in the absence of external anagenic stimuli. This result suggests that tissue stem cells expressing E6 or E7 are more poised to proliferate, explaining in part the reduced numbers of LRCs detected in telogen conditions (Fig. 1).
Fig. 2. Expression of the HPV16 oncogenes leads to more rapid mobilization of LRCs in response to acute anagen induction.
(A) In order to induce anagen in mice where LRCs were labeled, TPA was applied on mice every 48 h for four times. H&E staining was performed on all tail hair follicles. Hair follicle length was quantified to verify effective anagen induction. (B) ~70 Hair follicles were selected from at least 3 mice of each genotype, NTG, E6, E7 and E6E7 mice. The mean hair follicle length in μm was measured and plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. Statistical significance was also observed between NTG and transgenic mice under resting (no TPA) conditions. (C) In order to track the mobilization of LRCs in response to acute anagen induction, the percentage reduction of LRCs was tracked per genotype. At least 3 mice of each genotype at anagen (TPA) and telogen (no TPA), NTG, E6, E7 and E6E7 mice, were selected and hair follicle bulge regions were quantified. The relative reduction of BrdUrd positive cells per hair follicle bulge was plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. Statistical significance was also observed between E6 and E6E7 mice.
Combined expression of the HPV16 E6 and E7 oncogenes gives a robust proliferative ability in hair follicles
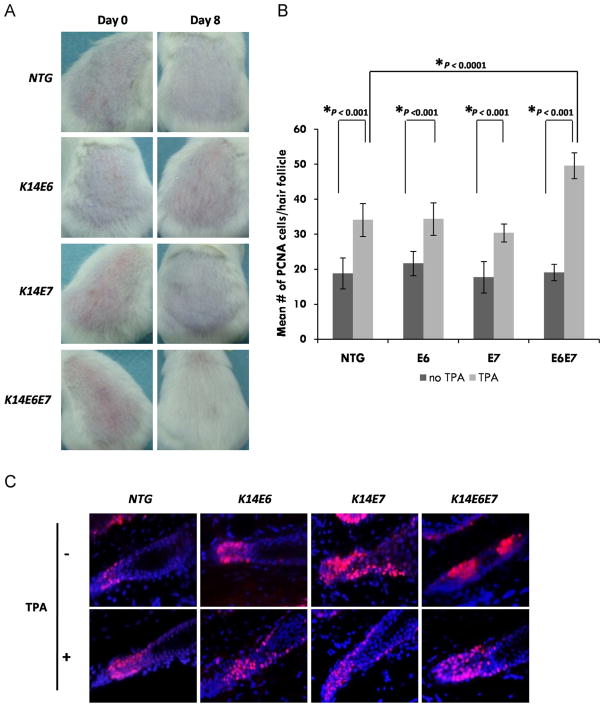
To investigate LRC mobilization outcome, hair re-growth was monitored. The backs of transgenic and control mice were shaved at day 65, previously characterized to correspond to the end of second telogen. Hair re-growth was monitored for up to 8 days post-shaving and was enhanced in mice expressing both E6 and E7 (Fig. 3A). Complete hair re-growth, consistent with increased tissue stem cell activity in those animals was only evident in mice bitransgenic for both E6 and E7. Also consistent with that result a greater increase in PCNA staining in anagen hair follicles from the bitransgenic animals was seen (Fig. 3B and C).
Fig. 3. Increased mobilization of LRCs correlates with increased hair growth in mice expressing the HPV16 E6 and E7 oncogenes.
(A) Backs of telogen mice of all genotypes were shaved and pictures were taken at days 0 and 8 after shaving. (B) ~50 Hair follicles were selected from at least 3 mice of each genotype, NTG, E6, E7 and E6E7 mice. The mean number of PCNA positive cells at the base of each hair follicle was quantified and plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. (C) Representative immunofluorescent figures of hair follicles showing PCNA positive cells (red). Counterstaining was done with DAPI (blue).
HPV16 oncogene expression does not lead to an overall reduction of other bulge stem cell markers
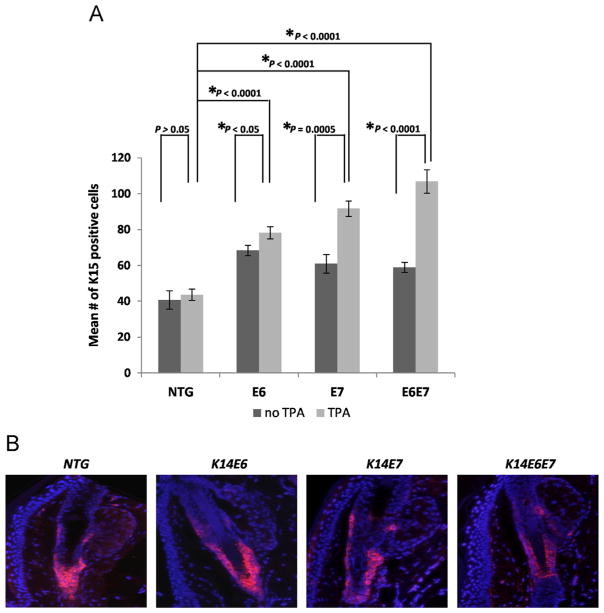
In order to eliminate the possibility that reduced LRC numbers (Fig. 1) are indicative of aberrant reduction in numbers of stem cells, endogenous markers of bulge stem cells, such as the expression of K15 was tested (Liu et al., 2003). The results showed an increase in the numbers of K15 positive cells and thus expansion of the K15 layer in both conditions of telogen and anagen (Fig. 4A and B). Thus, the reduction in LRC numbers is not consistent with an overall decrease in stem cell markers. On the contrary, an aberrant expansion in the K15 compartment is detected, suggesting that an increased ability to proliferate might not be the only change induced in stem cells by E6 and E7 expression.
Fig. 4. Other markers of bulge stem cells are not reduced in response to HPV16 oncogene expression.
(A) Immunofluorescence was performed using a K15-specific antibody. ~50 Hair follicles were selected from at least 3 mice of each genotype, NTG, E6, E7 and E6E7 mice. The mean number of K15 positive cells of each hair follicle was quantified and plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. Statistical significance was also observed between the NTG and the transgenic mice (no TPA) as well as between the various transgenics (TPA-treated). (B) Representative immunofluorescence of K15 staining (red) in the hair follicles of the genotypes examined. Counterstaining was done with DAPI (blue).
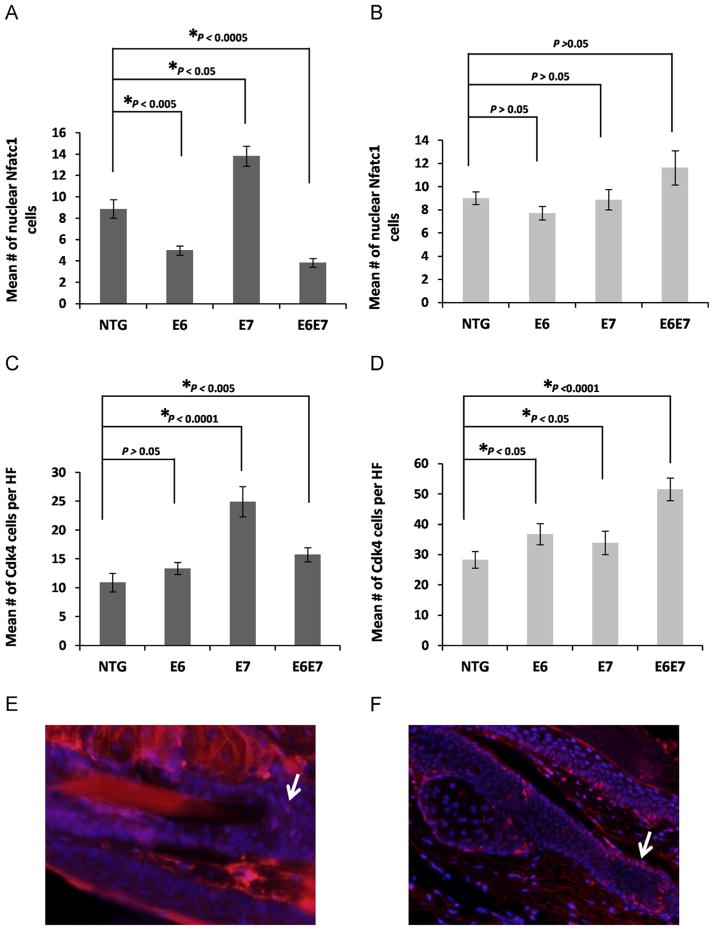
The Nfatc1 pathway is perturbed upon viral oncogene expression
In order to test whether the quiescence of bulge stem cells is affected by oncogene expression, the nuclear localization of the Nuclear factor of activated T-cells (Nfatc1), shown to be the gatekeeper of bulge stem cell quiescence, was tested in both telogen and anagen conditions (Horsley et al., 2008). No significant changes were observed in nuclear localization in conditions of follicle growth when the oncogenes were expressed (Fig. 5B). However, under resting conditions the expression of E6 or both E6 and E7 led to a significant reduction in the numbers of stem cells with nuclear Nfatc1 (expression of E7 alone did not have the same result, in fact it led to a significant increase of nuclear Nfatc1) (Fig. 5A). Furthermore, the expression of Cdk4, a downstream target of Nfatc1 and also a cell cycle gene implicated in the Rb pathway was tested showing an increase in both telogen and anagen conditions when the oncogenes are expressed (Fig. 5C and D).
Fig. 5. The quiescence of the bulge stem cell population is affected upstream as well as downstream of the Nfatc1 pathway by HPV16 oncogene expression.
(A–D) ~50 Hair follicles were selected from at least 3 mice of each genotype, NTG, E6, E7 and E6E7 mice. The mean number of Nfatc1 (A,B) and Cdk4 (C,D) positive cells of each hair follicle in telogen (no TPA) (A,C) and anagen (TPA) (B,D) conditions was quantified and plotted for each genotype (columns); bars, SD. All statistical comparisons were performed using a two-sided Wilcoxon rank sum test. (E,F) Representative immunofluorescense staining of (E) nuclear Nfatc1 and (F) Cdk4 positive cells is depicted by arrows. Counterstaining was done with DAPI (blue).
Discussion
Tissue stem cells have been implicated as the cells of origin in several cancer types. In the case of cervical cancers, they have been proposed to derive from multipotent cervical progenitors because tissue stem cells provide a reservoir of latently infected cells that support the viral life cycle (Maglennon et al., 2011), or because of differential infection or infection outcome in these types of cells. Despite the sustained interest around this topic i.e. the cell of origin in cancers, very little work addressing the expression of viral oncogenes in tissue stem cells has been done, mostly due to lack of understanding of what constitutes a true cervical multipotent cell, even though the prevailing dogma suggests that these reside in the so-called transformation zone. We have chosen the quiescent epithelial stem cells found in the hair follicle bulge region to assess the effects of E6 and E7 expression in vivo because this stem cell population is well-characterized. We propose that the expression of viral oncogenes in tissue stem cells promotes aberrant mobilization consistent with an improved ability of these cells to repopulate the tissue and possible roles in cancer initiation.
Two recent publications propose an alternate hypothesis to tissue stem cells as the cells of origin in cancers. These authors suggest that specific cell subpopulations of embryonic origin are the precursors of some premalignant, and consequently malignant lesions including some cervical malignancies (Herfs et al., 2012; Wang et al., 2011). This is due, at least in part, to the ability of these cells to outcompete neighboring populations in situations of tissue insult such as HPV infection. Of course, the proposed mechanism, and the traditionally held view that at least some cancers may be derived from the transformation zone are not mutually exclusive. The exact role of these squamocolumnar populations during normal tissue homeostasis is not known, nor is it known whether this anatomic location harbors cells with stem-like characteristics. In either case, our results support the notion that specific cell subpopulations which express the HPV16 oncogenes are more poised to repopulate the tissue, consistent with the cell-competition theory proposed.
In this study, we observed that expression of E6 and E7, the main viral oncogenes of HPV16, can compromise the relative quiescence of epidermal stem cells and lead hair follicles to precocious anagen entry as seen by the reduction in LRC numbers in both telogen and acute anagen conditions (Figs. 1 and 2C). This demonstration of disruption specifically of quiescent tissue stem cell homeostasis in vivo represents a novel phenotype associated with the expression of these oncogenes. Another recent study focusing on the effects of all HPV16 genes in non-quiescent populations of the hair follicle confirmed our findings (da Silva-Diz et al., in press). While the reduction in LRCs is a direct measure of proliferation in relatively quiescent populations which tracks the loss of label, staining for PCNA correlates to a great extent but not fully. The reason for this is that PCNA staining reflects the cumulative proliferation in the hair follicle including that derived from other populations.
To further verify that a reduction in LRCs is not indicative of a reduction in tissue stem cells overall, we characterized the expression of other stem cell markers. Expression of other stem cell markers such as K15 is not reduced in the presence of the oncogenes diminishing the possibility that the results seen are the result of reduced stem cell numbers (Fig. 4A). In contrast, there is an expansion of the K15 layer in both telogen and anagen conditions which might indicate the expansion of an aberrant population which shares some but not all stem cell characteristics. The expression of other proposed stem cell markers such as Lgr5 and Lgr6 (Jaks et al., 2008; Snippert et al., 2010), were tested by immunofluorescence but results were inconclusive.
In an attempt to explain the reduction in the LRC numbers, the nuclear localization of the quiescence marker Nfatc1 was also tested (Fig. 5). The results showed that the quiescence of the follicle cells can be compromised in resting conditions when E6 is expressed suggesting that the Nfatc1 pathway could be a possible direct or indirect target of the oncogene. Surprisingly, the number of Nfatc1 positive cells in hair follicles expressing E7 only was higher, despite all other evidence contrary to quiescence. Furthermore, in some cases (Fig. 5B and D) we observe an uncoupling of the expected relationship between the detection of nuclear Nfatc1 and its downstream target CDK4. This leads us to the conclusion that while under normal conditions anagen induction is marked by loss of nuclear Nfatc1, forced anagen induction need not occur by direct action on Nfatc1 but may in fact be marked by perturbation of the pathway downstream of it. In those cases, CDK4 is actually a more reliable marker of the balance between proliferation and quiescence. The expression of Cdk4, a downstream target of Nfatc1 and also a cell cycle regulatory gene involved in Rb homeostasis, increased both in the resting telogen and anagen conditions when E7 or both oncogenes are expressed (when E6 only is expressed the increase is statistically significant only in anagenic conditions) (Fig. 5C and D). The lack of exact correlation between the Nfatc1 and Cdk4 results in the E6 and E7 transgenic animals may suggest that E6 and E7 are affecting cellular quiescence in independent ways. For example it is more likely, that at least in resting conditions E7 is affecting factors downstream of Nfatc1 including Cdk4 which could be targeted directly (Fig. 5A and C). The increase in Nfatc1 positive cells in E7-expressing hair follicles may represent a yet-to be described compensation mechanism.
Our results indicate that relative quiescence and K15 are independent determinants of stemness and appear to be differentially regulated by the HPV oncogenes. We speculate that E6 and E7 may lead to cellular reprogramming giving rise to an aberrant stem-like population that is not relatively quiescent but expresses some markers of stemness such as K15. Future studies may shed some light on this interesting possibility.
Our work shows that the proliferation defects seen apply specifically to the quiescent label retaining cells and can be attributed to the viral oncogenes. Moreover, both oncogenes seem to be contributing to this phenotype, consistent with an important role for both in the context of carcinogenesis. Furthermore, the aberrant expansion of other markers of stem cells supports the development of an aberrant stem cell compartment, in the presence of HPV oncogenes, capable of enhanced proliferation.
The relevant cellular targets of E6 and E7 that are associated with this phenotype are not known, however, both E6 and E7 have the ability to modulate the function of cellular targets implicated in tissue stem cell biology. These include telomerase, pRb, E2F6 and polycomb group complexes and also histone demethylases. E6 can upregulate telomerase activity by intricate regulation at the transcriptional and posttranscriptional level (Liu et al., 2009). It has been shown that increased telomerase expression in the progenitor cell niche leads to quantitative and functional changes in this compartment (Flores et al., 2005; Sarin et al., 2005). The same applies for E7, whose prominent target pRb has also been shown to directly affect the progenitor cell niche (Ruiz et al., 2004). In conditional knockouts for RB, a parallel to the degradation seen by E7 expression, the LRCs of the skin are shown to be decreased. Furthermore, E7 has been shown to interact with E2F6 and in part inhibit its association with polycomb group complexes, which are crucial to cell fate decisions (McLaughlin-Drubin et al., 2008). These complexes can also be inhibited from binding to histone 3 by induction of histone demethylases KDM6A and KDM6B in E7 expressing cells (McLaughlin-Drubin et al., 2011).
To conclude, this study reveals the ability of the virus to modulate stem cells which could be crucial to their ability to contribute to carcinogenesis since these types of cells were shown to be the cells of origin in epithelial cancers. The enhanced ability of stem cells expressing HPV oncogenes to proliferate could put them in a better position to repopulate wounded tissue at the expense of non-infected cells. Continuous repopulation of tissue by cells expressing the oncogenes may be linked to ensuing carcinogenesis. Further studies to identify the exact molecular targets and pathways that are affected in the presence of the viral oncogenes could aid in the better understanding of stem cell homeostasis in the context of viral infection.
Materials and methods
Mice
Mouse strains were generated in the lab of Dr. Paul Lambert (University of Wisconsin, Madison). The mice used in the experiments were kept on a pure FVB/N inbred genetic background and were K14E6/E7TTL referred to as K14E6 or E6 and K14E7/E6TTL referred to K14E7 or E7 as previously described (Herber et al., 1996; Song et al., 1999). For the generation of bitransgenic mice K14E6 were crossed with K14E7 and the genotypes were confirmed by means of PCR. All the experimental mice used were in a heterozygous state. Mice were housed at the University of Cyprus, in accordance with regulations and protocols approved by the Cyprus Ministry of Agriculture.
BrdU incorporation, anagen induction and hair shaving
5-Bromo-2-deoxyuridine (BrdU) was administered peritoneally in mice at a final concentration of 50 mg/kg as first described previously (Bickenbach et al., 1986; Cotsarelis et al., 1990). For pulse chase experiments ten-day-old mice received an injection every 12 h for a total of four doses and they were euthanised 60 days after injections. For induction of anagen in age-matched mice, at day 60 after BrdU injection, hair was shaved and mice were treated every 48 h with TPA (20 nmol in acetone) for a total of four doses.
Immunohistochemistry
Mice were sacrificed, and tissues obtained were fixed in 4% paraformaldehyde overnight at 4 °C. Dehydration of the samples was performed in a graded series of ethanol concentrations and xylene before they were embedded in paraffin wax. Sections were obtained at 10 μm thickness on a microtome and left overnight to dry at room temperature. Samples were deparaffinised in xylene and rehydrated in a graded series of ethanol solutions. Antigen retrieval was done in a microwave using 10 mM citrate buffer and for BrdU immunohistochemistry, samples were also incubated for 20 mins in 2 M HCl. Blocking and antibody incubations were variable and optimal for each different antibody used. Primary antibodies used include: BrdU (Abcam), K15 (SantaCruz), Nfatc1 (SantaCruz), PCNA (SantaCruz). Following primary antibody incubation samples were washed in PBS. The following secondary antibodies were used: FITC-rabbit, Cy3-rat, Cy3-mouse, Cy3-streptavidin and biotin-rat all from Jackson ImmunoResearch and also Vectastain universal secondary (Vector laboratories). All images were acquired using a Zeiss Axio Observer.A1 microscope. Quantification was performed in a blinded fashion.
Statistical tests
To determine the statistical significance between the genotypes in each experiment, 3 mice of each genotype were used and 50–70 hair follicles were counted. Statistical analysis was done using “Mstat” software (version 5.5.3, McArdle Laboratory for Cancer Research, University of Wisconsin–Madison [http://mcardle.oncology.wisc.edu/mstat/]). Results were compared using a Wilcoxon rank sum test. For all statistical tests differences were considered statistically significant at p≤0.05.
Acknowledgments
The study was funded by University of Cyprus. The funding body had no role in the design of this study. We would like to acknowledge Ms. Amy Liem for her help with mouse care and shipping.
References
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994;68:4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auewarakul P, Gissmann L, Cid-Arregui A. Targeted expression of the E6 and E7 oncogenes of human papillomavirus type 16 in the epidermis of transgenic mice elicits generalized epidermal hyperplasia involving autocrine factors. Mol Cell Biol. 1994;14:8250–8258. doi: 10.1128/mcb.14.12.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickenbach JR, McCutecheon J, Mackenzie IC. Rate of loss of tritiated thymidine label in basal cells in mouse epithelial tissues. Cell Prolif. 1986;19:325–333. doi: 10.1111/j.1365-2184.1986.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun T, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- da Silva-Diz V, Sole-Sanchez S, Valdes-Gutierrez A, Urpi M, Riba-Artes D, Penin RM, Pascual G, Gonzalez-Suarez E, Casanovas O, Vinals F, Paramio JM, Batlle E, Munoz P. Progeny of Lgr5-expressing hair follicle stem cell contributes to papillomavirus-induced tumor development in epidermis. Oncogene. 2012 doi: 10.1038/onc.2012.375. http://dx.doi.org/10.1038/onc.2012.375, in press. [DOI] [PubMed]
- Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Nat Acad Sci. 2000;97:12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD, Xian W, Crum CP. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Nat Acad Sci. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jabbar SF, Abrams L, Glick A, Lambert PF. Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res. 2009;69:4407–4414. doi: 10.1158/0008-5472.CAN-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Nat Acad Sci. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PF, Pan H, Pitot HC, Liem A, Jackson M, Griep AE. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Nat Acad Sci. 1993;90:5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C. Identifying the cellular origin of squamous skin tumors. Proc Nat Acad Sci. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Nat Acad Sci. 2009;106:18780–18785. doi: 10.1073/pnas.0906357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111:3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Crum CP, Münger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Nat Acad Sci. 2011;108:2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Huh K, Münger K. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J Virol. 2008;82:8695–8705. doi: 10.1128/JVI.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D, Blanton R, Gown A, Mcdougall J. Altered expression of proliferation and differentiation markers in human papillomavirus-16 and papillomavirus-18 immortalized epithelial-cells grown in organotypic culture. Am J Pathol. 1992;140:167–177. [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morris R, Potten C. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Pitsouli C, Apidianakis Y, Perrimon N. Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe. 2009;6:301–307. doi: 10.1016/j.chom.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Santos M, Segrelles C, Leis H, Jorcano JL, Berns A, Paramio JM, Vooijs M. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development. 2004;131:2737–2748. doi: 10.1242/dev.01148. [DOI] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Rochat A, Zeltner R, Borenstein L, Barrandon Y, Wettstein FO, Iftner T. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells. J Virol. 1996;70:1912–1922. doi: 10.1128/jvi.70.3.1912-1922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgård R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Song S, Pitot HC, Lambert PF. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–5893. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ouyang H, Yamamoto Y, Kumar P, Wei T, Dagher R, Vincent M, Lu X, Bellizzi A, Ho K, Crum C, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]