Abstract
Sphingosine kinase signaling has become of increasing interest as a cancer target in recent years. Two sphingosine kinase inhibitors, SKI-II and ABC294640, are promising as potential breast cancer therapies. However, evidence for their therapeutic properties in specific breast cancer subtypes is currently lacking. In this study, we characterize these drugs in luminal, endocrine resistant (MDA-MB-361) and Basal-A, triple negative (MDA-MB-468) breast cancer cells and compare them with previously published data in other breast cancer cell models. Both SKI-II and ABC294640 demonstrated greater efficacy in Basal-A compared to luminal breast cancer. ABC294640, in particular, induced apoptosis and blocked proliferation both in vitro and in vivo in this triple negative breast cancer system. Furthermore, Sphk expression promotes survival and endocrine therapy resistance in previously sensitive breast cancer cells. Taken together, these results characterize sphingosine kinase inhibitors across breast cancer cell systems and demonstrate their therapeutic potential as anti-cancer agents.
Keywords: sphingolipids, chemoresistance, sphingosine kinase, breast cancer, ceramide, experimental therapeutics, sphingosine-1-phosphate
Introduction
The sphingolipid signaling pathway has been of increasing interest in recent years as a cancer therapeutic target (1). The sphingolipid, ceramide, is known to be an important element of both endogenous and chemotherapy-induced apoptosis. Furthermore, its metabolite, sphingosine-1-phosphate (S1P), is known to promote cancer survival, proliferation, and metastasis (2, 3). The sphingosine kinase (Sphk) converts ceramide into S1P and is known to be overexpressed in a number of cancer tissues, including breast, kidney, thyroid, and prostate (1). Of particular interest, breast cancer has become a model tissue for aberrant sphingolipid signaling in cancer (4, 5). Sphingosine kinase has been shown to increase proliferation and survival in estrogen receptor (ER)-positive breast cancer as well as poor prognosis in ER-negative breast cancer (3, 6).
Breast cancer is a complex disease consisting of multiple genotypes resulting in various phenotypes in the clinic. There is much debate in the literature concerning the relevance and translational potential of breast cancer cells, however, their significance and contribution to our understanding of the biology of cancer is crucial. There are numerous breast cancer cell lines representing dozens of phenotypes and determining the proper cell to best represent the clinical environment can be difficult (7). These cell lines originate from various biopsy sources (Table 1) and exhibit varying genetic profiles (Table 2). Considering the restrictions on using genetic mouse models of cancer because of the ‘OncoMouse patents’ held by private companies, many human breast cancer cell lines are also used in vivo in the form of xenografts in immunocompromised mice (8). The ability of a drug to target a broad range of breast cancer subtype is an important feature of any experimental therapeutic.
Table 1.
Tumor Type and Clinical Source of Human Breast Cancer Cell Lines (7)
| Cell Line | Tissue | Tumor type | Age | Ethnicity | Source |
|---|---|---|---|---|---|
| MCF10A | Breast | Normal | 36 | Caucasian | Primary Breast |
| MCF-7 | Breast | Invasive Ductal | 69 | Caucasian | Pleural effusion |
| MCF-7TN-R | Breast | Invasive Ductal | 36 | Caucasian | Pleural effusion |
| MDA-MB-231 | Breast | Adenocarcinoma | 51 | Caucasian | Pleural effusion |
| MDA-MB-361 | Breast | Adenocarcinoma | 40 | Caucasian | Primary Breast |
| MDA-MB-468 | Breast | Adenocarcinoma | 51 | African American | Pleural effusion |
Table 2.
Genetic and Phenotypic Characterization of Human Breast Cancer Cell Lines (7)
| Cell Line | Gene Cluster | ER Status | PR Status | Metastatic | Drug Status |
|---|---|---|---|---|---|
| MCF10A | Basal B | Negative | Negative | No | Sensitive |
| MCF-7 | Luminal | Positive | Positive | No | Sensitive |
| MCF-7TN-R | Basal-like | Negative | Negative | Yes | Chemoresistant |
| MDA-MB-231 | Basal B | Negative | Negative | Yes | Endocrine Resistant |
| MDA-MB-361 | Luminal | Positive | Negative | Yes | Endocrine Resistant |
| MDA-MB-468 | Basal A | Negative | Negative | Yes | Endocrine Resistant |
Although clinical breast cancer is a heterogeneous disease, a new genetic classification of breast cancer was established to characterize tumors. Four broad subtypes of clinical breast cancer have been defined based on genetic studies of tumor samples: luminal (generally ER-positive), basal-like (ER-negative), HER2-postive and, most recently, Claudin (7, 9–12)]. These cancers have distinct genetic profiles as well as clinical outcomes. Luminal cancers are the most common, making up approximately 69% of breast tumors whereas basal-like tumors account for 12–15% (10). Basal-like cancers are fairly heterogeneous but basal-like cancers can be further broken down into Basal-A and Basal-B groups. These tumor subtypes possess varying clinical characteristics, with basal cancers being more aggressive and correlating with increased mortality compared with the luminal and HER2 subtypes. Basal-A and Basal-B have distinct protein and gene expression profiles (7, 13). In general, Basal-B cells are less differentiated, exhibit greater epithelial-to-mesenchymal transition changes, and are more invasive compared to Basal-A breast cancer (7). The Basal-A gene expression profile is more similar to luminal cells compared to Basal-B. Furthermore, the response to both endocrine and chemotherapies varies depending on subtype (9, 14). For example, HER2 cancers are responsive to Herceptin, making the overall mortality rate of this subtype low compared with others. Luminal cancers usually respond to first line endocrine therapies such as tamoxifen. Yet, there are currently no targeted therapies for basal or endocrine therapy resistant luminal cancers.
There are multiple non-cancerous human mammary cell lines commercially available. One commonly used cell model is the Michigan Cancer Foundation 10A (MCF10A) cell line. Michigan Cancer Foundation 10A cells were originally isolated from fibrocystic breast tissue obtained from a reduction mammoplasty of a 36 year old woman with no evidence or family history of breast cancer. Subsequently, MCF10A have been characterized as immortalized, semitransformed breast epithelial cells and generally represent a normal breast phenotype for comparison with breast cancer cell lines (7, 15–18). Alternatively, several ER-positive cell lines are frequently studied in context of human breast cancer. The mainstay of endocrine responsive, endocrine therapy sensitive luminal breast cancer is the MCF-7 cell line. The MCF-7 cell line was isolated from the pleural effusion of a 69 year-old, postmenopausal, Caucasian woman with metastatic breast cancer in 1973. These cells are ER, PR positive, and HER2/Neu negative and have become the model for ER-positive breast adenocarcinoma in the laboratory (19). MCF-7 cells represent a drug sensitive, minimally invasive, ductal phenotype of breast carcinoma found in the clinic. Due to the wide dissemination of this cell line and its prolonged use in the laboratory, the sensitivity of MCF-7 cells to various agents is variable. In general, these cells are estrogen responsive and sensitive to endocrine therapy in the form of SERMs and SERDs as well as the apoptotic effects of TNF and chemotherapeutic agents (5, 20). Alternately, the M.D. Anderson-Metastatic Breast-361 (MDA-MB-361) cell line was derived from a metastatic brain site of a adenocarcinoma tumor of a 40 year old Caucasian woman. These cells are Basal-A subtype, ER-positive, and PR-negative and are metastatic in vivo. The MDA-MB-361 cell line poses as an alternative to MCF-7 as an ER-positive, breast cancer cell line and represents an estrogen dependent, endocrine therapy resistant, invasive breast cancer (7, 18, 21).
There are a multitude of cell lines used to represent hormone independent, endocrine therapy resistant breast cancer, often referred to as ‘triple negative’ because of their lack ER/PR/HER2 expression. The most studied of these cell lines is the MDA-MB-231 cell line, in which the Basal-B adenocarcinoma cells are derived from the pleural effusion of a 51 year-old Caucasian woman with metastatic breast cancer. Morphologically, these cells exhibit EMT changes, such as loss of E-cadherin and increased expression of N-cadherin. These cells are highly invasive and metastatic both in vitro and in vivo (7, 22). Similarly, MDA-MB-468 cells are triple negative and metastatic, though genetically they belong to the Basal-A subtype. The MDA-MB-468 cells were isolated in 1977 from a primary breast tumor tissue of a 51 year-old African American woman after treatment with adriamycin and cytoxan. These cells overexpress EGF, have decreased expression of PTEN and p53, and exhibit a constitutively active ERK/MAPK signaling pathway. These cells are highly aggressive and, similar to MDA-MB-231 cells, form large metastatic tumors in vivo that are SERM, SERD, and herceptin resistant (7, 23, 24). MDA-MB-231 and MDA-MB-468 cell lines represent varying genetic profiles of hormone independent, endocrine therapy resistant breast cancer in the laboratory.
The basal-like MCF-7TN-R cell line is a MCF-7 derived, ER/PR/HER2 negative, chemoresistant breast cancer cell line. These cells were derived by growing MCF-7 cells in increasing concentrations of TNFα until resistance was established (25–27). Altered death receptor signaling is a hallmark of these cells because they exhibit increased TNFR1 and p50 expression and high basal NF-κB transcriptional activity relative to MCF-7 cells (28). These cells exhibit morphologic EMT changes and are highly aggressive both in vitro and in vivo. The MCF-7TN-R cells display increased resistance to a several chemotherapeutic agents compared with MCF-7 cells, including doxorubicin, etoposide, and paclitaxel (5, 26). These cells represent a model of transition to chemoresistance and aberrant death receptor signaling in breast carcinoma (4, 5, 27, 29–31).
A number of sphingosine kinase inhibitors (SKI) have been developed in recent years, with the two most prevalent being SKI-II and ABC294640. SKI-II is the first commercially available inhibitor and is known to target both isoforms of Sphk (32, 33). ABC294640 is a Sphk2 selective inhibitor and was developed using SKI-II as the parent compound (34). Both SKI-II and ABC294640 are orally bioavailable and exhibit limited toxicity in animal model experiments. Currently, both of these inhibitors have been characterized in a number of breast model systems, ranging from normal breast tissue to multidrug resistant metastatic cancer cell systems (32–38). Both inhibitors selectively target particular types of breast cancer cells without affecting normal breast cells (35–37). Unfortunately, research with these inhibitors is noticeably lacking in several breast cancer subtypes. These include luminal, endocrine-resistant Basal-A, triple negative breast cancers. Both of these cancers are metastatic clinical tumors which exhibit endocrine therapy resistance. We further examine the use of sphingosine kinase inhibitors as a potential treatment in breast cancer cell systems that represent these cancers, MDA-MB-361 and MDA-MB-468. In addition, to facilitate our understanding of the overall potential of these inhibitors as breast cancer therapeutics we compare the use of SKIs in these cell systems to previously published data in other breast cancer cell systems (MCF10A, MCF-7, MDA-MB-231, and MCF-7TN-R).
Materials and Methods
Reagents
ABC294640 (3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)-amide) was provided by Apogee Biotechnology Corporation (Hummelstown, PA). SKI-II (4-(4-(4-chloro-phenyl)-thiazol-2-ylamino)-phenol) was purchased from Sigma-Aldrich. Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific.
Cell Culture
The MCF-7 human breast adenocarcinoma cell line from the American Type Culture Collection (ATCC; Manassas, VA) was generously provided by Louise Nutter (University of Minnesota, Minneapolis, MN) (20). MCF-7TN-R cells were generated by exposing MCF-7 cells to increasing concentration of TNFα until resistance was established (27). MDA-MB-468 cells were obtained from ATCC. ER-positive MDA-MB-361 were generously provided by Dr. Hongwu Chen (University of California Davis, Sacramento, CA). All cancer cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; pH 7.4; Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Salt Lake City, UT), 1% NEAA, MEMAA, sodium pyruvate, antibiotic/anti-mitotic, and insulin under mycoplasma-free conditions at 37°C in humidified 5% CO2 and 95% air. For studies examining the role of estrogen, cells were washed with phosphate buffered saline (PBS) 3 times and grown in phenol red-free Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% dextran-coated charcoal-treated fetal bovine serum (5% CS-FBS) for 72 h before plating for each particular experiment.
Generation of Sphk1 Overexpressing Cell Line
The protocol for the generation of stable cells lines was modified from previously published transfection methods (26, 39). MCF-7-GFP cells were plated in 10cm2 polystyrene dishes and allowed to adhere at 37°C. After 24 hours, plates were transfected with Homo sapiens sphingosine kinase 1 (SPHK1), transcript variant 1 or empty vector control (Origene Technologies, Inc., Rockville, MD). Transfection was accomplished using Lipofectamine (3:1 ratio) as per manufacturer’s instructions. Three days post transfection, cells were treated with neomycin (200 µg/mL; Invitrogen Corp., Carlsbad, CA). Cells were fed with media every 3 days and monitored for fluorescence. When fluorescent colonies formed, multiple clonal colonies were selected, passaged and growth monitored. Clones were then visually assessed for stable expression and Sphk1 expressions were confirmed by RT-PCR.
Clonogenic Survival Assay
Colony assays were performed as described in previously published methods (27, 40). Cells were plated in 6-well plates at a density of 1000 cells per well in full DMEM media. After twenty-four hours cells were treated with ABC294640 (0.1–10 µM) and then monitored for colony growth. After ten days the cells were fixed with 3% glutaraldehyde. Following fixation for 15 min, the plates were washed and stained with a 0.4% solution of crystal violet in 20% methanol for 30 min, washed with PBS, and dried. Colonies of ≥ 30 cells were counted as positive. Results were normalized to DMSO vehicle treated control cells. Statistical analysis of IC50 values were calculated from concentration-response curves using GraphPad Prism 5.0 (Graphpad Software, San Diego, CA), using with the equation: Y = Bottom + (Top-bottom)/ 1 + 10LogEC50-X
Cell Viability Assay
Viability assays were performed as described previously (25, 27). Briefly, cells were plated at a density of 7.5 × 105 cells per well in a 96-well plate in phenol-free DMEM supplemented with 5% FBS and allowed to attach overnight. Cells were then treated with SKI (ranging from 10nM to 100µM) for 24 h. Following treatment, 20 µL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml) reagent was incubated in each well for 4 h. Cells were lysed with 20% SDS in 50% dimethylformamide. The pH and absorbances were read on an ELx808 Microtek plate reader (Bio-Tek Instruments, Winooski, VT) at 550 nm, with a reference wavelength of 630 nm.
Cell Death Detection ELISA
The induction of apoptosis was determined as previously described using the nucleosome ELISA kit (Roche) (37). Cells were plated at 10,000 cells per well in 96-well plates and treated for 24 hours with SKI. The induction of apoptosis was determined by the amount of nucleosomes in the cytoplasm according to the manufacturer’s protocol. Absorbances were read on an ELx808 Microtek plate reader (Bio-Tek Instruments, Winooski, VT) at 405 nm.
Cell Proliferation Immunofluorescence Assay
Proliferation assays were performed as previously described (35, 37). Cells were plated at a density of 10,000 cells per well in a 96 well plate in 10% DMEM media and allowed to attach overnight. The following day, cells were treated with DMSO or SKI for 24 h. At endpoint, cells were fixed using 100 µL of 3.7% formaldehyde in PBS for 10 min. Formaldehyde was removed and cells were permeabilized using cold methanol for 5 min at room temperature and washed twice with PBS. 100 µL of 3% FBS in PBS blocking buffer was then added. After 30 min, blocking buffer was removed and cells were incubated for 1h with Ki-67 (BD Pharmingen, San Diego, CA) antibody. Cells were washed with PBS and stained with DAPI nuclear stain for 5 min before imaging. For staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view/image. The vehicle control was set to 1 for comparison with SKI-II treatment.
Animals
All procedures involving animals were conducted in compliance with State and Federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Tulane University Animal Care and Use Committee. The facilities and laboratory animal program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All the animal surgeries and other procedures were performed under anesthesia using a mix of isofluorane and oxygen delivered by mask.
Xenograft models were performed similar to previously reported studies (41). In brief, Nu/nu or SCID/BEIGE immunocompromised female ovariectomized mice (29–32 days old) were obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment ad libitum. Placebo or estradiol pellets (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL) were implanted s.c. in the lateral area of the neck in the middle point between the ear and shoulder using a precision trochar (10 gauge). MDA-MB-468 cells in the exponential growth phase were ed using PBS/EDTA solution and washed. Viable cells (5 × 106) in a 50 µL sterile PBS suspension were mixed with 100 µL Matrigel (BD Biosciences, Bedford, MA). Cells were injected in the mammary fat pad through a 5 mm incision in the hypogastric region, and the incision was closed using staples. Tumors were allowed to form over 10 days and mice were randomized to two treatment groups, vehicle control and ABC294640, with 5 mice per group. The ABC294640 mixture was suspended in a solution of DMSO and PBS and was given i.p. at 50 mg/kg for 15 days starting after tumors were measureable. Control mice were injected with vehicle daily for 15 days. Tumor size was measured every 2 days using a digital caliper. The volume of the tumor was calculated using the following formula: 4/3π LS2 (L = larger radius; S = shorter radius). At necropsy on day 24, animals were euthanized by cervical dislocation after exposure to a CO2 chamber. Tumors were removed and either frozen in liquid nitrogen or fixed in 10% formalin for further analysis.
Immunohistochemistry
Immunohistochemistry was performed as described in previously published methods (36, 37). Tumor explants were collected at necropsy, and fixed in 10% buffered formalin phosphate. FFPE 4-lM-thick tumor sections were analyzed by immunohistochemistry using primary monoclonal antibodies against human Ki-67 and BCL-2 (DAKO North America, Inc., Carpinteria, CA). The mouse antibodies on mouse tissue polymer detection kit (Biocare Medical, LLC, Concord, CA) were used to perform IHC. Briefly, FFPE sections were deparaffinized and hydrated in a graded series of ethanol solutions followed by 3% H2O2 for 5 min to inactivate endogenous peroxides, then rinsed. Slides were subjected to 10 min incubation in Avidin followed by 10 min incubation in Biotin. For antigen retrieval, sections were exposed to Rodent decloaker (Biocare Med.) at 95°C for 25 min, rinsed, and allowed to cool to room temperature for 20 min. Slides were incubated with Rodent block for 30 min and then with primary antibodies or serum alone (negative control) for 75 min. Mouse-on-mouse HRP-polymer secondary antibody was added to the sections and incubated for 15 min. After rinsing, DAB solution (Biocare Med.) was applied and incubated for 1 min, and sections were counterstained with hematoxylin (Biocare Med) followed by Tacha Blueing reagent (Biocare Med.) for 30 s each. Slides were then allowed to air dry and then cover slipped using Acrymount (Fisher Scientific Inc., Waltham, MA). Sections were viewed and photographed using the Leica DM IRB Inverted Research microscope and SPOT RT color camera. Five images at 40x were taken of each tumor. Care was taken to avoid areas of necrosis. For staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view/image.
Statistical analysis
Statistical analyses were performed as previously described (36, 37). Briefly, IC50 values were calculated from concentration-response curves using GraphPad Prism 5.0 (GraphPad Software), using with the equation:
Y = Bottom + (Top-bottom)/ 1 + 10LogEC50-X
assuming a standard slope, where the response goes from 10% to 90% of maximal as X increases over two log units. Differences in IC50 were compared using Student’s unpaired t-test with p<0.05 as the limit of statistical significance. Experiments comparing multiple concentrations to the control were tested with one-way ANOVA with Bonferroni post-test to compare individual concentrations. All statistical analyses were done using GraphPad Prism 5.0 (GraphPad Software).
Quantitative Real Time RT-PCR
Real time RT-PCR was performed similar to previously reported studies (42, 43). In brief, total cellular RNA was extracted using the RNeasy® mini column (Qiagen, Valencia, CA), following the manufacturer’s instructions. The concentration of RNA was determined using an ultraviolet spectrophotometer. Reverse transcription (RT) was performed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). The level of Sphk1 and Sphk2 transcripts was determined using the iQ5 real-time quantitative PCR detection system (BioRad Inc., Hercules, CA). Primers for PCR were designed to span intron/exon junctions to minimize amplification of residual genomic DNA. The primer sequences for genes studied are as follow: (sense and anti-sense, respectively): Actin: (F) 5’- TGA GCG CGG CTA CAG CTT -3’, (R) 5’ – CCT TAA TGT CAC ACA CGA TT – 3’; SPHK1: (F) 5’- GCCGATACTTCTCACTCTC - 3’ (R) 5’ – ATGAACCTGCTGTCTCTG - 3’; SPHK2: (F) 5’- GCCACCTACGAAGAGAAC - 3’ (R) 5’ – TGACCAATAGAAGCAACCG - 3’. All primers were obtained from Invitrogen (Carlsbad, CA). The PCR reaction was carried out in the following manner: step 1) 95°C 3 minutes; step 2) for 40 cycles 95°C 20 seconds, 60°C 1 minute; step 3) 70°C 10 seconds, hold at 4°C. Each reaction tube contained: 12.5 µl 2× SYBR Green supermix + 6.5 µl nuclease-free water + 1 µl 0.1 µg/µl primer (pair) + 5 µl cDNA (0.2 µg/µl). Genes were amplified in triplicate. Data was analyzed by comparing relative target gene expression to actin control. Relative gene expression was analyzed using 2−ΔΔCt method. RNA isolation, cDNA synthesis and qPCR were performed as previously described and outlined above.
Results
Overexpression of Sphk increases long-term survival and confers endocrine resistant in invasive luminal carcinoma cells
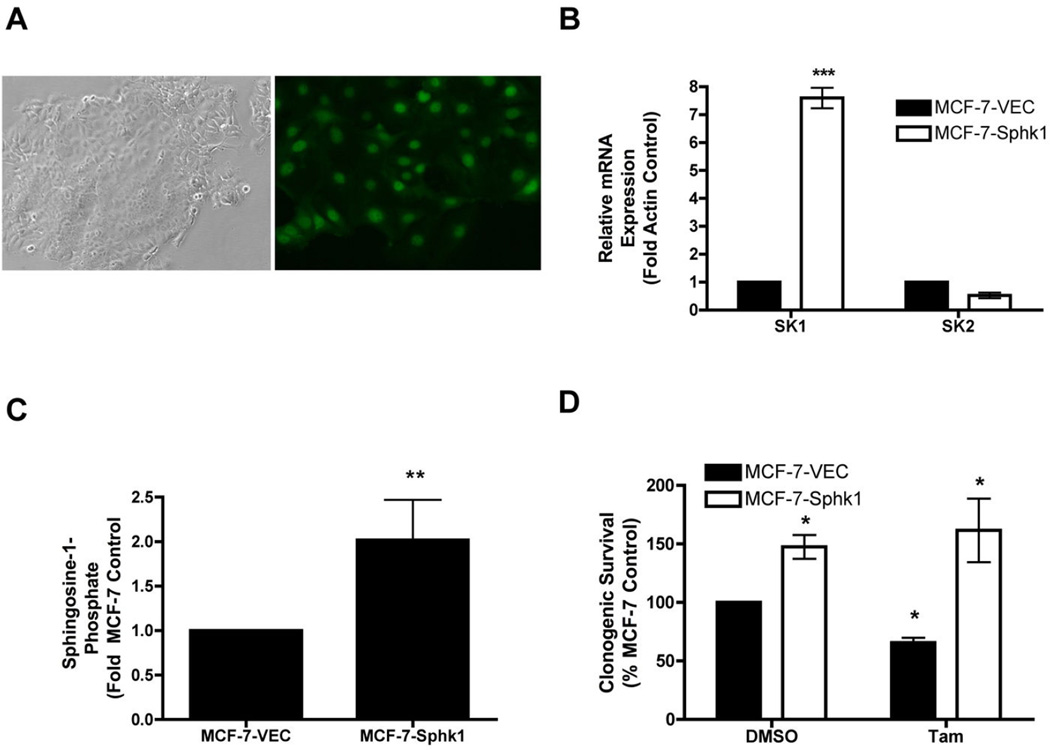
Sphingosine kinase is known to mediate the promotion and proliferation of breast cancer (4). Furthermore, studies have shown that Sphk1 overexpression confers resistance to TNF and doxorubicin (44). Unfortunately, there have been few studies concerning the effects of sphingosine kinase on endocrine therapy resistance (3). Therefore, to determine the effect of Sphk1 on endocrine therapy, we utilized the well-studied luminal MCF-7 system. We stably overexpressed Sphk1 in MCF-7 cells expressing a GFP tag (Figure 1a). These MCF-7-Sphk1 cells have a 7.60 ± 0.36 fold (p<0.05) increase in Sphk1 mRNA expression (Figure 1b) and a 2.02 ± 0.34 (p<0.05) fold increase in downstream sphingosine-1-phosphate levels (Figure 1c). Using these cells we determined the effect of Sphk on clonogenic survival and response to endocrine therapy treatment in comparison to parental MCF-7-VEC cells. Interestingly, overexpression of Sphk1 increased overall cancer cell clonogenic survival (Figure 1c) and conferred resistance to tamoxifen compared with parental MCF-7-VEC cells (Figure 1d). Thus, increased Sphk may play a role in endocrine therapy resistance in luminal subtype cancers. These results provide proof of principle that Sphk is a potential therapeutic target for endocrine resistant, ER-positive cancers, such as the MDA-MB-361 cell system.
Figure 1. Generation and characterization of Sphk1 overexpressing MCF-7 cell line.
MCF-7-GFP cells were stably transfected with Sphk1 or Vector control. (A) Images of MCF-7-Sphk1 cells taken at 100×. (B) qRT-PCR expression of Sphk1 and Sphk2 in MCF-7-VEC and MCF-7-Sphk1 cells. Results shown as mean fold change of three independent experiments ± S.E.M. (C) Endogenous S1P levels in MCF-7-VEC and MCF-7-Sphk1 cells. S1P levels normalized to MCF-7-VEC control. Mean values of ± S.E.M. of three different experiments in duplicate are reported. (D) MCF-7 and MCF-7-Sphk1 cells were plated at 500 cells per 60mm2. The following day, cells were treat with ICI or tamoxifen for 10–14 days. Colonies greater than 50 cells were scored as positive for colony formation. Data are presented as percent of MCF-7 vehicle treated samples. Mean values of ± S.E.M. of three different experiments in duplicate are reported.
Characterization of SKIs in ER-positive, endocrine resistant breast cancer
Our findings suggest a potential link between Sphk and endocrine resistance. Therefore, we utilized the unstudied MDA-MB-361 cell system to determine the biological effects of SKIs in ER-positive, endocrine resistant breast cancer. As discussed above, MDA-MB-361 cells are a luminal and resistant to endocrine therapies such as tamoxifen (7, 39). Previous breast cancer studies determined that SKIs can decrease breast cancer viability in various cell systems (26, 45, 46). We initially screened both SKI-II and ABC294640 on ER-positive, metastatic breast cancer viability. Unlike previous findings, neither SKI nor ABC294640 exhibited potent anti-viability properties in this breast cancer subtype, with IC50 values of >1000 µM (p<0.001) and 765 ± 8.62 µM (p<0.001), respectively. As shown in Table 3, these results were somewhat surprising, considering that previous studies demonstrated opposing viability effects compared to MDA-MB-361 cell lines.
Table 3.
| Cell System | SKI-II | ABC294640 | p-value |
|---|---|---|---|
| MCF10A | >1000 | >1000 | p<0.01 |
| MCF-7 | 13.0 ± 1.21 | 21.85 ± 3.24 | p<0.001 |
| MCF-7TN-R | 4.43 ± 1.25 | 8.83 ± 1.13 | p<0.001 |
| MDA-MB-231 | 11.77 ± 217 | 22.39 ± 5.11 | p<0.001 |
| MDA-MB-361 | >1000 | 765 ± 8.62 | p<0.001 |
| MDA-MB-468 | 15.8 ± 3.09 | 19.16 ± 3.06 | p<0.001 |
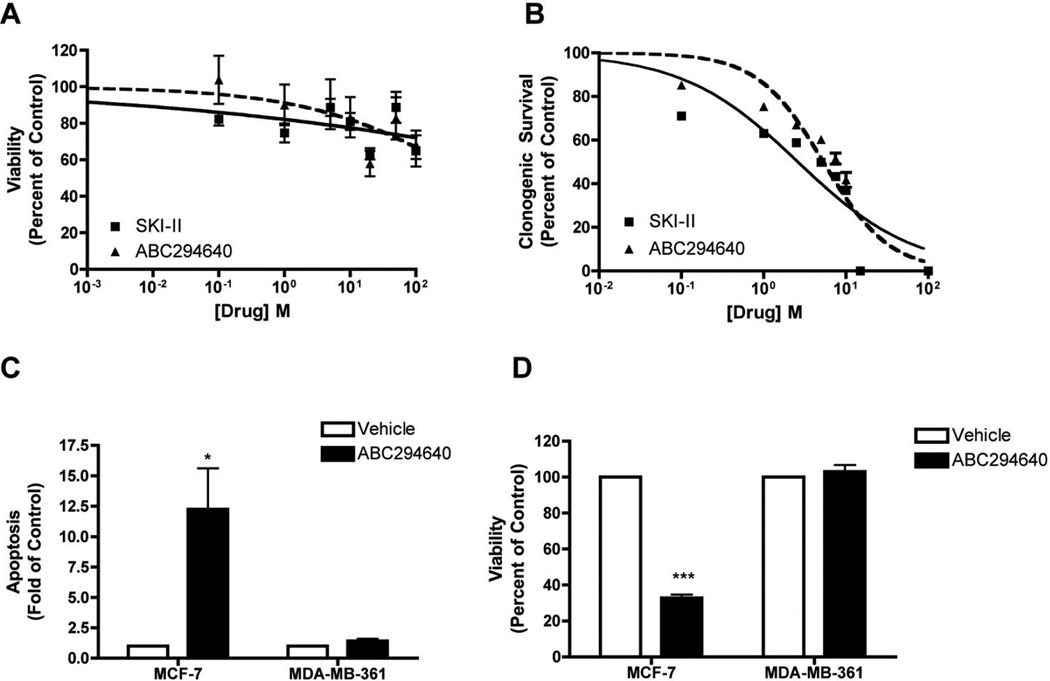
The efficacy of short-term viability assays in predicting therapeutic potential has been disputed in the literature, with some researchers suggesting that long-term assays provide a better clinical model (47). The ability of SKIs to block breast cancer colony formation in other breast cancer cell lines. Therefore, we determined the effects of these inhibitors on long-term clonogenic survival in MDA-MB-361 cells. As predicted, both SKI-II and ABC294640 dose-dependently decrease breast cancer clonogenic survival (Figure 2b). SKI-II exhibited an IC50 value of 2.63 ± 1.25 µM (p<0.001), while ABC294640 had an IC50 value of 5.32 ± 1.13 µM (p<0.001). These low micromolar results are similar to the IC50 values in other breast cancer model systems (Table 4).
Figure 2. Effect of SKIs on MDA-MB-361 metastatic breast cancer.
(A) Cells were plated at 7.5 × 105 cells per 96 well plate. The following day cells were treated with indicated concentrations of SKI-II (solid line) or ABC294649 (dotted line) for 24 h. Data are presented as percent of vehicle treated samples. Mean values of ± S.E.M. of five different experiments in quadruplicate are reported. (B) Cells were plated at 500 cells per 60 mm2. The following day, cells were treated with SKI-II (solid line) or ABC294649 (dotted line) for 10–14 days. Data are presented as percent of vehicle treated samples. Mean values of ± S.E.M. of three different experiments in duplicate are reported. (C) MCF-7 and MDA-MB-361 cells plated at 10,000 cells per well in a 96 well plate and treated with 50µM of ABC294640 for 24h. Following treatment, cells were measured for defragmented oligonucleotides as a measure of apoptosis using ELISA analyses and (D) cells were analyzed for viability using MTT analyses. Mean values of ± S.E.M. of three different experiments in duplicate are reported.
Table 4.
| Cell System | SKI-II | ABC294640 | p-value |
|---|---|---|---|
| MCF-7 | 2.04 ± 1.08 | 3.15 ± 1.16 | p<0.001 |
| MCF-7TN-R | 2.70 ± 1.05 | 5.21 ± 1.10 | p<0.001 |
| MDA-MB-231 | 2.51± 1.08 | 2.9 ± 1.16 | p<0.001 |
| MDA-MB-361 | >1000 | 765 ± 8.62 | p<0.001 |
| MDA-MB-468 | 1.20 ± 1.20 | 4.88 ± 1.14 | p<0.001 |
While Sphk1 is known to inhibit apoptosis in breast cancer, the role of Sphk2 in programmed cell death is less clear. Recent studies have demonstrated ABC294640 to have pro-apoptotic properties in ER-negative MCF-7TN-R cells (26). Therefore, we determined whether Sphk2 plays a similar role in ER-positive breast cancers, which are phenotypically distinct from triple negative breast cancer. Both endocrine sensitive MCF-7 and endocrine resistant MDA-MB-361 cells were treated with equivalent doses of ABC294640 and assessed for levels of fragmented DNA oligonucleotides as a measure of cell death. Interesting, we found that ABC294640 increased apoptosis 12.24 ± 3.38 fold (p<0.05) compared with control in MCF-7 cells. The decrease in viability at the same dose suggests that induction of apoptosis may be an anti-viability mechanism of ABC294640 in ER-positive breast cancer. These results are similar to those we found in chemoresistant breast cancer (37). Interestingly, pharmacological inhibition of Sphk2 does not alter apoptosis, or corresponding viability, in the MDA-MB-361 cell system. Since ABC294640 is more effective in long-term clonogenic survival assays than in short-term viability assays, it is possible that pharmacological induction of apoptosis in the MDA-MB-361 cell system takes longer than 24 h. However, these data demonstrate that SKIs are not as efficacious in luminal, endocrine resistant breast cancer compared to other breast cancer subtypes, such as luminal endocrine sensitive and basal-like tumors.
Characterization of SKIs in Basal-A, triple negative breast cancer
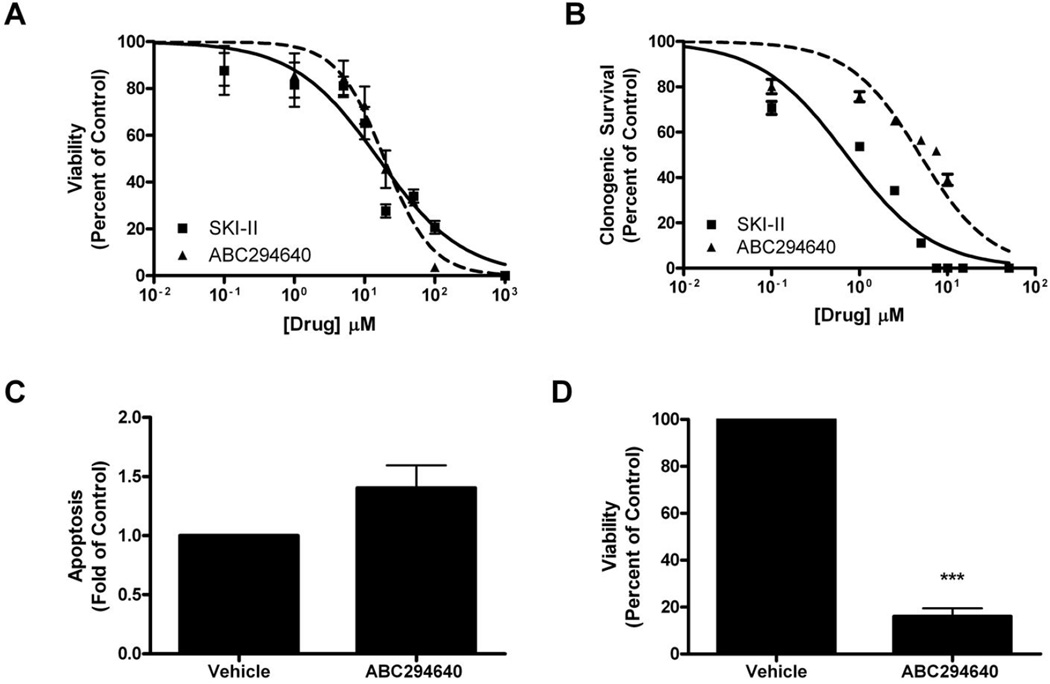
Previous studies have demonstrated that SKIs can block viability and survival in Basal-B, triple negative, as well as chemoresistant basal-like breast cancer systems (26). However, the effect of these drugs on Basal-A subtype breast cancer has not been investigated previously. Therefore, we utilized the MDA-MB-468 cell line to better characterize SKIs in this breast cancer system. As shown in Figure 3a, both SKIs dose-dependently blocked viability in these cells. SKI-II was slightly more effective, with an IC50 value of 15.8 ± 3.09 µM (p<0.001) compared to 19.16 ± 3.06 µM (p<0.001) with ABC294640 treatment (Figure 3a). Our results are similar to previously published values in other breast cancer systems (Table 3). We next determined the effect of these drugs on long-term clonogenic survival in these cells. Similar to other published results, treatment with both SKIs resulted in potent decreases in cancer colony formation, with IC50 values of 1.20 ± 1.20 µM (p<0.001) and 4.88 ± 1.14 µM (p<0.001) for SKII and ABC294640, respectively (Figure 3b).
Figure 3. Effect of SKIs on endocrine resistant MDA-MB-468 breast cancer.
(A) Cells were plated at 7.5 × 105 cells per 96 well plate. The following day cells were treated with indicated concentrations of SKI-II (solid line) or ABC294649 (dotted line) for 24 h. Data are presented as percent of vehicle treated samples. Mean values of ± S.E.M. of five different experiments in quadruplicate are reported. (B) Cells were plated at 500 cells per 60 mm2. The following day, cells were treated with SKI-II (solid line) or ABC294649 (dotted line) for 10–14 days. Data are presented as percent of vehicle treated samples. Mean values of ± S.E.M. of three different experiments in duplicate are reported. (C) Cells plated at 10,000 cells per well in a 96 well plate and treated with 50µM of ABC294640 for 24h. Following treatment, cells were measured for defragmented oligonucleotides as a measure of apoptosis using ELISA analyses and (D) cells were analyzed for viability using MTT analyses. Mean values of ± S.E.M. of three different experiments in duplicate are reported.
The apoptotic role of Sphk2 in breast cancer is less clear than that of Sphk1. We determined whether pharmacologic inhibition of Sphk2 with ABC294640 would induce apoptosis in Basal-A, triple negative breast adenocarcinoma. Treatment with ABC294640 resulted in a 12.09 ± 3.47 fold (p<0.05) increase in apoptosis, and a corresponding decrease in viability, in MDA-MB-468 cells (Figure 3c,d). These results suggest that Sphk2 plays a similar role in Basal-B subtype breast cancer as it does with invasive ductal (MCF-7) and basal-like, chemoresistant (MCF-7TN-R) breast cancers (35–37). Our findings are also in stark contrast to luminal breast cancer (MDA-MB-361) cells wherein inhibition of Sphk2 had no statistical effect on apoptosis.
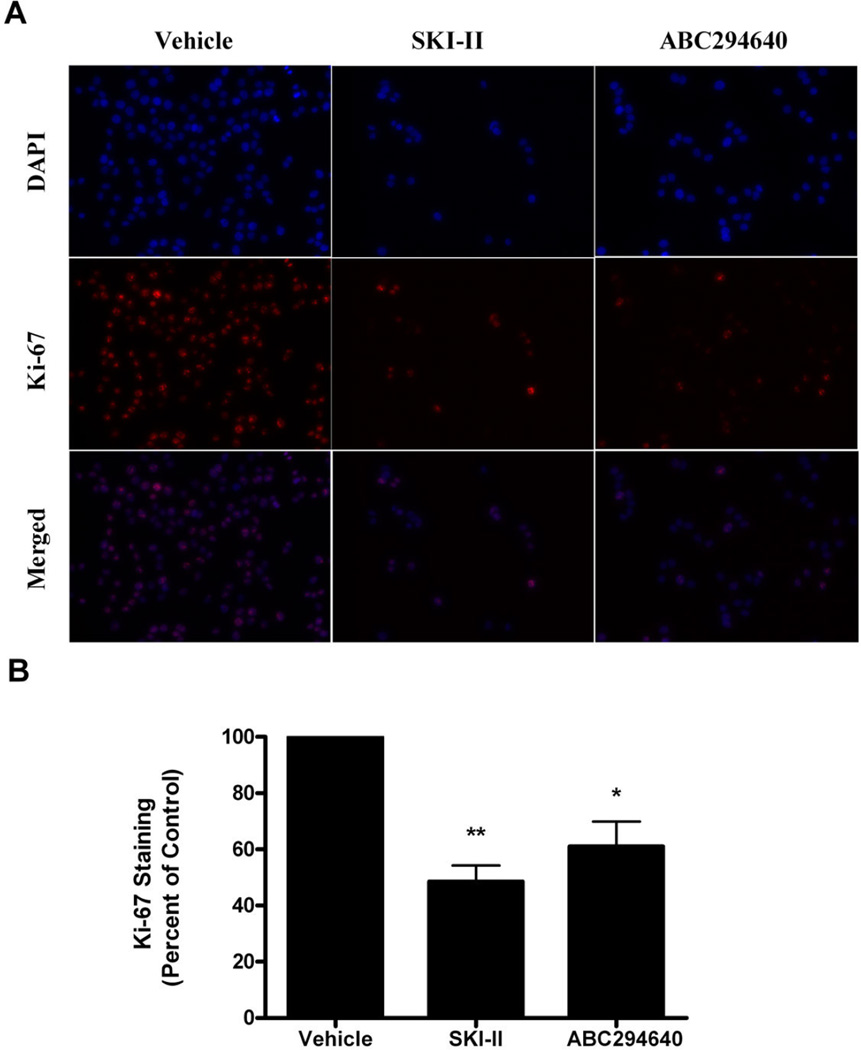
The ability of SKIs to block both viability and survival in MDA-MB-468 cells suggests that pharmacologically targeting Sphk in Basal-A breast cancer may be a promising therapeutic option. The ability of anti-cancer agents to block proliferation, in addition to inducing apoptosis, is an important biological characteristic. SKI-II and ABC294640 have varying anti-proliferative properties in other breast cancer cell systems. For example, ABC294640 has potent anti-proliferative properties in MCF-7 and MDA-MB-231 cells, while only marginally decreasing proliferation in MCF-7TN-R cells (26, 35, 36). Therefore, we determined the anti-proliferative properties of SKIs in MDA-MB-468 cells. We used nuclear Ki-67 staining as a measure of proliferation, similar to previously published experiments (26, 35, 36). As seen in Figure 4, treatment with SKI-II and ABC204640 decreased cellular proliferation of MDA-MB-468 cells. SKI-II was slightly more potent, with treatment resulting in a 51.50 ± 5.81% (p<0.001) reduction in Ki-67 staining compared to a 38.96 ± 8.87% (p<0.001) with ABC294640 (Figure 4b). Our results suggest that SKIs are more efficacious in MDA-MB-468 than in the MCF-7TN-R cell system, but less than MCF-7 cell systems.
Figure 4. Anti-proliferative properties of ABC294640.
(A) MDA-MB-468 cells treated with vehicle or ABC394640 for 48 h were fixed using 3.7% formaldehyde in PBS, permeabilized using cold methanol and incubated with anti-Ki-67 antibody (red). Cells were washed (with PBS) and DAPI (blue) nuclear stained before imaging. Three independent experiments were performed with representative pictures shown here. (B) Quantitation of Ki-67 staining was determined as a percentage of total positive cells/image. The vehicle control was set to 1 for comparison with ABC294640 treatment.
Anti-Tumor Activity of ABC294640 in Basal-A, triple negative breast cancer
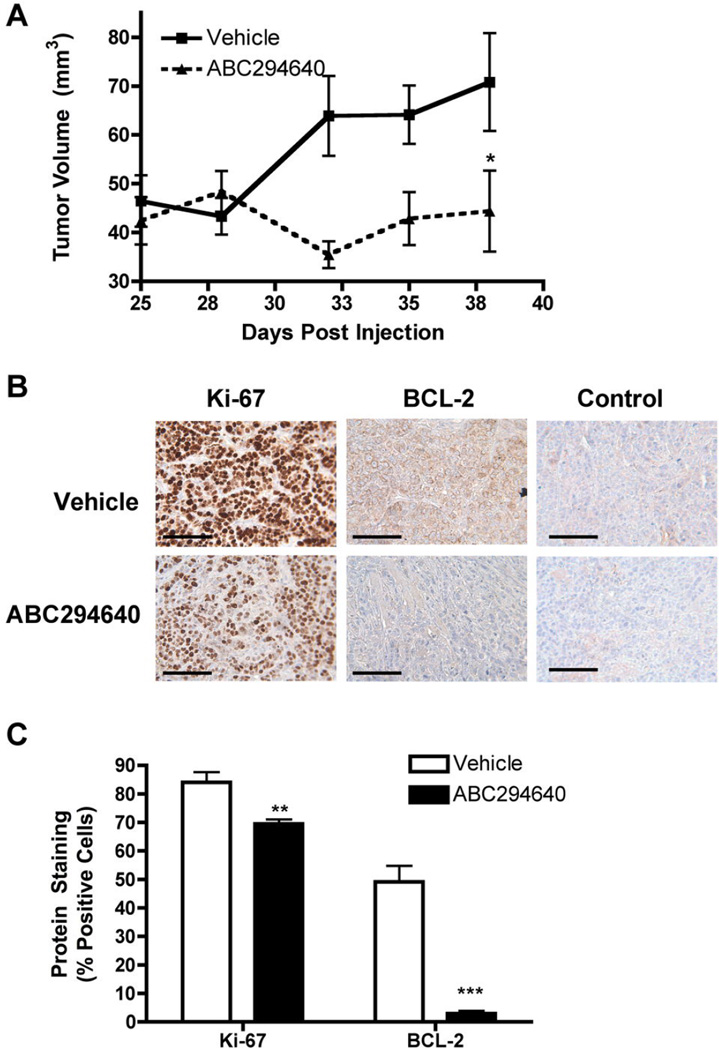
ABC294640 is currently entering clinical trials for the treatment of advanced solid tumors. This drug was chosen over SKI-II because of the more desirable pharmacokinetic and pharmcodynamic properties of ABC294640 (48). There have been several recent studies demonstrating anti-tumor effects of ABC294640 in breast cancer xenograft models (48, 49). For example, treatment with the inhibitor decreased tumor growth by 68.4% in MCF-7 and 67.4% (p<0.001) in MCF-7TN-R animal models (26, 46). Because of the promising in vitro therapeutic properties of this drug, we studied the effect of ABC294640 on MDA-MB-468 tumor growth in vivo. Using well-established, immunocompromised mouse xenograft models for tumor growth, MDA-MB-468 cells were injected subcutaneously in female overiectomized mice and mice were subsequently measured for tumor growth. Treatment with ABC294640 (50 mg/kg) for 14 days decreased MDA-MB-468 tumor volume by 37.3% (n=10, p<0.001) (Figure 5). The anti-tumor properties of ABC294640 were somewhat less than those reported in previously published studies using other cells systems.
Figure 5. ABC294640 decreases endocrine therapy resistant breast cancer tumor growth.
(A) MDA-MB-468 cells were injected in the mammary fat pads of female mice. Tumors were allowed to form over 9 days. Mice were treated i.p. with 50mg/kg of ABC294640 for 15 days. Tumor volume was measured every two days. Treatment tumors at endpoint were statistically significant from vehicle (*p<0.05). (B) Tumors from vehicle and ABC294640 treated mice were processed and stained for Ki-67 and BCL-2. Representative images staining and internal negative control in tumor sections are shown. Scale bar equal to 500 µm. (C) Quantitation of Ki-67 and BCL-2 staining is expressed as percent positive of total number of cells per field of view (***p < 0.001, **p < 0.01, *p < 0.05).
Because of the anti-proliferative and pro-apoptotic properties of ABC294640 in vitro, we further investigated whether these properties contributed to the anti-tumor effects of the drug. Endpoint tumors from vehicle and ABC294640 treated mice were first analyzed for protein expression of Ki-67 using immunohistochemistry. As shown in Figure 5b, cellular proliferation is markedly decreased in mice exposed to ABC294640. Specifically, there was an 17.30 ± 2.96 % (p<0.01) decrease in Ki-67 expression in treatment tumors compared to vehicle control. We next analyzed expression of BCL-2 as a measure of programmed cell death. BCL-2 is known to mediate intrinsic breast cancer apoptosis through sequestration of the pro-apoptotic proteins Bax/Bid and decreased expression of BCL-2 correlates with increased cellular apoptosis (50–52). Treatment tumors displayed a 93.11 ± 1.34% (p<0.01) decrease in BCL-2 expression compared to control tumors (Figure 5c). These results suggest that ABC294640 inhibits tumor proliferation and induces apoptosis to exert its anti-tumor effects. Our results also correlate well with our cellular findings, thus providing proof of principle that the in vitro anti-cancer effects of ABC294640 translate in vivo. Taken together, our findings demonstrate that ABC294640 has therapeutic potential in the treatment of Basal-A, triple negative breast cancer.
Discussion
The sphingolipid pathway, particularly Sphk signaling, has become of increasing interest as a breast cancer therapeutic target (1, 4, 5). The most commonly used SKIs, SKI-II and ABC294640, have demonstrated promising results in various breast cancer cell systems (32, 34–37). Specifically, both drugs have little effect on normal breast MCF10A cells, while proving to be highly effective in MCF-7 luminal, ER-positive breast adenocarcinoma cells (26, 36, 45). These drugs exhibit biological activity in the Basal-B, triple negative MDA-MB-231 and basal-like, multi-drug resistant MCF-7TN-R cells (26). However, pharmacologically inhibiting Sphk in luminal, endocrine therapy resistant and Basal-A, triple negative breast cancers has not been investigated. Here, for the first time, we have characterized both SKI-II and ABC294640 in these breast cancer subtypes.
Unlike previously published studies, SKIs were not effective in luminal, endocrine therapy resistant breast cancer compared to other cell systems. Both SKI-II and ABC294640 had little effect on short-term viability compared to endocrine sensitive ER-positive breast cancer (35, 46). Our laboratory previously demonstrated that SKIs can directly bind the ER and inhibit downstream estrogen signaling to promote its anti-cancer effects (35, 46). Because MDA-MB-361 cells are ER-positive, yet tamoxifen resistant, it is possible that mutations in the ER pathway that result in endocrine resistance may also affect the binding of SKIs to the ER. This decreased anti-estrogenic effect of SKIs in MDA-MB-361 cells could account for the differential activity of these inhibitors across ER positive cells lines. Furthermore, our finding that overexpression of Sphk promotes endocrine resistance in MCF-7 cells suggests that alterations in Sphk expression between MCF-7 and MDA-MB-361 may contribute to short-term SKI resistance in these cells. Supporting this hypothesis, Hollestelle et al performed gene expression profiling across a number of breast cancer cell lines, including MDA-MB-361 and MDA-MB-468. Array data from this study suggests that MDA-MB-361 cells exhibit increased mRNA expression of Sphk1, but not Sphk2, compared to MDA-MB-468 cells (53). This hypothesis would correlate with our findings that long-term exposure dose-dependently blocked colony formation while short-term exposure had little effect on cell viability. Further study is needed to determine the mechanism of short-term SKI resistance in endocrine resistant compared with endocrine sensitive breast cancer.
Unlike MDA-MB-361 cells, both SKI-II and ABC294640 decreased viability, survival, and proliferation in Basal-A, triple negative breast cancer cells. Similar to recent studies, ABC294640 induced apoptosis and blocked proliferation in vitro, though these results were quantitatively less than luminal, endocrine sensitive and basal-like, chemoresistant breast cancer cells (26, 36). The anti-proliferative and pro-apoptotic effects of ABC294640 translated into animal models, resulting in diminished tumor growth in vivo. Further studies are needed to better elucidate the differential effects of SKIs across the various cell lines. For example, if Sphk overexpression or activity is greater in MCF-7 and MCF-7TN-R cells compared to MDA-MB-468, then the presented results would be supported. However, another complicating factor may account for the disparity among breast cancer subtypes, such as differential location of Sphk2 within the cell. It is known that the subcellular localization of Sphk isoforms varies depending on tissue type and pathological state (1). Sphk1 is found primarily in the cytoplasm of most cells, whereas Sphk2 can be cytoplasmic or nuclear. Sphk2 is found mainly in the nucleus of MCF-7 breast cancer cells but in the cytosol of HEK293 embryonic kidney cells and MDAMB-453 breast cancer cells (54–56). Currently, the cellular localization of Sphk2 in many breast cancer systems is unknown.
Alternatively, a proliferative pathway may increase Sphk activity in MDA-MB-361 compared to other cells systems. Sphk1 is stimulated by a number of extracellular and intracellular factors such as, EGF, VEGF, TNF, phorbol esters, estrogen, calcium modulators, and interleukins (57–61). Sphk1 can also be directly phosphorylated by various growth factors such as EGF, protein kinase C, and ERK which induce its translocation to the cell membrane, enhancing S1P generation (62–65). On the other hand, Sphk2 can be activated by EGF, PKC, and phorbol esters and is phosphorylated by ERK1/2 and protein kinase D (PKD), initiating its nuclear export (54, 66, 67). Differential activity of growth pathways across breast cancer subtypes may alter the phosphorylation and activation profiles of Sphk, thus affecting the efficacy of our sphingosine kinase inhibitors. This possibility may account for the lack of effect on viability and apoptosis MDA-MB-361 compared with MDA-MB-468 cells.
Although we and others have investigated SKI-II and ABC294640 across the major breast cancer subtypes, most of these studies have been performed in vitro. Animal studies have been used to determine the pharmacokinetics and dynamics of these drugs, as well as characterize their anti-tumor properties (36, 37, 48). Recent evidence suggests a role for Sphk in tumor and migration and metastasis in a variety of cancers, including thyroid, kidney, esophageal, liver, and breast (68–72). Furthermore, increased Sphk expression correlates with increased tumor aggressiveness and decreased prognosis for clinical breast cancer patients (6). To date, there have been few studies using SKI-II or ABC294640 analyzing their effects on invasion, migration, or metastasis of breast cancer cell systems. More studies are necessary to better evaluate the clinical potential of these inhibitors. Taken together, our findings demonstrate the therapeutic promise of targeting sphingolipid signaling as a breast cancer treatment strategy.
Acknowledgements
We thank Ms. Nancy Busija for editing of the manuscript. This work was supported by The Center for Bioenvironmental Research at Tulane and Xavier Universities and the National Institutes of Health Grant CA125806.
Abbreviations
- DAB
Diaminobenzidine
- DAP
4,6-diamidino-2-phenylindole
- DCC
Dicyclohexylcarbodiimide
- MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Sphk1
sphingosine kinase-1
- Sphk2
sphingosine kinase-2
- SKI
sphingosine kinase inhibitor
- S1P
sphingosine-1-phosphate
Footnotes
Author Contributions
All authors participated in the design, interpretation of results and analysis of the data. Further contributions were as follows: JA performed experiments and drafted the manuscript, MW performed IHC, LR performed qRT-PCR, and JD performed animal experiments.
References
- 1.Antoon JW, Beckman BS. Sphingosine kinase: a promising cancer therapeutic target. Cancer Biol Ther. 2011;11(7):647–650. doi: 10.4161/cbt.11.7.14921. [DOI] [PubMed] [Google Scholar]
- 2.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 3.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring Endocrine Response in Breast Cancer Cells by Inhibition of the Sphingosine Kinase-1 Signaling Pathway. Endocrinology. 2009 doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 4.Meacham WD, Antoon JW, Burow ME, Struckhoff AP, Beckman BS. Sphingolipids as determinants of apoptosis and chemoresistance in the MCF-7 cell model system. Exp Biol Med (Maywood) 2009;234(11):1253–1263. doi: 10.3181/0902-MR-77. [DOI] [PubMed] [Google Scholar]
- 5.Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 2003;228(9):995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- 6.Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast cancer research and treatment. 2008;112(1):41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 7.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5(9):741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 9.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100(22):1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, Garcia-Closas M. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 11.Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst. 2007;99(22):1657–1659. doi: 10.1093/jnci/djm228. [DOI] [PubMed] [Google Scholar]
- 12.Eroles P, Bosch A, Alejandro Perez-Fidalgo J, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer treatment reviews. 2011 doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PloS one. 2009;4(7):e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson WF, Chu KC, Chatterjee N, Brawley O, Brinton LA. Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the surveillance, epidemiology, and end results database. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(1):18–27. doi: 10.1200/JCO.2001.19.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer research. 1990;50(18):6075–6086. [PubMed] [Google Scholar]
- 16.Martin SS, Leder P. Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Molecular and cellular biology. 2001;21(19):6529–6536. doi: 10.1128/MCB.21.19.6529-6536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller FR, Bukowski J. Mammary tumor stimulatory factors as well as mammastatin are produced by the normal human breast epithelial cell line MCF10A. Int J Cancer. 1994;59(2):296–297. doi: 10.1002/ijc.2910590224. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast cancer research and treatment. 2004;83(3):249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 19.Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. The Journal of biological chemistry. 1973;248(17):6251–6253. [PubMed] [Google Scholar]
- 20.Burow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, Hammond TG, Clejan S, Beckman BS. Differences in susceptibility to tumor necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer research. 1998;58(21):4940–4946. [PubMed] [Google Scholar]
- 21.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Molecular and cellular biology. 2004;24(12):5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker-Nasir E, Codington JF, Jahnke MR, Fuller TC, Jeanloz RW. Isolation and partial characterization of surface components of cell line MDA-MB-231 derived from a human metastatic breast carcinoma. J Natl Cancer Inst. 1982;69(2):371–380. [PubMed] [Google Scholar]
- 23.Oliveras-Ferraros C, Vazquez-Martin A, Lopez-Bonet E, Martin-Castillo B, Del Barco S, Brunet J, Menendez JA. Growth and molecular interactions of the anti-EGFR antibody cetuximab and the DNA cross-linking agent cisplatin in gefitinib-resistant MDA-MB-468 cells: new prospects in the treatment of triple-negative/basal-like breast cancer. International journal of oncology. 2008;33(6):1165–1176. [PubMed] [Google Scholar]
- 24.Pathak S, Siciliano MJ, Cailleau R, Wiseman CL, Hsu TC. A human breast adenocarcinoma with chromosome and isoenzyme markers similar to those of the HeLa line. J Natl Cancer Inst. 1979;62(2):263–271. [PubMed] [Google Scholar]
- 25.Antoon JW, Liu J, Ponnapakkam AP, Gestaut MM, Foroozesh M, Beckman BS. Novel D: - erythro N-octanoyl sphingosine analogs as chemo- and endocrine-resistant breast cancer therapeutics. Cancer Chemother Pharmacol. 2010;65(6):1191–1195. doi: 10.1007/s00280-009-1233-0. [DOI] [PubMed] [Google Scholar]
- 26.Antoon JW, White MD, Slaughter EM, Davis JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NF-kB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer biology & therapy. 2011;11(7) doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309(2):523–532. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- 28.Frigo DE, Vigh KA, Struckhoff AP, Elliott S, Beckman BS, Burow ME, McLachlan JA. Xenobiotic-induced TNF-alpha expression and apoptosis through the p38 MAPK signaling pathway. Toxicol Lett. 2005;155(2):227–238. doi: 10.1016/j.toxlet.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Weldon CB, Scandurro AB, Rolfe KW, Clayton JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe BM, Beckman BS, Burow ME. Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray. Surgery. 2002;132(2):293–301. doi: 10.1067/msy.2002.125389. [DOI] [PubMed] [Google Scholar]
- 30.Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M. Design, Synthesis, and Biological Activity of a Family of Novel Ceramide Analogues in Chemoresistant Breast Cancer Cells. Journal of medicinal chemistry. 2009 doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 31.Weldon CB, Parker AP, Patten D, Elliott S, Tang Y, Frigo DE, Dugan CM, Coakley EL, Butler NN, Clayton JL, Alam J, Curiel TJ, Beckman BS, Jaffe BM, Burow ME. Sensitization of apoptotically-resistant breast carcinoma cells to TNF and TRAIL by inhibition of p38 mitogen-activated protein kinase signaling. International journal of oncology. 2004;24(6):1473–1480. [PubMed] [Google Scholar]
- 32.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer research. 2003;63(18):5962–5969. [PubMed] [Google Scholar]
- 33.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318(2):596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 34.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and Antitumor Activity of ABC294640, a Selective Inhibitor of Sphingosine Kinase-2. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME, Beckman BS. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. Journal of molecular endocrinology. 2011;46(3):205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, Rhodes LV, Ashe HB, Wiese TE, Smith CD, Burow ME, Beckman BS. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151(11):5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11(7):678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antoon JW, White MD, Burow ME, Beckman BS. Dual Inhibition of Sphingosine Kinase Isoforms Ablates TNF-Induced Drug Resistance. Oncol Rep. 2012 doi: 10.3892/or.2012.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes LV, Short SP, Neel NF, Salvo VA, Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, Fonseca JP, Bratton MR, Segar C, Tilghman SL, Sobolik-Delmaire T, Horton LW, Zaja-Milatovic S, Collins-Burow BM, Wadsworth S, Beckman BS, Wood CE, Fuqua SA, Nephew KP, Dent P, Worthylake RA, Curiel TJ, Hung MC, Richmond A, Burow ME. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer research. 2011;71(2):603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M. Design, synthesis, and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem. 2009;52(18):5748–5752. doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 41.Salvo VA, Boue SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, Wood CE, Erhardt PW, Beckman BS, McLachlan JA, Cleveland TE, Burow ME. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(23):7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey MR, Beckman BS, McLachlan JA, Rowan BG, Pochampally R, Burow ME. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast cancer research and treatment. 2009 doi: 10.1007/s10549-009-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boue SM, Tilghman SL, Elliott S, Zimmerman MC, Williams KY, Payton-Stewart F, Miraflor AP, Howell MH, Shih BY, Carter-Wientjes CH, Segar C, Beckman BS, Wiese TE, Cleveland TE, McLachlan JA, Burow ME. Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max) Endocrinology. 2009;150(5):2446–2453. doi: 10.1210/en.2008-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Experimental cell research. 2002;281(1):115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 45.Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME, Beckman BS. Pharmacological Inhibition of Sphingosine Kinase Isoforms Alters Estrogen Receptor Signaling in Human Breast Cancer. Journal of molecular endocrinology. 2011 doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, Rhodes LV, Hasina AB, Wiese TE, Smith CD, Burow ME, Beckman BS. Anti-Estrogenic Effects of the Novel Sphingosine Kinase-2 Inhibitor ABC294640. Endocrinology. 2010 doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer research. 1999;59(7):1391–1399. [PubMed] [Google Scholar]
- 48.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. The Journal of pharmacology and experimental therapeutics. 2010;333(1):129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A language and enviroment for statistical computing: Foundation for statistical computing. Vienna, Austria: Available from: http://www.R-project.org. [Google Scholar]
- 50.Buchholz TA, Davis DW, McConkey DJ, Symmans WF, Valero V, Jhingran A, Tucker SL, Pusztai L, Cristofanilli M, Esteva FJ, Hortobagyi GN, Sahin AA. Chemotherapy-induced apoptosis and Bcl-2 levels correlate with breast cancer response to chemotherapy. Cancer J. 2003;9(1):33–41. doi: 10.1097/00130404-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Buchholz TA, Garg AK, Chakravarti N, Aggarwal BB, Esteva FJ, Kuerer HM, Singletary SE, Hortobagyi GN, Pusztai L, Cristofanilli M, Sahin AA. The nuclear transcription factor kappaB/bcl-2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(23):8398–8402. doi: 10.1158/1078-0432.CCR-05-0885. [DOI] [PubMed] [Google Scholar]
- 52.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X( L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 53.Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, Riaz M, Koopman DG, Ten Hagen TL, de Leeuw BH, Zwarthoff EC, Teunisse A, van der Spek PJ, Klijn JG, Dinjens WN, Ethier SP, Clevers H, Jochemsen AG, den Bakker MA, Foekens JA, Martens JW, Schutte M. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast cancer research and treatment. 2010;121(1):53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- 54.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280(33):29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 55.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of biological chemistry. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275(26):19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 57.Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. The Journal of biological chemistry. 2004;279(43):44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 58.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Molecular and cellular biology. 2002;22(22):7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173(2):301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291(5509):1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 61.Meyer zu Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rumenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414(2–3):145–154. doi: 10.1016/s0014-2999(01)00789-0. [DOI] [PubMed] [Google Scholar]
- 62.Lidington D, Peter BF, Meissner A, Kroetsch JT, Pitson SM, Pohl U, Bolz SS. The phosphorylation motif at serine 225 governs the localization and function of sphingosine kinase 1 in resistance arteries. Arterioscler Thromb Vasc Biol. 2009;29(11):1916–1922. doi: 10.1161/ATVBAHA.109.194803. [DOI] [PubMed] [Google Scholar]
- 63.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. Embo J. 2003;22(20):5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. The Journal of experimental medicine. 2005;201(1):49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) The Journal of biological chemistry. 2002;277(38):35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 66.Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Jahangeer S, Okada T, Nakamura S. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. The Journal of biological chemistry. 2007;282(37):27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 67.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. The Journal of biological chemistry. 2007;282(16):12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 68.Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang S, Liu L, Chen T, Li J, Tu H, He X. Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 2011 doi: 10.1111/j.1478-3231.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- 69.Gao P, Smith CD. Ablation of sphingosine kinase-2 inhibits tumor cell proliferation and migration. Molecular cancer research : MCR. 2011;9(11):1509–1519. doi: 10.1158/1541-7786.MCR-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergelin N, Blom T, Heikkila J, Lof C, Alam C, Balthasar S, Slotte JP, Hinkkanen A, Tornquist K. Sphingosine kinase as an oncogene: autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase C-alpha and ERK1/2. Endocrinology. 2009;150(5):2055–2063. doi: 10.1210/en.2008-0625. [DOI] [PubMed] [Google Scholar]
- 71.Doll F, Pfeilschifter J, Huwiler A. Prolactin upregulates sphingosine kinase-1 expression and activity in the human breast cancer cell line MCF7 and triggers enhanced proliferation and migration. Endocr Relat Cancer. 2007;14(2):325–335. doi: 10.1677/ERC-06-0050. [DOI] [PubMed] [Google Scholar]
- 72.Pan J, Tao YF, Zhou Z, Cao BR, Wu SY, Zhang YL, Hu SY, Zhao WL, Wang J, Lou GL, Li Z, Feng X, Ni J. An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma. J Transl Med. 2011;9:157. doi: 10.1186/1479-5876-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]