Abstract
Object
Functional MRI (fMRI) has the potential to be a useful presurgical planning tool to treat patients with primary brain tumor. In this study the authors retrospectively explored relationships between language-related postoperative outcomes in such patients and multiple factors, including measures estimated from task fMRI maps (proximity of lesion to functional activation area, or lesion-to-activation distance [LAD], and activation-based language lateralization, or lateralization index [LI]) used in the clinical setting for presurgical planning, as well as other factors such as patient age, patient sex, tumor grade, and tumor volume.
Methods
Patient information was drawn from a database of patients with brain tumors who had undergone preoperative fMRI-based language mapping of the Broca and Wernicke areas. Patients had performed a battery of tasks, including word-generation tasks and a text-versus-symbols reading task, as part of a clinical fMRI protocol. Individually thresholded task fMRI activation maps had been provided for use in the clinical setting. These clinical imaging maps were used to retrospectively estimate LAD and LI for the Broca and Wernicke areas.
Results
There was a relationship between postoperative language deficits and the proximity between tumor and Broca area activation (the LAD estimate), where shorter LADs were related to the presence of postoperative aphasia. Stratification by tumor location further showed that for posterior tumors within the temporal and parietal lobes, more bilaterally oriented Broca area activation (LI estimate close to 0) and a shorter Wernicke area LAD were associated with increased postoperative aphasia. Furthermore, decreasing LAD was related to decreasing LI for both Broca and Wernicke areas. Preoperative deficits were related to increasing patient age and a shorter Wernicke area LAD.
Conclusions
Overall, LAD and LI, as determined using fMRI in the context of these paradigms, may be useful indicators of postsurgical outcomes. Whereas tumor location may influence postoperative deficits, the results indicated that tumor proximity to an activation area might also interact with how the language network is affected as a whole by the lesion. Although the derivation of LI must be further validated in individual patients by using spatially specific statistical methods, the current results indicated that fMRI is a useful tool for predicting postoperative outcomes in patients with a single brain tumor.
Keywords: functional magnetic resonance imaging, brain tumor, aphasia
Functional MRI is a noninvasive method used to localize critical cognitive functions. Accordingly, recent interest has grown toward understanding whether fMRI—in particular, the BOLD signal—can be used to aid presurgical planning of lesion resections involving eloquent cortex to minimize disruption of cognitive function and ensure maximal tumor tissue removal. The current gold-standard method of localization is electrical cortical mapping, which, while extremely precise and predictive of postoperative deficits, is also invasive and time consuming.3,5,7,18,30,33 Thus, it would be beneficial to derive fMRI-based measures that not only match the utility of electrical cortical mapping, but also help to minimize patient morbidity. Although fMRI-based mapping and electrical cortical mapping have shown varied concordance7 and individual differences in the topography of language-related eloquent cortex can be significant,20 2 fMRI-based measures in the literature have shown particular promise in surgical planning: the lesion-to-activation distance (LAD) and the lateralization index (LI).
Numerous authors have shown the fMRI-derived LAD measure to be a potential estimator of postoperative functional outcomes, and this relationship has been further confirmed by electrocortical mapping–derived distance measures.8,9,13,37 Case reports and observational studies with small numbers of patients have suggested that functional motor deficits relate to a tumor’s proximity to motor task–related cortical activation.6,37 A recent study of ours replicated these findings and demonstrated that language function is also influenced by tumor proximity but has a relationship different from that between LAD and motor function, in which deficits scale linearly with motor area LAD but nonlinearly with language area LAD.35
Lateralization of function between the 2 hemispheres is another important parameter to consider for surgical planning.38 Language dominance is often surgically assessed using the intracortical sodium amobarbital (Wada) test, which has a complication rate of up to 11%.16 Consequently, much work has been done to determine language dominance using fMRI. Language-related activation has been suggested to shift in the presence of a slow-growing lesion that encroaches on a functional area and hypothetically causes recruitment of surrounding brain areas, or even the homologous contralateral areas, to compensate for functional loss.22,25 Language lateralization can be quantified by calculating the LI, which is the difference in the ratio of significantly activated voxels between the left and right hemispheres, determined while the individual is performing a task.29 While LI seems to depend on numerous factors, such as the task used,37 statistical thresholding,24 and lesion type, some studies suggest that LI is a good predictor of surgical outcomes,2 but see the study by Giussani et al.7
Our aim in the present study was to investigate whether the individually thresholded fMRI maps provided for clinical preoperative planning had prognostic utility. Specifically, for patients with primary brain tumors, we evaluated whether measures of LAD and/or LI, retrospectively estimated from preoperative fMRI maps, related to postoperative language deficits. We did so by focusing on 2 major language areas of the human brain: the Broca area (Brodmann Areas 44 and 45) and the Wernicke area (Brodmann Area 22). We hypothesized that a shorter LAD and more bilaterally oriented LI would relate to increased postoperative deficits.
Methods
Patient Selection
Forty-nine cases were selected from a database of 423 neurosurgical patients who had undergone fMRI as part of presurgical planning between June 1999 and July 2009. Inclusion criteria selected patients who were right-handed (verbal self report) had a diagnosed left-sided primary neoplasm within the frontal, temporal, or parietal lobe, and had undergone lesion resection. Right-handed patients were chosen because the majority of right-handed persons (> 95%) have left hemisphere language dominance.11,29 Therefore, a critical assumption was that the LI in these patients was left lateralized prior to tumor development. Patients with left hemisphere tumors were chosen for the study to coincide with the language-dominant hemisphere. Patients with multiple lesion foci, as determined from presurgical structural imaging, and those who were younger than 18 years of age were excluded. Preoperative and postoperative neurological deficits were determined from patient medical records. Any record of preoperative or postoperative language deficits (all types of aphasia, including Broca, Wernicke, and conduction subtypes) was considered, but nonspecific speech difficulties were not. Postoperative deficits were grouped as either transient (lasting < 6 months) or persistent (lasting ≥ 6 months) loss of function. Tumor grade was categorized as either high, which included Grade III and IV tumors, or low, which included Grade I and II tumors, as determined from the surgical pathological report. Grading was based on the WHO system. All patients gave informed consent according to a protocol approved by the local institutional review board.
Language Paradigms
The language paradigms used to assess patients are described in detail elsewhere.19 Task efficacy and reliability for producing language-related activation has been demonstrated.4 In brief, activation of the Broca area was assessed with word-generation tasks. We used 2 types of word-generation tasks: 1) alternating 20-second blocks of antonym word generation versus rest, and 2) alternating 20-second blocks of letter word generation versus rest. The Wernicke area was identified with alternating 20-second blocks of text reading versus symbols reading. In this task, the patient silently read a short paragraph in the text reading block. During the control/symbols block, the patient was shown a paragraph of symbols and asked to scan and find specific symbols. The symbols block controlled for eye movements during reading, which presumably helps discriminate eye movement–related activation from the true language areas.19 Multiple tasks were used to assess language production, because not all patients showed language-related activation with any one task. In this case, we selected the task that resulted in the largest amount of language-related activation for each patient.
Functional MRI Acquisition and Processing
Imaging was done with either a 1.5- or 3-T commercial MR scanner (GE Medical Systems) equipped with high-speed gradients. The BOLD-weighted single-shot EPIs were obtained repeatedly at intervals defined by the repetition time for each patient during task performance. Technical parameters were as follows: field of view 24 cm, matrix 64 × 64, TR 2000 msec, TE 40 msec (for 1.5 T) or 27 msec (for 3 T), fractional anisotropy 85° (for 1.5 T) or 75° (for 3 T), and 6-mm coronal plane sections (for 1.5 T) or 5-mm axial plane sections (for 3 T); spatial coverage was sufficient to provide mapping of the entire cortex. The number of images and the duration of imaging varied with the paradigm. Imaging duration ranged from 3 to 5 minutes. Additional high-resolution anatomical scans, including 3D volumetric T1- and T2-weighted sequences, were acquired as part of the preoperative assessment. All postprocessing was done using AFNI, freely available at http://afni.nimh.nih.gov/afni/.
Postprocessing in AFNI
Postprocessing of the EPI data sets included both spatial smoothing and temporal alignment. Spatial smoothing was applied to the EPI data sets using a full width at half maximum Gaussian kernel. Eight-millimeter smoothing was applied to the 1.5-T data sets that were acquired with a 6-mm section thickness and 1-mm skip. Six-millimeter smoothing was applied to the 3-T data set that was acquired with a 5-mm section thickness. Temporal alignment was done with AFNI 3dTshift function that shifts voxel time series from the input data set so that the separate slices are aligned to the same temporal origin. This helped account for differences in temporal acquisition at each slice location within the 2-second repetition time. The AFNI 3Dvolreg was used to correct for head motion in the reconstructed time courses. This was done by spatially coregistering all data to the middle time point of the fMRI run. The EPIs were coregistered with structural images. They were manually “nudged” for perceived optimal spatial coregistration with the corresponding 3D anatomy. The AFNI plug-in “nudge dataset” allowed visualization in 3 planes of the anatomy with an overlay showing the EPI volume. “Nudging” was applied in 6 planes to align the disparate EPI and anatomical data sets into a best fit. Once one of the EPI data sets was thus manually aligned, all other EPI data sets from the same scan session were automatically coregistered using AFNI 3dvolreg.
Activation was determined by cross-correlation of the time course of the EPI signal at each voxel with a generalized least squares fitting algorithm to a smoothed and temporally delayed boxcar reference function modeling the presumed hemodynamic response. This comparison provided a voxel-wise t-statistic with which images were thresholded individually to optimize visualization of language areas and overlaid on the coregistered anatomical brain volume maps. Thresholding was individually applied with the intent of optimizing localization of the activation foci. Cluster sizes were a minimum of 20 contiguous voxels (p ≤ 0.05) with a t-value ranging from 2.15 to 42 (mean 12.63). Clinicians would then view and use these thresholded maps for surgical planning as needed.
Parameter Measurements and Analysis
Tumor location and volume were assessed from the structural scans. Measurements were taken in the transverse (x), anterior/posterior (y), and superior/inferior (z) axes using PACS software. The tumor margin was set as the edge of signal for enhancing tumors and determined by architectural distortion on both the T1- and T2-weighted scans for nonenhancing tumors. Tumor volume was calculated using the equation commonly used by clinicians to estimate tumor volume: (x × y × z)(π)/6. The extent of resection was derived from radiology records and categorized as partial (> 10% tumor remained), subtotal (< 10% tumor remained), or total (there was no tumor remaining after resection). Note that such information was not available for all patients and thus was analyzed for only a subset of patients (44 patients).
We focused on the Broca area (Brodmann Areas 44 and 45) and Wernicke area (Brodmann Area 22). The minimum distance from tumor edge to activation edge or activation center in the Broca area and Wernicke area was measured using PACS and took into account the distance in the 3 anatomical planes. Language-related activation areas in the left and right hemispheres were determined using the image slice showing the largest area of activation in the relevant anatomical area (Fig. 1). The diameter of the activation area in each hemisphere (that is, homologous locations relative to activation) was used to calculate an area of activation.
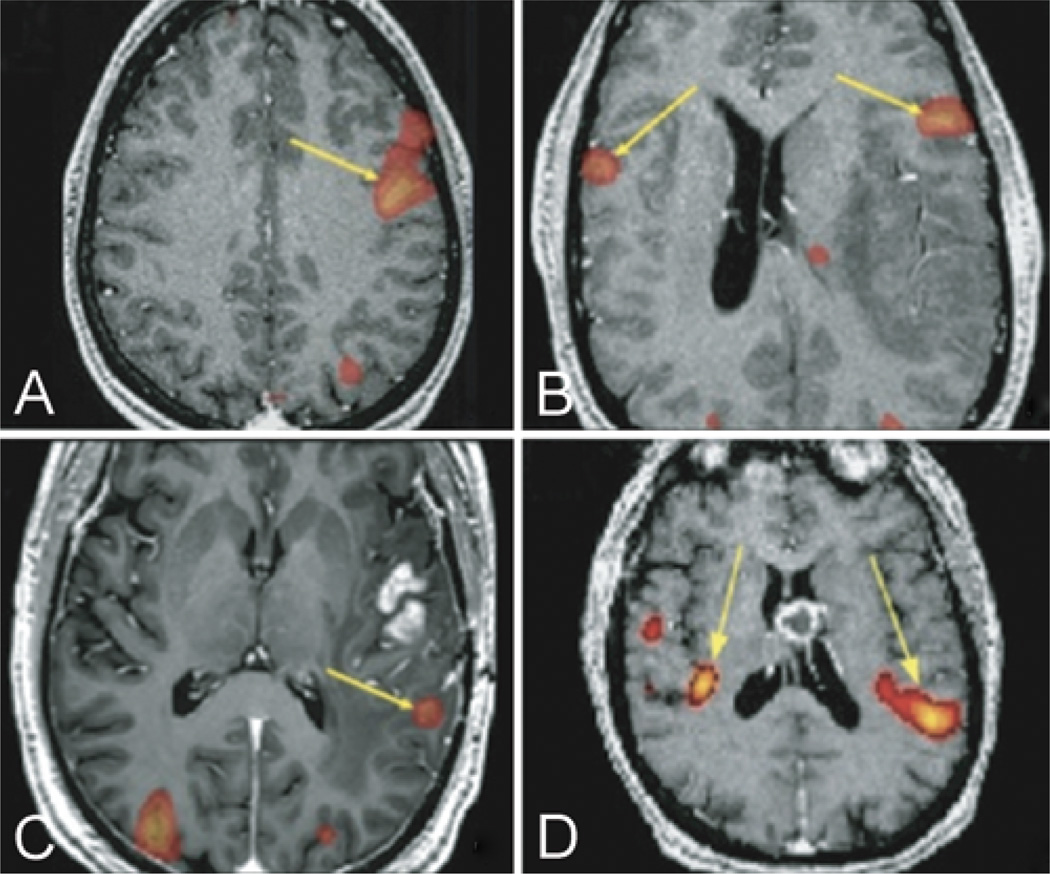
Fig. 1. Representative fMR images.
A: Unilateral activation of Broca area (yellow arrow) during the antonym word-generation task in a 21-year-old male with a Grade II astrocytoma of the left frontal lobe, Broca area LI = 1. B: Bilateral activation of Broca area (yellow arrows) during antonym word-generation task in a 43-year-old male with a Grade IV astrocytoma of the left temporal lobe, Broca area LI = 0.64. C: Unilateral activation of Wernicke area (yellow arrow) during text-reading task in a 52-year-old male with a Grade IV astrocytoma of the left temporal lobe, Wernicke area LI = 1. D: Bilateral activation of Wernicke area (yellow arrows) during text-reading task in a 61-year-old male with mixed oligoastrocytoma of the left frontal lobe, Wernicke area LI = 0.77.
The LI was calculated using the equation (L − R)/(L + R), where L and R refer to the activation area in the left or right hemisphere in either the Broca or the Wernicke area. It is often determined by setting the activation threshold to a value and comparing the number of voxels in the left or right hemisphere. In the present study, for both LAD and LI, since we focused on drawing clinically relevant conclusions, we retrospectively chose to estimate these measures from individually thresholded maps, which had been provided for clinical use during individual patient assessments.
All statistical analyses were performed using R statistical software (freely available at http://www.r-project.org/). All data were analyzed using the chi-square test of association, Fisher exact test, Kruskal-Wallis test, or 1-way ANOVA, as appropriate. In particular, the existence of a relationship between LAD and deficits (pre- or postoperative) or LI and deficits was determined using a 1-way ANOVA, with deficits as a factor and patients as a repeated measure. The existence of a relationship between LAD and LI was assessed using the Kruskal-Wallis test with LAD categorized as < 1, 1–2, and > 2 cm. Logistic regression– based multivariate models were used to predict postoperative outcomes. These models tested the predictive value of different combinations of clinical and fMRI parameters. Overall model fit was determined using the Bayesian Information Criterion index, and individual parameter fits with each model were assessed based on the significance of the parameter’s coefficients (p ≤ 0.05).
Results
All patients (71% male) were between the ages of 20 and 72 years (median age 43 years). The mean tumor volume was 37.0 ± 45.6 cm3, and 51% of the tumors were high grade on histological analysis (Table 1). Roughly 40% of tumors were in the left frontal lobe, 40% in the left temporal lobe, and 12% in the left frontotemporal area. Significant Broca area activation—as determined by the minimum cluster size and a p < 0.05—was found in all 49 patients. Significant Wernicke area activation was found in all but 3 patients, that is, 46 patients. There was a trending association between preoperative and postoperative deficits (χ2 = 4.3, df = 2, 49 patients, p = 0.12); that is, if a patient presented with aphasia, then they were more likely to have aphasia postoperatively (Table 2). Most patients with no deficits preoperatively had no deficits postoperatively (20 of 33 patients). Thirteen patients had new deficits postoperatively, 3 of whom experienced persistent deficits.
TABLE 1.
Summary of demographics in 49 patients
| Characteristic | Value |
|---|---|
| % male sex | 71 |
| mean age in yrs | 44 |
| age range in yrs | 20–72 |
| mean tumor vol in cm3 | 37.0 ± 45.6 |
| % high-grade tumors | 51 |
| tumor lobe location (% of patients) | |
| lt frontal | 38.7 |
| lt frontotemporal | 12.2 |
| lt temporal | 36.8 |
| lt insula | 6 |
| lt parietal | 6 |
| tumor pathology (% of patients) | |
| high-grade astrocytoma | 45 |
| oligodendroglioma | 35 |
| low-grade astrocytoma | 8 |
| dysembryoplastic neuroepithelial tumor | 6 |
| mixed glioma & oligoastrocytoma | 4 |
| pilocytic astrocytoma | 2 |
TABLE 2.
Language deficits preoperatively versus postoperatively in 49 patients
| Postop Deficit (no. of patients) | |||
|---|---|---|---|
| Preop Deficit | Absent | Transient | Persistent |
| absent | 20 | 10 | 3 |
| present | 5 | 7 | 4 |
Age, Sex, and Lesion-Derived Parameters Versus Language Deficits
There was no relation between postoperative language deficits and patient age, patient sex, tumor grade, or tumor volume (p > 0.05; Table 3). An increased age related to increased preoperative deficits (Table 4). A high tumor grade showed a marginally significant relationship with the presence of preoperative deficits.
TABLE 3.
Relation between postoperative language deficits and patient and tumor parameters in 49 patients*
| Parameter | Statistic | p Value |
|---|---|---|
| sex | χ2(1,N=49) = 0.74 | 0.39 |
| age | F(1,47) = 0.04 | 0.84 |
| tumor grade | χ2(1,N=49) = 0.02 | 0.88 |
| tumor vol | F(1,47) = 1.36 | 0.18 |
| Broca LAD (from edge of activation) | F(1,47) = 4.73 | 0.03† |
| Broca LAD (from center of activation) | F(1,47) = 4.16 | 0.05† |
| Wernicke LAD (from edge of activation) | F(1,47) = 1.12 | 0.30 |
| Wernicke LAD (from center of activation) | F(1,47) = 1.23 | 0.27 |
| Broca LI | F(1,47) = 3.15 | 0.08‡ |
| Wernicke LI | F(1,47) = 1.02 | 0.31 |
Chi-square tests of association and 1-way ANOVA used. Absent versus present; transient and persistent deficits grouped together.
Abbreviation: N = number of patients.
Significant (p ≤ 0.05).
Trending toward significance (p ≤ 0.1).
TABLE 4.
Relation between preoperative language deficits and patient and tumor parameters in 49 patients*
| Parameter | Statistic | p Value |
|---|---|---|
| sex | χ2(1,N=49) = 0.002 | 0.96 |
| age | F(1,47) = 4.44 | 0.04† |
| tumor grade | χ2 (1,N=49) = 2.63 | 0.10‡ |
| tumor vol | F(1,47) = 2.30 | 0.13 |
| Broca LAD (from edge of activation) | F(1,47) = 0.13 | 0.72 |
| Broca LAD (from center of activation) | F(1,47) = 0.23 | 0.63 |
| Wernicke LAD (from edge of activation) | F(1,47) = 5.04 | 0.03† |
| Wernicke LAD (from center of activation) | F(1,47) = 6.07 | 0.02† |
| Broca LI | F(1,47) = 1.66 | 0.20 |
| Wernicke LI | F(1,47) = 0.08 | 0.77 |
Chi-square tests of association and 1-way ANOVA used. Absent versus present; transient and persistent deficits grouped together.
Significant (p ≤ 0.05).
Trending toward significance (p ≤ 0.1).
Broca Area LAD and LI Versus Postoperative Language Deficits
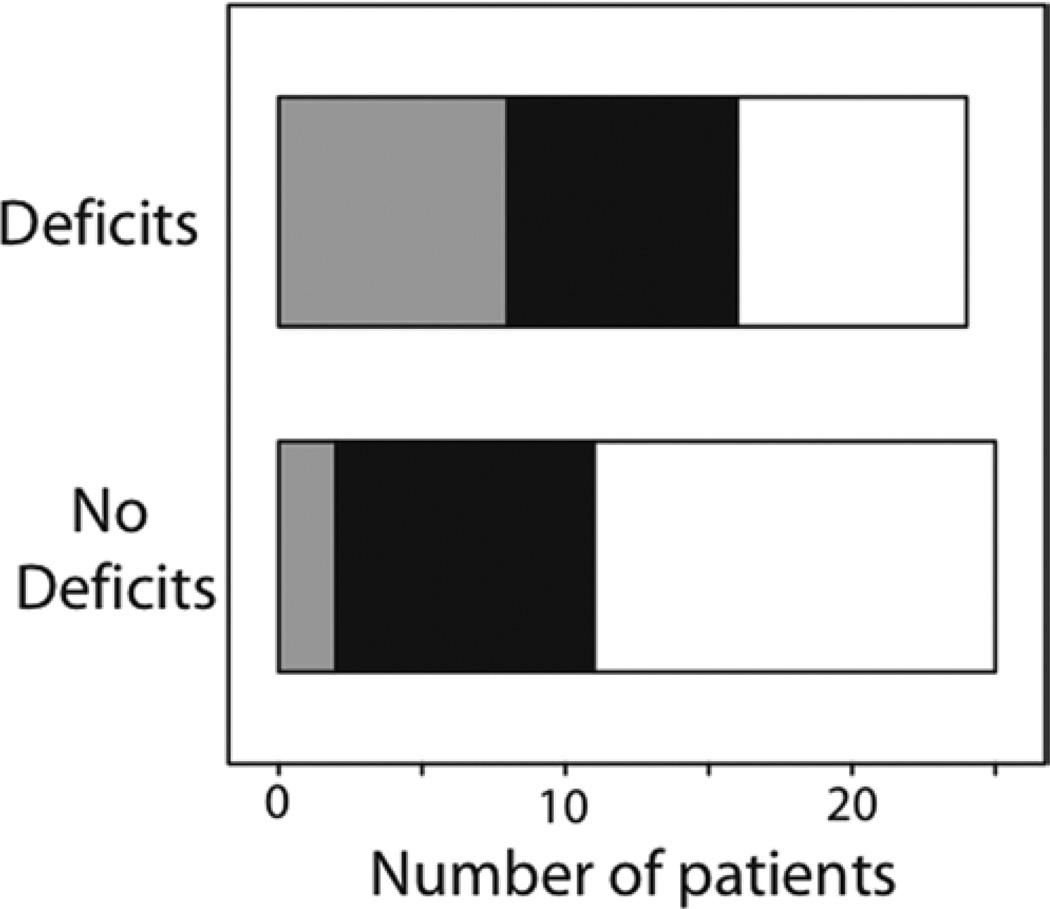
Postoperative aphasia was associated with the Broca area LAD (measured from the edge of the cluster of activation, p = 0.03; or measured from the center of the cluster of activation, p = 0.05; Table 3; Fig. 2), where a shorter LAD was related to the presence of postoperative aphasia. The mean Broca area LAD for the group with postoperative deficits was 1.75 ± 1.41 cm, and for the group with no postoperative deficits was 2.68 ± 1.76 cm. However, stratification by tumor location showed that this effect was not specific to tumors in either frontal [Broca area LAD measured from the center of the cluster of activation; F(1,16) = 0.17, p = 0.84] or posterior [left temporal and parietal tumors grouped together, measured from the center of the cluster of activation; F(1,22) = 2.56, p = 0.12] brain areas but instead was an overall effect of left hemisphere tumors.
Fig. 2.
Frequency histograms of patients with and without postoperative deficits (aphasia). Broca area LAD relates to postoperative language deficits across all patients. Broca area LAD categorized as < 1 cm (gray), 1–2 cm (black), and > 2 cm (white).
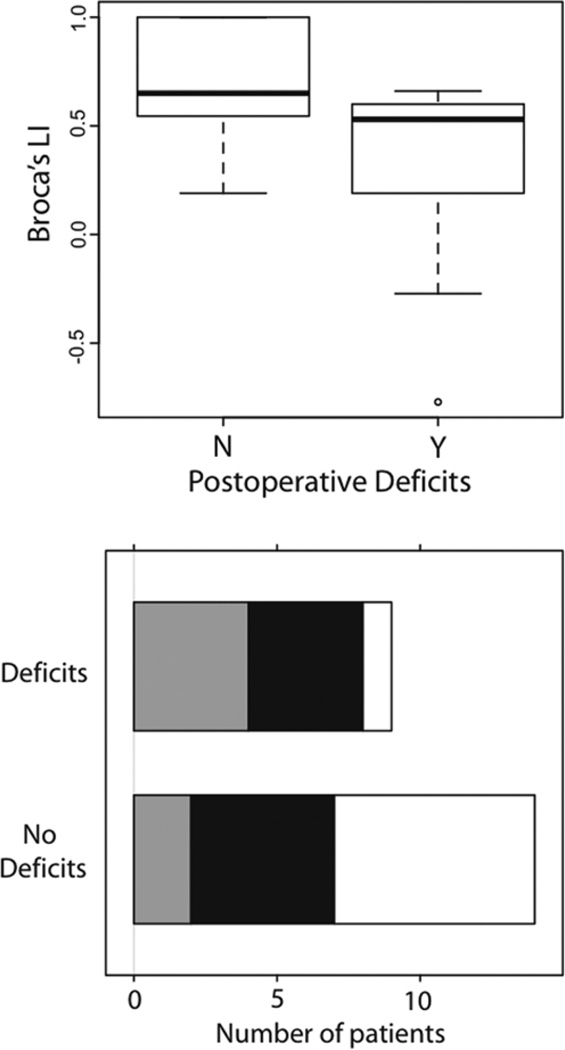
Additionally, more bilateral Broca area activation— that is, Broca area LI values close to 0—was associated with increased postoperative deficits and trended toward significance (p = 0.08; Table 3). However, stratification by tumor location indicated that this relationship was stronger when the tumor was present in posterior brain areas [F(1,22) = 7.80, p = 0.01] but not in frontal brain areas [F(1,16) = 0.35, p = 0.56]. The mean Broca area LI for the group with postoperative deficits was 0.28 ± 0.50 and for the group with no postoperative deficits was 0.72 ± 0.25 (Fig. 3 upper).
Fig. 3.
Frequency histograms of patients with and without postoperative deficits (aphasia). Functional MRI measures related to postoperative language deficits in the patient group with posterior brain tumors. Upper: Broca area LI versus presence of postoperative deficits. Lower: Wernicke area LAD categorized as < 1 cm (gray), 1–2 cm (black), and > 2 cm (white).
Post hoc analyses in a subset of patients addressed whether there was a relationship between LAD and extent of resection (partial, subtotal, or total). There was no relationship between extent of resection and Broca area LAD [F(2,42) = 0.63, p = 0.54].
Wernicke Area LAD and LI Versus Postoperative Language Deficits
While the Wernicke area LAD was not related to postoperative deficits in tumors across all brain areas, it did show a regionally specific effect trending toward significance in patients with posterior brain tumors [F(1,22) = 3.37, p = 0.08]. The mean Wernicke area LAD for the group with postoperative deficits was 1.39 ± 1.18 cm and for the group with no postoperative deficits was 2.96 ± 2.36 cm (Fig. 3 lower). Again, a shorter LAD was related to increased postoperative deficits. Moreover, in a subset of patients, we examined whether there was a relationship between LAD and extent of resection (partial, subtotal, or total). There was no relationship between extent of resection and Wernicke area LAD [F(2,38) = 0.37, p = 0.62].
There was no relationship between Wernicke area LI and postoperative deficits across all patients, nor within groups of patients with either posterior or frontal brain tumors.
Preoperative Language Deficits
Of note, a shorter Wernicke area LAD related to the presence of preoperative language deficits (p = 0.02). The mean Wernicke area LAD for the group with preoperative deficits was 1.90 ± 1.39 cm, and for the group with no preoperative deficits was 3.36 ± 2.15 cm (Table 4). An older patient age was related to the presence of preoperative deficits (p = 0.04). The mean age for the group with preoperative deficits was 49.94 ± 16.92 years and for the group with no preoperative deficits was 41.03 ± 12.18 years.
Lateralization Index Versus Other Parameters
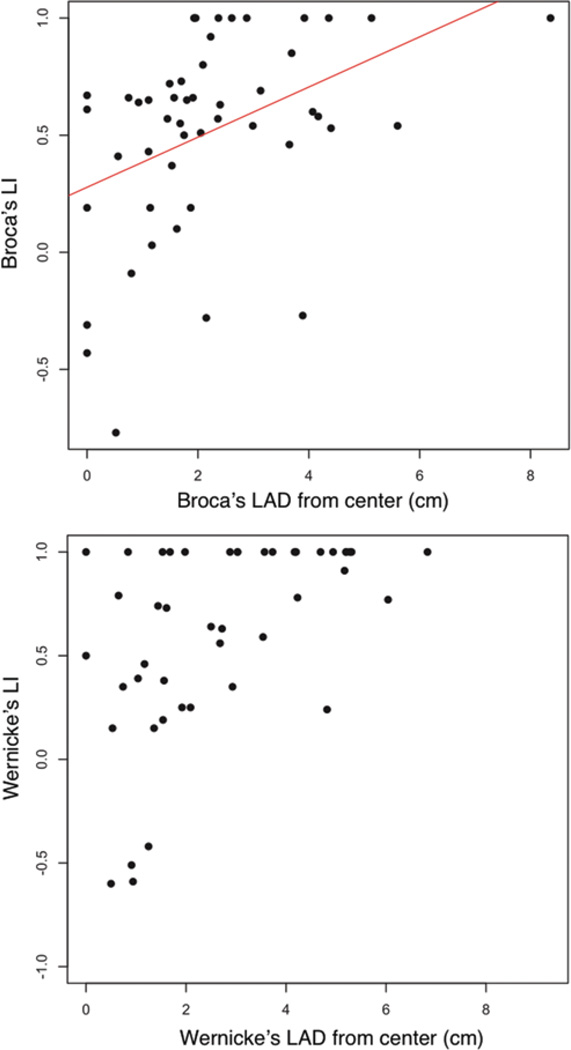
We assessed the relationship between LI and other patient and tumor parameters that related to preoperative and postoperative language deficits (Table 5). There was a significant association between Wernicke area LI and tumor volume, where larger tumors were associated with more bilateral activation (p = 0.004). In addition, there was an association between Broca area LAD and Broca area LI as well as Wernicke area LAD and Wernicke area LI such that more bilaterally distributed activation was related to a closer distance between the tumor and the area of activation (Fig. 4). Furthermore, the Broca LAD correlated with the Broca LI [r(47) = 0.42, p = 0.002], but Wernicke LAD did not correlate with Wernicke LI [r(44) = 0.18, p = 0.22]. Stratification by tumor location showed that across posterior brain tumors, a shorter Wernicke area LAD was related to a more bilateral Broca area LI (marginally significant effect, H = 4.73, df = 2, p = 0.09), where Wernicke LAD < 2 cm showed a mean Broca area LI of 0.34 ± 0.56 (LAD 0–1 cm) or 0.41 ± 0.32 (LAD 1–2 cm), but Wernicke area LAD > 2 cm showed a Broca area LI of 0.82 ± 0.25. No such crossover effects were observed in frontal brain areas.
TABLE 5.
Patient and tumor parameters versus LI*
| Statistic | ||
|---|---|---|
| Parameter | Broca LI | Wernicke LI |
| patient sex | F(1,47) = 4.84; p = 0.49 | F(1,47) = 0.03; p = 0.87 |
| patient age | H = 1.48, 1 df, p = 0.22 | H = 1.67, 1 df, p = 0.20 |
| tumor grade | F(1,47) = 0.20; p = 0.66 | F(1,47) = 0.10; p = 0.75 |
| tumor vol | H = 2.18, 1 df, p = 0.14 | H = 8.26, 1 df, p = 0.004† |
| Broca LAD (from center of activation) | H = 7.44, 2 df, p = 0.02† | H = 2.64, 2 df, p = 0.26 |
| Wernicke LAD (from center of activation) | H = 1.52, 2 df, p = 0.47 | H = 7.53, 2 df, p = 0.02† |
Comparisons between LI and sex or tumor grade were done using 1-way ANOVA. Comparisons between LI and age, tumor volume, and LAD were done by grouping the independent variables. Median split was performed on age and tumor volume. The LAD was grouped into 3 categories: LAD < 1 cm, 1–2 cm, and > 2 cm. Chi-square tests of association, Kruskal-Wallis test, and 1-way ANOVA were used.
Significant (p ≤ 0.05).
Fig. 4.
Relationship between LAD and LI. Upper: Broca area LAD versus Broca area LI [r(47) = 0.42, p = 0.002]. Lower: Wernicke area LAD versus Wernicke area LI.
Note that further analysis incorporating multiple parameters into different logistic regression models predicting postoperative deficits did not yield better descriptions of these data. This may be attributable to power limitations based on the number of patients in the study.
Discussion
In the present study we examined whether 2 fMRIbased measures (LAD and LI) related to postoperative language deficits in a cohort of patients with primary brain tumors. Patients were right-handed and had left hemisphere tumors in frontal, parietal, or temporal lobes. Prior to surgery, all patients underwent fMRI and performed a number of language tasks. Our results showed that the proximity of the tumor to the Broca area is related to postoperative deficits, where shorter distances are related to the presence of postoperative aphasia. This is true of tumors located across lobes within the left hemisphere. A similar relationship between LAD and language deficits in patients with temporal lobe tumor was shown by Håberg et al.,8 where an LAD > 1 cm predicted better patient outcomes. Similar results have also been shown for motor deficits;13,35,37 however, motor and language areas may respond to lesion encroachment differently.35 Furthermore, tumor invasion of eloquent cortex (that is, a shorter LAD) predicts poor patient survival in the case of recurrent glioblastoma multiforme.21
Stratification by tumor location (frontal or posterior regions) revealed no relationship between Broca area LAD and postoperative aphasia in either frontal or posterior tumor location subsets. However, in the posterior tumor subset, Wernicke area LAD was related to the presence of postoperative deficits, where a shorter LAD was related to the presence of aphasia. Moreover, in the posterior tumor subset, a more bilateral Broca area LI was related to the presence of deficits. These results speak to the question of whether plasticity resulting from the presence of a lesion in the language-dominant hemisphere, which seems to lead to more bilateral functional activation, is protective or not against postoperative language deficits. In healthy humans, bilateral activation confers protection against disruption of language function by repetitive transcranial magnetic stimulation to either the left or right hemispheres.12 Furthermore, patients with left-sided tumors show more bilateral activation compared with healthy controls,32 and the degree of lateralization is related to how well language is disrupted by repetitive transcranial magnetic stimulation in these patients. Lateralization may be a protective step taken in the process of tumor-related reorganization, as evidenced by the fact that slow-growing lesions show more lateralization than fast-growing lesions.31 In stroke patients, who may show bilateral activation with left-sided stroke, Saur and Hartwigsen27 asserted that it remains unclear whether strong right hemisphere activation early after stroke represents functionally relevant activation compensating for the lesioned left hemisphere areas. The authors suggested that this may alternatively be the result of disinhibition, which would be consistent with the assumption that left hemisphere areas inhibit their homotopic right hemisphere counterparts in the healthy brain.
We also found that the Wernicke area LAD and Broca area LI were related such that a shorter Wernicke LAD was related to more bilateral Broca LI across posterior tumors. This finding suggests a possible interaction effect between LAD and LI so that as the tumor encroaches on eloquent cortex in posterior areas, it may disrupt the language network as a whole (indicated by more bilateral Broca area activation). In such cases, postoperative language deficits may be more likely. Assessment of whether LAD and LI interact to causally result in functional deficits must be explored in a larger sample with more robust methods for determining LI. Preliminary analysis in this cohort showed a relationship between LAD and LI for either the Broca area or the Wernicke area, where a decreased LAD was related to a more bilateral LI.
We also found that old age, high tumor grade, and increased Wernicke area LAD were related to the existence of preoperative deficits. The effects of tumor grade are probably a result of more aggressive tissue infiltration by high-grade tumors as compared with benign tumors.
The difference in effects between Broca and Wernicke area parameters may be attributable to differences in functional topography. The Broca area shows less anatomical variation across individuals compared with the Wernicke area.26 The predictive value of structural measures using diffusion tensor imaging14,17,36 or diffusion weighted imaging1,10 in combination with fMRI measures may better predict functional language outcomes following surgery by additionally accounting for differences in connectivity between the areas. For example, using the same patient database that we used in the present study, Lazar et al.15 reported that after tumor and arteriovenous malformation resection, improvement in the appearance of white matter tracts correlated with improvement in motor function.
The current study has a number of limitations. Language-related activation was statistically thresholded on an individual basis instead of at one value across the group. This was done to optimize visualization of individual activation maps, since the original intent of imaging was to provide patient-specific information for preoperative planning. Additionally, some threshold-independent methods require consideration of the whole hemisphere, which does not allow spatially specific determination of functional cortex within a hemisphere.24 Changes in a set group threshold may change LI values for certain tasks, because changes in the t-value would mainly influence the area of activation. In the present study, LAD was measured from the center of the activation cluster, which is less sensitive to thresholding, as well as from the activation edge. Both measures yielded similar results. Moreover, 2 scanners of different strengths (1.5 T vs 3 T) were used to collect the data and may have introduced some inconsistencies in the data that were not specifically accounted for in the analyses. Additionally, to what degree clinicians used the imaging data provided to them was not specifically considered. However, in a controlled clinical study, Petrella et al.23 used individually thresholded maps and showed that when clinicians used fMRI data, their surgical treatment plans were more aggressive and less time consuming. In the current study, however, post hoc analyses in a subset of patients showed no relationship between LAD and extent of resection for either Broca area or Wernicke area LADs. Additionally, the extent of resection did not relate to postoperative deficits, preoperative deficits, or tumor grade individually, although we cannot rule out a more complex interaction effect between resection extent and LAD, LI, tumor volume, and tumor grade. There are also limitations associated with neurovascular uncoupling that would affect BOLD measures in such a patient population. Neurovascular uncoupling can mimic activation normally indicative of functional reorganization.28,34 For this study, a small percent of patients underwent scanning during breath holding to assess the degree of neurovascular uncoupling present. Unfortunately, not all patients underwent this type of imaging. Finally, having access to a detailed account of the time course and severity of postoperative deficits would have been beneficial in determining the sensitivity of LAD and LI in predicting language-related deficits. Future studies must address these important limitations to further validate the utility of LAD and LI.
Conclusions
In this study we begin to address whether individually thresholded fMRI maps provided for clinical use have prognostic utility. We showed evidence that 2 such estimated measures from clinical fMRI maps, the LAD between tumor and language area as well as that language area’s LI, relate to postoperative functional language outcomes in patients with an isolated primary brain tumor. While further validation of their true predictive value is needed, our results indicate that these measures may help inform clinicians about surgical outcomes and thus aid presurgical planning and reduce patient morbidity.
Acknowledgments
Disclosure
The authors thank Dr. Alejandro Munoz del Rio and Jeremy Weiss for their advice regarding the statistical analysis of these data.
This project was supported by the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health Grant No. 1UL1RR025011 (V.P), and National Institute of Mental Health Grant No. MH095428 (B.K.).
Abbreviations used in this paper
- AFNI
Analysis of Functional NeuroImages
- BOLD
blood oxygen level–dependent
- EPI
echo planar image
- fMRI
functional MRI
- LAD
lesion-to-activation distance
- LI
lateralization index
- PACS
picture archiving and communication system
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Kundu, Penwarden, Wood, Nair, Kuo, Field, Moritz, Meyerand, Prabhakaran. Acquisition of data: Nair, Moritz, Prabhakaran. Analysis and interpretation of data: Kundu, Penwarden, Wood, Andreoli, Voss, Meier, Nair, Field, Prabhakaran. Drafting the article: Kundu, Penwarden, Gallagher, Nair, Kuo. Critically revising the article: Kundu, Meier, Prabhakaran. Reviewed submitted version of manuscript: Kundu. Approved the final version of the manuscript on behalf of all authors: Kundu. Statistical analysis: Kundu, Andreoli, Voss. Administrative/ technical/material support: Field, Meyerand, Prabhakaran. Study supervision: Kuo.
Portions of this paper were presented in talks at the annual meetings of both the American Society for Functional Neuroradiology (Boston, 2010) and the American Society for Neuroradiology (Las Vegas, 2010), and in poster form at the annual meeting of the Society for Neuroscience (Chicago, 2010).
References
- 1.Baehring JM, Bi WL, Bannykh S, Piepmeier JM, Fulbright RK. Diffusion MRI in the early diagnosis of malignant glioma. J Neurooncol. 2007;82:221–225. doi: 10.1007/s11060-006-9273-3. [DOI] [PubMed] [Google Scholar]
- 2.Binder JR. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2011;20:214–222. doi: 10.1016/j.yebeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizzi A, Blasi V, Falini A, Ferroli P, Cadioli M, Danesi U, et al. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping. Radiology. 2008;248:579–589. doi: 10.1148/radiol.2482071214. [DOI] [PubMed] [Google Scholar]
- 4.Brannen JH, Badie B, Moritz CH, Quigley M, Meyerand ME, Haughton VM. Reliability of functional MR imaging with word-generation tasks for mapping Broca’s area. AJNR Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 5.Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- 6.Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg. 1999;91:238–250. doi: 10.3171/jns.1999.91.2.0238. [DOI] [PubMed] [Google Scholar]
- 7.Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- 8.Håberg A, Kvistad KA, Unsgård G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. 2004;54:902–915. doi: 10.1227/01.neu.0000114510.05922.f8. [DOI] [PubMed] [Google Scholar]
- 9.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Khan RB, Gutin PH, Rai SN, Zhang L, Krol G, DeAngelis LM. Use of diffusion weighted magnetic resonance imaging in predicting early post-operative outcome of a new neurological deficit after brain tumor resection. Neurosurgery. 2006;59:60–66. doi: 10.1227/01.neu.0000243284.68297.f2. [DOI] [PubMed] [Google Scholar]
- 11.Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 12.Knecht S, Flöel A, Dräger B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan R, Raabe A, Hattingen E, Szelényi A, Yahya H, Hermann E, et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery. 2004;55:904–915. doi: 10.1227/01.neu.0000137331.35014.5c. [DOI] [PubMed] [Google Scholar]
- 14.Laundre BJ, Jellison BJ, Badie B, Alexander AL, Field AS. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005;26:791–796. [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar M, Alexander AL, Thottakara PJ, Badie B, Field AS. White matter reorganization after surgical resection of brain tumors and vascular malformations. AJNR Am J Neuroradiol. 2006;27:1258–1271. [PMC free article] [PubMed] [Google Scholar]
- 16.Loddenkemper T, Morris HH, Möddel G. Complications during the Wada test. Epilepsy Behav. 2008;13:551–553. doi: 10.1016/j.yebeh.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto R, Okada T, Mikuni N, Mitsueda-Ono T, Taki J, Sawamoto N, et al. Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008;255:1703–1711. doi: 10.1007/s00415-008-0005-9. [DOI] [PubMed] [Google Scholar]
- 18.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 19.Moritz C, Haughton V. Functional MR imaging: paradigms for clinical preoperative mapping. Magn Reson Imaging Clin N Am. 2003;11:529–542. doi: 10.1016/s1064-9689(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 20.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–3843. doi: 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partovi S, Jacobi B, Rapps N, Zipp L, Karimi S, Rengier F, et al. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. AJNR Am J Neuroradiol. 2012;33:2151–2157. doi: 10.3174/ajnr.A3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrella JR, Shah LM, Harris KM, Friedman AH, George TM, Sampson JH, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006;240:793–802. doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- 24.Pillai JJ, Zaca D. Relative utility for hemispheric lateralization of different clinical fMRI activation tasks within a comprehensive language paradigm battery in brain tumor patients as assessed by both threshold-dependent and threshold-independent analysis methods. Neuroimage. 2011;54(Suppl 1):S136–S145. doi: 10.1016/j.neuroimage.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 25.Sailor J, Meyerand ME, Moritz CH, Fine J, Nelson L, Badie B, et al. Supplementary motor area activation in patients with frontal lobe tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 2003;24:1837–1842. [PMC free article] [PubMed] [Google Scholar]
- 26.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 27.Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Arch Phys Med Rehabil. 2012;93(1 Suppl):S15–S25. doi: 10.1016/j.apmr.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A, Hubbe U, Ziyeh S, Henning J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhacement. AJNR Am J Neuroradiol. 2007;21:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 29.Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 30.Sunaert S. Presurgical planning for tumor resectioning. J Magn Reson Imaging. 2006;23:887–905. doi: 10.1002/jmri.20582. [DOI] [PubMed] [Google Scholar]
- 31.Thiel A, Habedank B, Herholz K, Kessler J, Winhuisen L, Haupt WF, et al. From the left to the right: how the brain compensates progressive loss of language function. Brain Lang. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Habedank B, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol. 2001;50:620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- 33.Tieleman A, Deblaere K, Van Roost D, Van Damme O, Achten E. Preoperative fMRI in tumour surgery. Eur Radiol. 2009;19:2523–2534. doi: 10.1007/s00330-009-1429-z. [DOI] [PubMed] [Google Scholar]
- 34.Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–581. doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- 35.Wood JM, Kundu B, Utter A, Gallagher TA, Voss J, Nair VA, et al. Impact of brain tumor location on morbidity and mortality: a retrospective functional MR imaging study. AJNR Am J Neuroradiol. 2011;32:1420–1425. doi: 10.3174/ajnr.A2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JS, Mao Y, Zhou LF, Tang WJ, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neurnavigation: a prospective, controlled study in patients with gliomas involving pyradimal tracts. Neurosurgery. 2008;61:935–949. doi: 10.1227/01.neu.0000303189.80049.ab. [DOI] [PubMed] [Google Scholar]
- 37.Yetkin FZ, Ulmer JL, Mueller WM, Cox RW, Klosek MM, Haughton VM. Functional magnetic resonance imaging assessment of the risk of postoperative hemiparesis after excision of cerebral tumors. Internat Neuroradiol. 1998;4:253–257. [Google Scholar]
- 38.Zacà D, Nickerson JP, Deib G, Pillai JJ. Effectiveness of four different clinical fMRI paradigms for preoperative regional determination of language lateralization in patients with brain tumors. Neuroradiology. 2012;54:1015–1025. doi: 10.1007/s00234-012-1056-2. [DOI] [PubMed] [Google Scholar]