Abstract
The Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) is caused by a bunyavirus known as the FTLS virus (FTLSV), which was recently discovered in China. We examined the epidemiological and etiological features of 637 laboratory-confirmed cases of FTLS with onset from January 2011 to December 2012 in Henan Province, China. The highest incidence of FTLS occurred between May and August: 76.5% of all laboratory-confirmed cases occurred during those four months. Of the laboratory-confirmed cases, 60.9% were in the 46–69 years old age groups; 96.1% (612/637) occurred in farmers; 98.1% (625/637) were reported from Xinyang Prefecture. During the same time period, 2047 cases were reported in China. The nucleotide and amino acid sequences of FTLSV strains identified during 2011–2012 in Henan Province were ≥96% identical. This findings provides insight for developing public-health interventions for the control and prevention of FTLS in epidemic area.
Introduction
The Fever, Thrombocytopenia and Leukopenia Syndrome virus (FTLSV), also known as the Severe Fever with Thrombocytopenia Syndrome virus (SFTSV) and Huaiyangshan virus, belongs to the phlebovirus genus, the family of Bunyaviridae [1]–[3]. Likes other bunyaviruses [4]–[6], the L segment encodes RNA-dependent RNA polymerase; the M segment has an open reading frame (ORF) coding for a GnGc precursor in the order Gn-Gc; whereas the S segment uses ambisense coding to express two proteins: one is a nucleocapsid (N) protein encoded by the 5′ half of viral complementary sense S RNA, and the other is a nonstructural (NS) protein encoded by viral sense S RNA [7]. The virus has been found to cause the Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS), also known as the Severe Fever with Thrombocytopenia Syndrome (SFTS) and Huaiyangshan Hemorrhagic Fever in China since 2007 [1]–[3]. The disease has been reported in 11 Chinese provinces (Henan, Hubei, Shandong, Anhui, Jiangshu, Liaoning, Yunnan, Guangxi, Jiangxi, Shanxi and Zhejiang Provinces) and Shanghai City [1]–[3], [8], [9]. 2047 cases of FTLS were reported during 2011–2012 in China. Henan Province has the highest case count, and accounts for 48.2% of all reported cases in China [10]. Recently, this virus has also been found to cause severe disease and death in other countries such as Japan and Korea [11]. Major clinical presentations of FTLS included fever, thrombocytopenia, leukocytopenia, gastrointestinal symptoms, neurological symptoms, bleeding tendency, as well as less specific clinical manifestations. It is difficult to differentiate FTLS from other infectious diseases, especially hemorrhagic fever with renal syndrome and human granulocytic anaplasmosis [1]. This disease has a case-fatality rate ranging from 2.5% to 30% in different areas of endemicity [12]. No vaccine or antiviral drugs are currently available [13]. FTLS has been a Category B reportable infectious disease in mainland China since September 29, 2010.
Using surveillance data from January 1, 2011 to December 31, 2012 in Henan Province, we studied the epidemiological and etiological characteristics of FTLS, to inform the development of effective prevention and control measures.
Materials and Methods
Case Definitions and Specimen Collection
All clinically diagnosed FTLS cases in Henan Province are reported to the Henan Center for Disease Control and Prevention by medical practitioners, who make the clinical diagnosis based on the guidelines by the National Health and Family Planning Commission [1], [14], as follows: (1) Epidemiological characteristics, including history of tick bites, working in the mountainous areas or the forest, or direct contact with the blood of a confirmed case-person during the two weeks prior to symptom onset; (2) Clinical presentations, including at least one of the following: Fever, headache, muscle aches, nausea, vomiting, diarrhea, skin bruises, bleeding, multiple organ damage, disseminated intravascular coagulation (DIC); (3) Laboratory findings, including decrease of leukocyte count and thrombocytopenia; and (4) Ruling out other diseases, such as human granulocytic anaplasmosis, hemorrhagic fever with renal syndrome, dengue fever, thrombocytopenic purpura, septicaemia. A patient who met all of the above criteria was a clinically diagnosed FTLS case-patient. We collected and tested blood specimens from clinically diagnosed FTLS case-patients who were hospitalized. Both acute and convalescent phase sera were collected by hospital staff and transported to the laboratories using cold chain. A laboratory-confirmed case-patient met at least one of the following criteria: Isolation of SFTSV from the patient’s serum; detection of FTLSV RNA in the patient’s serum during the acute phase of the illness; detection of anti-FTLSV IgM antibody by enzyme-linked immunosorbent assay (ELISA); or seroconversion or a four-fold elevation in serum IgG antibodies against FTLSV by indirect immunofluorescence assay (IFA). We analyzed the data for laboratory-confirmed FTLS case-patients to assess their epidemiological and etiological characteristics.
We used a structured questionnaire to collect the case-patients’ sociodemographic and clinical data. The data were verified by checking against medical records.
Real-time RT-PCR Detection
Total RNA was extracted from acute serum samples using a QIAamp viral RNA mini Kit (Qiagen, Germany), following the manufacturer’s instructions. The real-time RT-PCR assay was performed using primers (S3-137F, 5′-ATGGATGCAAGGTCCAGAAT-3′, S3-268R, 5′-CTTCCTCAGAGCTGCTTGCT-3′), probe (C437-PROBE2, 5′-TGCCGCTGAGCAATTTCTCTC TCCA-3′) [15] and the QIAGEN QuantiTectTM Probe RT-PCR Kit (Qiagen, Germany). The conditions for real-time RT-PCR reaction were as follows: 50°C for 30 min, 95°C for 15 min, 45 cycles of 95°C for 15 s,60°C for 45 s. Data were analyzed using the software supplied by the manufacturer.
ELISA Assay
Anti-FTLSV IgM antibodies were detected in acute-phase sera of case-patients using an ELISA kit (Xinlianxin Biomedical Technology CO., LTD, Wuxi, Jiangsu, China) according to the manufacturer’s protocol. For this ELISA kit, we used 50 µl of positive control, negative control, and sera diluted to 1∶40. The test results were determined to be valid if the criteria for the positive control and the negative controls were fulfilled. The absorbance values of the positive control must be more than 1.5 at 450 nm after blanking; the absorbance values of the negative control must be less than 0.1 at 450 nm after blanking. If the absorbance values of the controls did not meet the quality control range specifications, the test was determined to be invalid and must be repeated. Calculation of the cut-off value was the mean absorbance value for negative controls times 2.1; if the mean absorbance value for negative controls was lower than 0.05, then the value 0.05 was used. The positive/negative determination of samples was performed using the cut-off value.
Indirect IFA
FTLSV-specific IgG antibodies were detected in the patients’ sera during the acute and convalescent phases by indirect IFA, as previously described [16]. Vero E6 cells-infected FTLSV showing cytopathic effect (CPE) were harvested and centrifuged at 1,000×g for 10 minutes. The centrifugal precipitate was re-suspended in 1×PBS, spotted onto 12-well glass slides, and fixed with acetone for 10 minutes. Two-fold serial dilutions (1∶10 to 1∶1280) of the sera were prepared in PBS buffer. A volume of 20 µl of each serum sample was added to the cell-spotted wells and incubated for 45 minutes at 37°C. After washing for 10 minutes in PBS, 20 µl of FITC-conjugated goat anti-human IgG (Sihuan Sci-Technics Company, Beijing, China) diluted 1∶40 in buffer containing Evans Blue was added to each well and incubated for 30 minutes at 37°C. After washing, slides were mounted in glycerin and examined by immunofluorescence microscopy.
Virus Isolation and Sequencing
All acute phase serum samples were used to inoculate Vero E6 cells. 100 µl of serum was inoculated onto Vero E6 cell monolayers in 25 cm2 flasks and incubated for 14 days at 37°C/5% CO2 in MEM/2% fetal calf serum with a media change after 7 days. All cultures were monitored daily for CPE. Each sample underwent at least three cell culture passages in Vero E6 cells before being considered negative [17]. Both virus-infected cells and negative control cells were examined for FTLSV by real-time RT-PCR at each passage. The whole genome sequences of FTLSV were amplified using primers described in previous studies by RT-PCR [3]. The RT-PCR products were sent to Sangon Biotech Co., Ltd (Shanghai, China) for DNA sequencing using an automated ABI 3730 DNA sequencer.
Phylogenetic Analysis
The genomes of FTLSV isolates were compiled using the SeqMan program in the LaserGene software package (DNASTAR). The percentage similarities of nucleotide identity or amino acid identity were calculated using the Clustalx software. Molecular phylogenetic analysis was conducted by using the maximum likelihood (ML) method based on the Kimura 2-parameter model in the MEGA 5 software [18]. The tree with the highest log likelihood was shown. The percentage of trees in which the associated taxa clustered together was shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically as follows: When the number of common sites was <100 or <1/4 of the total number of sites, the Maximum Parsimony method was used; otherwise the BIONJ method with MCL distance matrix was used. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. All available nucleotide sequences of genome segments of SFTSV isolates from GenBank were analyzed, together with our newly generated genome of SFTSV isolates in this study, and the phylogenetic trees were constructed in order to understand the evolutionary characterization of SFTSV.
Statistical Analysis
All epidemiologic and laboratory data were double-entered with the EpiData 3.1 software. All statistical analyses were performed using SAS v9.13 (SAS Institute Inc., Cary, NC). An association with p<0.05 was considered to be statistically significant.
Ethics Consideration
This research was approved by the Institutional Review Board at the Center for Disease Control and Prevention of Henan Province. All participants gave written informed consent for use of their samples for research purposes.
Results
Laboratory Findings
During January 2011 to December 2012, a total of 987 clinically diagnosed FTLS case-patients were reported; 812 (82.3%) of those patients gave acute phase sera and 529 (53.6%) gave convalescent phase sera. Of the 812 acute phase sera, 589 were positive for FTLSV RNA (using real-time RT-PCR) and/or anti-FTLSV IgM antibodies (using ELISA); these included 316 positive sera using both methods, 230 using FTLSV RNA only, and 43 using anti-FTLSV IgM antibodies only. An additional 48 paired sera had positive anti-FTLSV IgG antibody by the IFA test (including 29 seroconversions and 19 with ≥4-fold increase). In total, there were 637 ( = 589+48) laboratory-confirmed case-patients in this study.
Epidemiology Characteristics
Place
The annual incidence rate based on the laboratory-confirmed cases was 0.35 per 100,000 population in Henan Province. The vast majority (98.1%, 625/637) of the laboratory-confirmed cases were reported in Xinyang Prefecture in southern Henan Province (Figure 1).
Figure 1. Map of Henan Province, showing laboratory-confirmed FTLS cases in 2011–2012.
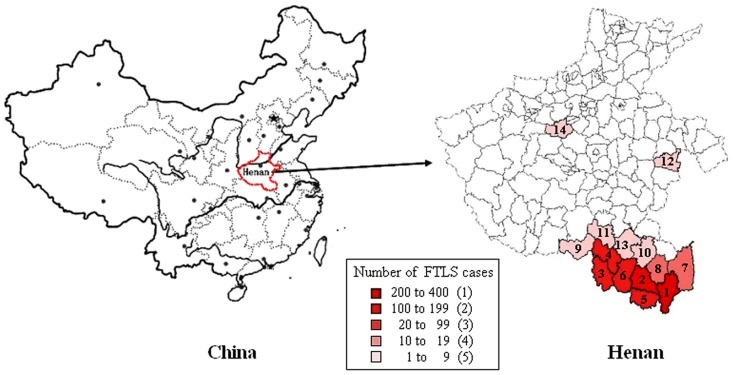
1 Shangcheng, 2 Guangshan, 3 Sihe), 4 Pingqiao, 5 Xinxian, 6 Luoshan, 7 Gushi, 8 Huangchuan, 9 Tongbai, 10 Xixian, 11 Queshan, 12 Luyi, 13 Zhengyang, 14 Dengfeng.
Time
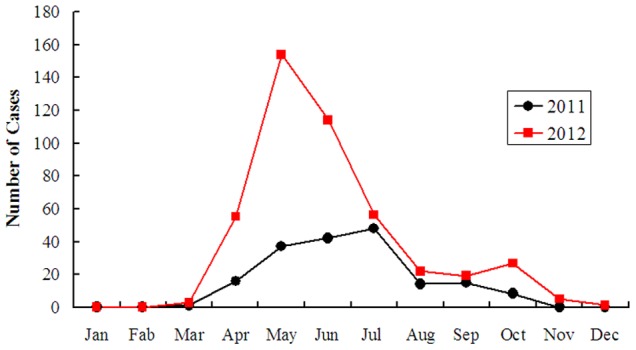
FTLS showed strong seasonality. In both 2011 and 2012, cases started to increase in March, and peaked between May and August; cases during those four months accounted for 76.5% (487/637) of all laboratory-confirmed cases. The monthly case count peaked in July during 2011 and May during 2012. Cases started to decline in October in both years (Figure 2).
Figure 2. Monthly distribution of reported FTLS cases: Henan Province, China, January 2011 to December 2012.
Person
Of the 637 laboratory-confirmed case-patients, 40.2% (256/637) were males and 59.8% (381/637) were females. The median age of the case-patients was 60 (range, 10–90) years; 60.9% (388/637) of the patients were 46–69 years in age. The sex distribution differed significantly among the age groups (χ2 = 147.8553, P<0.0001). The vast majority of the case-patients (96.1%, 612/637) were farmers, including agricultural and forest workers living in rural areas.
Deaths
During 2011 and 2012, 18 deaths due to FTLS were reported in Henan Province; the case fatality rate was 2.8% (18/637). The median age of deceased case-patients was 66.5 (range, 42–77) years. The mortality rate increased with age (Cochran-Armitage trend test, Z = 2.3622, P = 0.0182).
Molecular Characterization of FTLSV Strains
To characterize the FTLSV strains circulating in Henan Province and investigate their genetic origin, we analyzed the viruses isolated from acute serum samples tested positive for FTLSV RNA. Of the 133 FTLSV strains isolated and confirmed by real time RT-PCR, complete genomes were determined for 10 strains isolated during 2011 and 19 strains isolated during 2012. The GenBank accession numbers of SFTSV obtained in this study were listed in Table 1.
Table 1. The GenBank accession numbers of SFTSV obtained in this study.
| Name of FTLSVstrain | GenBank accessionNO. of L segment | GenBank accessionNO. of M segment | GenBank accessionNO. of S segment | Isolationsource | geographic origin |
| FLSV2011YPQ11 | KF711886 | KF711926 | KF711897 | Human serum | pingqiao |
| FLSV2011YPQ12 | KF711880 | KF711930 | KF711915 | Human serum | pingqiao |
| FLSV2011YPQ17 | KF711887 | KF711923 | KF711896 | Human serum | pingqiao |
| FLSV2011YGS22 | KF711888 | KF711927 | KF711894 | Human serum | guangshan |
| FLSV2011YGS45 | KF711870 | KF711925 | KF711893 | Human serum | guangshan |
| FLSV2011YGS5 | KF711884 | KF711945 | KF711899 | Human serum | guangshan |
| FLSV2011YGS52 | KF711861 | KF711922 | KF711895 | Human serum | guangshan |
| FLSV2011YGS60 | KF711863 | KF711947 | KF711900 | Human serum | guangshan |
| FLSV2011YGS7 | KF711885 | KF711928 | KF711898 | Human serum | guangshan |
| FLSV2011YXX9 | KF711889 | KF711924 | KF711909 | Human serum | xinxian |
| FLSV2012YGS10 | KF711872 | KF711937 | KF711904 | Human serum | guangshan |
| FLSV2012YGS4 | KF711875 | KF711933 | KF711902 | Human serum | guangshan |
| FLSV2012YSC6 | KF711879 | KF711931 | KF711911 | Human serum | shangcheng |
| FLSV2012YSH10 | KF711868 | KF711940 | KF711912 | Human serum | pingqiao |
| FLSV2012YSH104 | KF711867 | KF711943 | KF711914 | Human serum | shangcheng |
| FLSV2012YSH105 | KF711869 | KF711944 | KF711916 | Human serum | shihe |
| FLSV2012YSH107 | KF711864 | KF711942 | KF711910 | Human serum | shihe |
| FLSV2012YSH14 | KF711876 | KF711938 | KF711908 | Human serum | shangcheng |
| FLSV2012YSH16 | KF711874 | KF711934 | KF711903 | Human serum | shangcheng |
| FLSV2012YSH27 | KF711878 | KF711932 | KF711905 | Human serum | shihe |
| FLSV2012YSH37 | KF711871 | KF711936 | KF711918 | Human serum | guangshan |
| FLSV2012YSH6 | KF711882 | KF711921 | KF711892 | Human serum | xinxian |
| FLSV2012YSH86 | KF711877 | KF711939 | KF711907 | Human serum | gushi |
| FLSV2012YSH89 | KF711873 | KF711935 | KF711906 | Human serum | shangcheng |
| FLSV2012YSH9 | KF711881 | KF711919 | KF711890 | Human serum | xinxian |
| FLSV2012YSH91 | KF711883 | KF711920 | KF711891 | Human serum | shihe |
| FLSV2012YSH92 | KF711865 | KF711941 | KF711917 | Human serum | shihe |
| FLSV2012YSH93 | KF711866 | KF711946 | KF711913 | Human serum | shihe |
| FLSV2012YXX1 | KF711862 | KF711929 | KF711901 | Human serum | xinxian |
The nucleotide sequences of FTLSV isolates in this study were closely related to each other, with 95.8% to 99.9% nucleotide identity for the complete L segments, 98.0% to 100% nucleotide identity for the complete M segments, and 95.1% to 99.9% nucleotide identity for the partial S segments. The identity of amino acid sequences was 98.8% to 100% in RNA-dependent RNA polymerase, 96.6% to 100% in GnGc precursor, and 98.3% to 100% in N protein, respectively.
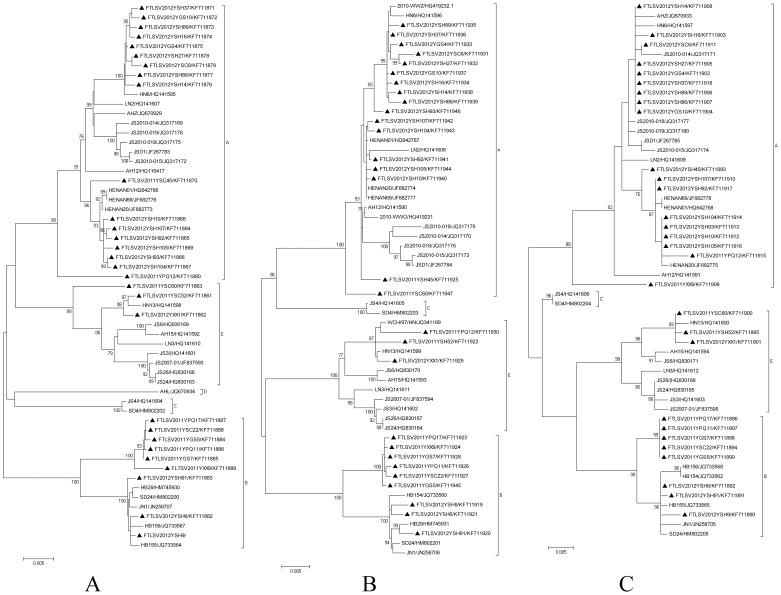
Using the ML method in MEGA software, phylogenetic trees were constructed with the sequences of 29 isolates in this study and 27 reference FTLSV strains from GenBank. Phylogenetic analysis of the L segment sequences revealed that the FTLSV strains were classified into four large groups in a previous study: A, B, C and E [19]. Our new isolates belonged to groups A, B, and E. Most of the 2011 isolates belonged in Group A, whereas most of the 2012 isolates belonged in Group B; a small number of strains belonged to Group E. The trees based on the S or M segment sequences had similar topologies as those based on the L segment sequences (Figure 3).
Figure 3. Phylogenetic analysis of FTLSV strains isolated from Henan Province during 2011 and 2012, compared with other bunyaviruses.
The phylogenetic tree was constructed by using the maximum likelihood method with the MEGA5 software. The reliability values indicated at the branch nodes were determined using 1,000 bootstrap replications. Isolated FTLSV strains in 2011 and 2012 from Henan Province were labeled by black solid triangles. Phylogenetic relationship of FTLSV with other bunyaviruses, based on the complete L, M, S segment sequences, are shown in panel A, B, C, respectively.
Discussion
From January 2011 to December 2012, 987 clinically diagnosed FTLS cases were reported in Henan Province. Although cases were detected each month, nearly three-quarters occurred during May-August, suggesting that surveillance and prevention efforts should be strengthened during summer months each year. The incidence rate was highest among persons aged 46–69 years; most of reported case-patients were farmers living in wooded and hilly areas or working in the fields. Some FTLS case-patients reported a history of tick bite before illness. Previously, FTLSV RNA had been detected in some ticks, and FTLSV stains were isolated from ticks collected from dogs and goats in the epidemic region [20]. Tick densities in the epidemic areas in Henan Province are high during May-August, coinciding with the peak of the cases [21], indicating that FTLSV may be mostly tick-borne. Therefore, prevention of tick bites should be promoted as important prevention and control measures to reduce FTLS in the epidemic area.
The vast majority of FTLS cases were reported in Xinyang Prefecture in southern Henan Province (114°01′–114°06′ E, 31°46′–31°52′ N). Of the 6,108,683 population in Xinyang Prefecture, 85% work in agriculture. The county consists of southwestern mountains, a central hilly district, and northern plains and depressions. It is in a subtropical to warm-temperate transitional zone, with a continental monsoon humid climate and 4 distinct seasons (annual average temperature: 15.2°C; average rainfall: 1,150 mm). The major sources of income for the farmers in this county included various crops, fruit trees, tea, and livestock breeding. These features create ideal conditions for tick-borne diseases such as FTLS to spread.
The high (>96%) identity of the virus isolates from the laboratory-confirmed FTLS cases during the study period indicated a lack of variability in the FTLSV strains circulating in Henan Province. Of these diagnostic methods, virus isolation is the most time-consuming, and requires expensive instruments or special techniques. Real-time RT-PCR and IFA can be completed in 2–4 h [22]–[24], but this technique requires elaborate methods for the detection of amplified products or sophisticated instruments. ELISA assays could offer a simple, convenient and efficient method of measuring antibodies in large-scale epidemiology studies in a wide range of settings, without requiring specialized and expensive measuring equipments [25], [26]. ELISA assays may also be applied in early diagnosis for FTLS patient.
The epidemiology, pathogenesis and mode of transmission of FTLS are still unclear because this disease was only recently discovered. FTLS is a serious public health problem in Henan Province, causing many severe diseases and deaths annually. Our study describes the characteristics of FTLS cases and makes recommendations for prevention measures, which can help to inform the prevention and treatment of FTLS in the endemic area.
Acknowledgments
We especially want to thank Dr. Xiangguo Qiu for critical reading and review of this manuscript. We sincerely acknowledge the cooperation of Xinyang Center for Disease Control and Prevention, Nanyang Center for Disease Control and Prevention, Zhumadian Center for Disease Control and Prevention, and Zhengzhou Center for Disease Control and Prevention.
Funding Statement
This research was supported by Henan Medical Science Project (200702016), Henan Province Health Department and National Health and Fimaly Planning Commission of the People’s Republic of China Co-build Project (201101017), and China-Australia Health and HIV/AIDS Facility (EID35). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Xu BL, Liu LC, Huang XY, Ma H, Zhang Y, et al. (2011) Metagenomic analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a new Bunyavirus. Plos Pathogens 11: e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang YZ, Zhou DZ, Xiong YW, Chen XP, He YW, et al. (2011) Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Chin J Epidemiol 32: 209–220. [PubMed] [Google Scholar]
- 3. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, et al. (2011) Fever with thrombocytopenia associated with a novel Bunyavirus in China. N Engl J Med 364: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amman BR, Manangan AP, Flietstra TD, Calisher CH, Carroll DS, et al. (2013) Association between movement and Sin Nombre virus (Bunyaviridae: Hantavirus) infection in North American deermice (Peromyscus maniculatus) in Colorado. J Wildl Dis 49: 132–142. [DOI] [PubMed] [Google Scholar]
- 5. Lihoradova OA, Indran SV, Kalveram B, Lokugamage N, Head JA, et al. (2013) Characterization of Rift Valley fever virus MP-12 strain encoding NSs of Punta Toro virus or sandfly fever Sicilian virus. PLoS Negl Trop Dis 7: e2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiao H, Tian HY, Cazelles B, Li XJ, Tong SL, et al. (2013) Atmospheric moisture variability and transmission of hemorrhagic fever with renal syndrome in changsha city, mainland china, 1991–2010. PLoS Negl Trop Dis 7: e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang YZ, Zhou DJ, Qin XC, Tian JH, Xiong Y, et al. (2012) The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. J Virol 86: 2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tommy TY, Liu W, Thomas AB, Cui N, Zhuang L, et al. (2013) Evolutionary and molecular analysis of the emergent severe fever with thrombocytopenia syndrome virus. Epidemics 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan H, Hu JY, Liu SL, Shen H, Zhu YY, et al. (2013) A reported death case of a novel bunyavirus in Shanghai, China. Virology Journal 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding F, Zhang WY, Wang LY, Hu WB, Soares Magalhaes RJ, et al. (2013) Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011–2012. Clinical Infectious Diseases 56: 1682–1683. [DOI] [PubMed] [Google Scholar]
- 11. Chang MS, Woo JH (2013) Severe fever with thrombocytopenia syndrome: tick-mediated viral disease. J Korean Med Sci 28: 795–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu W, Lu QB, Cui N, Li H, Wang LY, et al. (2013) Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in china who had severe fever with thrombocytopenia syndrome. Clin Infect Dis 57: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 13. Yu L, Zhang L, Sun LN, Lu J, Wu W, et al. (2011) Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS ONE 7: e38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(2010) National guideline for prevention and control of severe fever with thrombocytopenia syndrome. Beijing: National Health and Family Planning Commission of People’s Republic of China.
- 15. Huang XY, Liu LC, Du YH, Ma H, Mu YJ, et al. (2013) Detection of a novel bunyavirus associated with fever, thrombocytopenia and leukopenia syndrome in Henan Province, China, using real-time reverse transcription PCR. J Med Microbiol 62: 1060–1064. [DOI] [PubMed] [Google Scholar]
- 16. Huang XY, Du YH, Li XL, Ma H, Man RQ, et al. (2012) Establishment of indirect immunofluorescence assay (IFA) for detection of IgG antibody against new bunyavirus. Chin J Prev Med 46: 165–168. [PubMed] [Google Scholar]
- 17. Du YH, Huang XY, Deng WB, Ma HX, Ma H, et al. (2012) Culture, isolation and identification of new bunyavirus in African green monkey kidney (Vero) cells. Chin J Prev Med 46: 169–172. [PubMed] [Google Scholar]
- 18.Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol pp: 1596–1599. [DOI] [PubMed]
- 19. Zhang YZ, He YW, Dai YA, Xiong Y, Zheng H, et al. (2012) Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis 54: 527–533. [DOI] [PubMed] [Google Scholar]
- 20. Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, et al. (2012) Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals. Chin J Virol 28: 252–257. [PubMed] [Google Scholar]
- 21. Liu Y, Huang XY, Du YH, Wang HF, Xu BL (2012) Survey on ticks and detection of new bunyavirus in some vect in the endemic areas of fever, thrombocytopenia and leukopenia syndrome(FTLS) in Henan province. Chin J Prev Med 46: 500–504. [PubMed] [Google Scholar]
- 22. Neeraja M, Lakshmi V, Dash PK, Parida MM, Rao PV (2013) The clinical, serological and molecular diagnosis of emerging dengue infection at a tertiary care institute in southern India. J Clin Diagn Res 7: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam WY, Leung TF, Lee N, Cheung JL, Yeung AC, et al. (2010) Development and comparison of molecular assays for the rapid detection of the pandemic influenza A (H1N1) 2009 virus. J Med Virol 82: 675–683. [DOI] [PubMed] [Google Scholar]
- 24. Reddy V, Ravi V, Desai A, Parida M, Powers AM, et al. (2012) Utility of IgM ELISA, TaqMan real-time PCR, reverse transcription PCR, and RT-LAMP assay for the diagnosis of Chikungunya fever. J Med Virol 84: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 25. Ojogun N, Kahlon A, Ragland SA, Troese MJ, Mastronunzio JE, et al. (2012) Anaplasma phagocytophilum outer membrane protein A interacts with sialylated glycoproteins to promote infection of mammalian host cells. Infect Immun 80: 3748–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin RM, Patel R, Zinovik A, Kramer MS, Oken E, et al. (2012) Filter paper blood spot enzyme linked immunoassay for insulin and application in the evaluation of determinants of child insulin resistance. PLoS One 7: e46752. [DOI] [PMC free article] [PubMed] [Google Scholar]