Abstract
Purpose
Ketogenic diets (KDs) are high in fat and low in carbohydrates as well as protein which forces cells to rely on lipid oxidation and mitochondrial respiration rather than glycolysis for energy metabolism. Cancer cells (relative to normal cells) are believed to exist in a state of chronic oxidative stress mediated by mitochondrial metabolism. The current study tests the hypothesis that KDs enhance radio-chemo-therapy responses in lung cancer xenografts by enhancing oxidative stress.
Experimental Design
Mice bearing NCI-H292 and A549 lung cancer xenografts were fed a KD (KetoCal® 4:1 fats: proteins+carbohydrates) and treated with either conventionally fractionated (1.8-2 Gy) or hypofractionated (6 Gy) radiation as well as conventionally fractionated radiation combined with carboplatin. Mice weights and tumor size were monitored. Tumors were assessed for immuno-reactive 4-hydroxy-2-nonenal-(4HNE) modified proteins as a marker of oxidative stress as well as PCNA and γH2AX as indices of proliferation and DNA damage, respectively.
Results
The KD combined with radiation resulted in slower tumor growth in both NCI-H292 and A549 xenografts (p<0.05), relative to radiation alone. The KD also slowed tumor growth when combined with carboplatin and radiation, relative to control. Tumors from animals fed a KD in combination with radiation demonstrated increases in oxidative damage mediated by lipid peroxidation as determined by 4HNE-modified proteins as well as decreased proliferation as assessed by decreased immunoreactive PCNA.
Conclusions
These results show that a KD enhances radio-chemo-therapy responses in lung cancer xenografts by a mechanism that may involve increased oxidative stress.
Introduction
Over the past 2 decades, there has been little improvement in the overall survival trends in patients with locally advanced lung cancer despite the development of new chemotherapy agents and advances in immunotherapy. Therefore, complementary approaches that enhance the efficacy of radiation and chemotherapy while causing minimal toxicity would have a high therapeutic benefit.
It has long been known that malignant cells have high levels of glucose uptake, glycolysis and pentose phosphate activity even in the presence of oxygen. This glycolytic phenotype has been associated with higher grade lung neoplasms and poorer prognosis (1), while reversing this glycolytic phenotype using biochemical or pharmacological approaches has resulted in decreased metastases and tumor growth (2). Cancer cells also have alterations in their mitochondrial structure and function resulting in increased levels of reactive oxygen species (ROS) such as O2•− and H2O2, relative to normal cells (3-5). It has been proposed, with significant supporting data, that cancer cells utilize increased glucose metabolism to generate reducing equivalents that are necessary to facilitate decomposition of ROS as an adaptive response to metabolic oxidative stress caused by cancer cell specific dysfunctional mitochondrial O2 metabolism (3, 6-8). Therefore, approaches which further increase mitochondrial oxidative metabolism while limiting glucose metabolism, may selectively increase oxidative stress in cancer cells resulting improved responses to radiation and chemotherapy. One clinical approach that exploits mitochondrial metabolism is a ketogenic diet (KD).
KDs typically consist of 90% fat, 8% protein and 2% carbohydrate and are an established therapy for epilepsy (9). KDs derive their name from the generation of ketones, mainly beta-hydroxybutyrate (βHB) and acetoacetate (AA) which occur due to increased fatty acid oxidation in the liver and are precursors for acetyl Co A, the first step in the citric acid cycle (9). Because of the low carbohydrate content of KDs, there is often a reduction of blood glucose levels and overall greater glycemic control (10, 11). It has also been demonstrated that the rates of glucose uptake as well as rates of flux into the glycolytic pathway are reduced in rats fed a KD (12, 13). The combination of reduction in blood glucose and rise in ketones is believed to force cells to rely more heavily on mitochondrial respiration than on glycolysis for energy metabolism (14).
The current study tests the hypothesis that feeding a ketogenic diet to mice bearing lung cancer xenografts induces oxidative stress in tumors and improves responses to radio-chemo-therapy. The results show that KDs effectively enhance tumor growth inhibition and prolong animal survival in response to radio-chemo-therapy in mice with lung cancer xenografts by a mechanism that appears to involve oxidative stress. Furthermore, KDs effectively enhanced responses in lung cancer xenografts using both conventional (1.8-2 Gy) and hypo-fractionated (6 Gy) radiation dosing schemes without causing increased systemic toxicity as indicated by changes in animal weight or behavior. These data support the hypothesis that KDs may enhance lung cancer responses to radio-chemo-therapy by a mechanism that involves selective enhancement of oxidative stress in tumor tissues.
Methods
Cell and culture conditions
A549 and H292 cells were obtained from the American Type Culture Collection (ATCC). A549 cells were maintained in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (FBS) (Hyclone) and 0.01% gentamicin sulfate (Cellgro). H292 cells were maintained in Roswell Park Memorial Institute Medium (RPMI) containing 10% FBS (Hyclone) and 0.01% gentamicin sulfate (Cellgro). Glucose depleted media consisted of DMEM devoid of glucose and pyruvate but containing glutamine (Gibco) to which 70 mg/dL D-glucose was added.
Clonogenic survival analysis
A549 or H292 cells (120,000/60 mm dish) were plated and allowed to grow in their respective tissue culture media for 24 hours. All plates were then placed into DMEM glucose depleted media with 70 mg/dL glucose added back. Selected tissue culture dishes were supplemented with 3 mM (R)-(β)-3-hydroxybutyric acid sodium salt (βHB)(Sigma Aldrich) and 1.5 mM lithium acetoacetate (AA)(Sigma Aldrich) mimicing the maximum concentration and approximate ratio of ketones found in the blood of adult humans on a KD (14). After 24 hours of ketone treatment, cultures were exposed to sham, 0.5 Gy, 1 Gy, 2 Gy, 3 Gy or 4 Gy ionizing radiation (IR) after which clonogenic survival analysis was performed as previously described in standard complete media (15).
Tumor xenograft growth
Female 4-6 week old athymic-nu/nu mice were purchased from Harlan Laboratories (Indianapolis, IN). Mice were housed in the Animal Care Facility at the University of Iowa and all procedures were approved by the University of Iowa IACUC committee and conformed to NIH guidelines. H292 and A549 tumor cells were injected subcutaneously into the right flanks as previously described (15). Treatment began when tumor volumes measured approximately 30 mm3.
Ketogenic diet and ketone monitoring
The mice in the KD groups were fed ad libitum KetoCal® (Nutricia North America, a formula designed for children with epilepsy). This diet has a ketogenic ratio (fats: proteins + carbohydrates) of 4:1 (energy distribution: fat 90%, carbohydrate 1.60% and protein 8.40%) and the fat is derived from soybean oil (100% long chain fatty acids; 22% saturated fat, 24% monounsaturated fat, and 15% polyunsaturated fat). KetoCal® was prepared as a paste on a culture dish lid by adding water (water: KetoCal 1:2) then placed upside down in the food hopper and attached to assure the animals access (16). Control mice were fed a standard rodent diet (NIH-31 modified 25% protein, 21% fat, 54% carbohydrate). KD was started 2 days before the initiation of IR and continued for 24 hours after the last treatment. Blood βHB and glucose levels were measured using the Precision Xtra (Abbott Labs) machine via a tail vein stick. Previous results indicated that the average blood levels of βHB starting 3 days after beginning the KD diet and continuing through the end of treatment were 1.01 ± 0.64 mM for the KD mice versus 0.18 ± 0.08 mM for control mice (17).
Ionizing radiation in vivo
Mice in all treatment and sham groups were anesthetized using 87.5 mg/kg ketamine and 12.5 mg/kg xylazine mixture in the radiation facility at the University of Iowa. The mice in the ionizing radiation (IR) groups were placed in a lead box with only the right flank exposed. Radiation was delivered using a Pantak Therapax DXT 300 X-ray machine operated at 200 kVp with added filtration of 0.35 mm Cu + 1.5 mm Al resulting in a beam quality of 0.95 mm Cu. Tumors were measured daily using Vernier calipers (volume = (length × width2)/2) and euthanized when tumor length exceeded 1.5 cm in any dimension. For the 12 Gy total dose fractionated IR experiment (Figure 1), mice were divided into the following treatment groups (N represents the total number of animals per treatment group combined from two duplicative experiments); 1) Control group [N=10], 2) KD group [N=12], 3) IR group [12 Gy total dose in 6 × 2 Gy fractions every other day for 2 weeks; N=16], 4) IR + KD group [N=16], 5) carboplatin [15 mg/kg intra-peritoneal once per week times 3 doses; N=6], 6) carboplatin + KD [N=5], 7) IR + carboplatin [N=12], and 8) IR + carboplatin + KD [N=14]. For the 61.2 Gy total dose fractionated IR experiment (Figure 2) mice were divided into 4 groups; 1) Control [N=6], 2) KD [N=7], 3) IR [61.2 Gy in 34 doses, 1.8 Gy every other day; N=8], and 4) KD + IR [N=7]. The 4 treatment groups for the 12 Gy hypofractionation IR experiment (Figure 3) with 9 mice per treatment group were; 1) Control, 2) KD, 3) IR (12 Gy in 2 × 6 Gy fractions, 48 hours apart), and 4) KD + IR. At the completion of the KD, the 3 mice with the largest tumors from each treatment group were collected and frozen in liquid nitrogen for oxidative stress assessment.
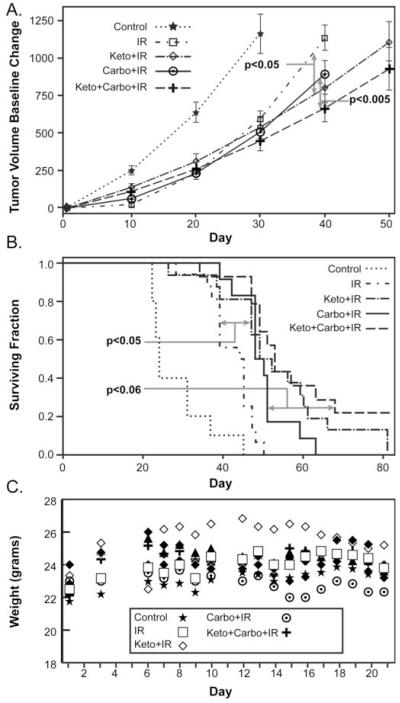
Figure 1. The ketogenic diet enhances chemo-radiation responses in H292 lung cancer xenografts.
Nude mice (5-16 animals per group) were injected with H292 cells in the flank and tumors allowed to grow to 30 mm3. Treated mice were given 3 × 15 mg/kg carboplatin doses on three consecutive Mondays. Following each of the first two doses of carboplatin the mice were irradiated with three × 2 Gy IR fractions Monday, Wednesday and Friday for 2 weeks (12 Gy total dose) followed one final carboplatin dose on the following Monday. The KD started 2 days prior to the first chemo-radiation dose and continued until two days following the last dose for a total of 16 days. When maximum tumor diameter exceeded 1.5 cm, mice were sacrificed. (A) Tumor volume growth curve estimates using mixed linear regression analysis demonstrate that KD significantly (P<0.05) decreases tumor growth rates when combined with IR and carboplatin-IR, relative to IR and carboplatin-IR alone, respectively. (B) Pair wise group comparisons of Kaplan-Meir survival curves demonstrate that KD significantly enhances IR response (p<0.05), relative to IR alone, and approaches significance for carbo-IR sensitivity (p<0.06). (C) Pair wise group comparisons of animal weights demonstrate that all treatments were well tolerated as demonstrated by a lack of significant weight change (errors omitted for clarity).
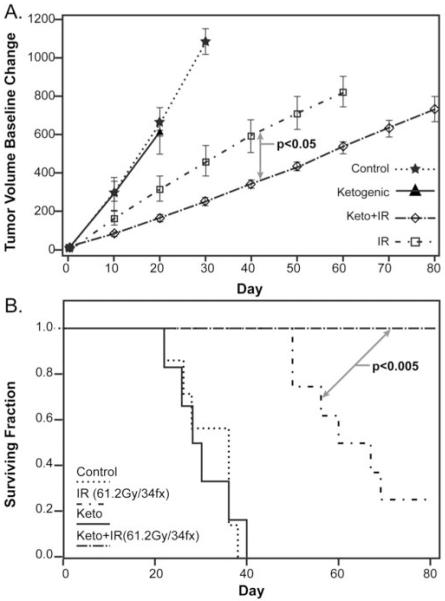
Figure 2. A protracted KD increases radiation response in H292 lung cancer xenografts given conventional fractionated radiation.
H292 xenografts were grown in nude mice as in Figure 2. Mice (6-8 per group) were treated with KD for the entire treatment time and/or a 61.2 Gy total dose of radiation in 34 × 1.8 Gy fractions three times per week (M, W , F). The experiment was terminated 81 days after the beginning of KD. (A) Tumor growth curve estimates demonstrated that the KD significantly enhances radiation response as assayed by tumor growth rates (p=0.0210). (B) Pair wise group comparisons of Kaplan-Meir survival curves also demonstrated that the KD significantly enhanced radiation response (p=0.0041).
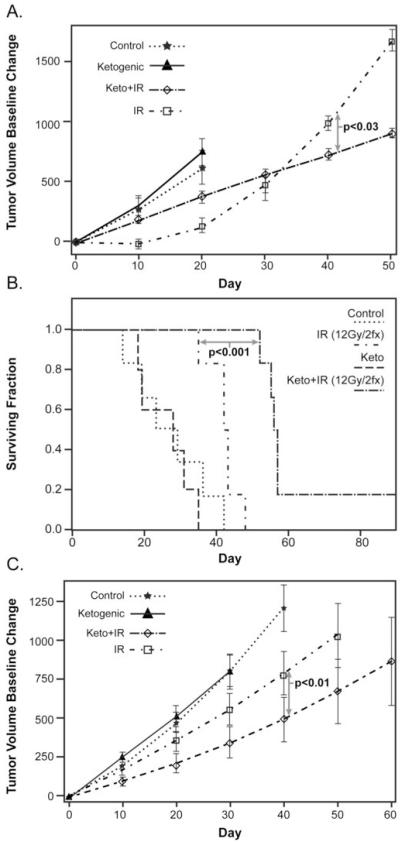
Figure 3. The responses of H292 and A549 lung cancer xenografts to hypo-fractionated radiation were enhanced by the KD.
H292 and A549 xenografts were grown in nude mice as in Figure 2. Mice (5-9 per group) were treated with KD for 2 days prior to radiation and continuing for a total of 7 days. Hypo-fractionated radiation (2 × 6 Gy fractions) was delivered on day 3 and 5 of the protocol. Tumor growth curves (A) and Kaplan-Meir survival plots (B) showed enhanced radiation responses in animals with H292 xenografts fed the KD (p<0.05), relative to IR alone. (C) Tumor volume growth curve estimates of A549 xenografts also demonstrated that KD + IR significantly slowed tumor growth when compared to radiation alone (p<0.05). All treatments were well-tolerated as demonstrated by the lack of weight loss during therapy (Supplemental Fig. 1A and B).
4-Hydroxy-2-nonenal-(4HHE)-modified protein immuno-blotting assay
Approximately 20 mg of mouse tumor was washed and homogenized in 300 μl 500 mM potassium phosphate, 50 mM EDTA buffer pH 7.0 with Complete Mini-protease inhibitor (Roche Diagnostics). Protein concentrations were determined by Lowry Assay. 25 μg of protein was placed in an equal volume with nano-pure H2O and blotted onto pre-wetted Sequi-Blot PVDF membrane (Bio-Rad) and allowed to dry. After re-wetting in methanol, the membrane was incubated in 250 mM sodium borohydride in 100 mM MOPS, pH 8.0 for 15 min to chemically reduce the Schiff base adduct to reveal the Michael addition product for antibody recognition. The membrane was then washed 3 times each with nano-pure H2O followed by PBS, then blocked for 30 minutes in 2 % albumin in PBS+ 1% Tween 20. The blot was incubated with the primary antibody recognizing the Michael addition product of 4HNE-modified cellular proteins (18) diluted 1/2000 overnight at 4°C, followed by 2 hours in secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit polyclonal antibody (1/20,000), and chemiluminescence detection (ECL Plus Western Blotting Detection System, GE Healthcare) with X-ray film. Immunoreactive protein on the dot blot was analyzed using integrated densities determined using Image J software.
Western Blot Assays
Approximately 100 mg piece of tumor was homogenized in 0.5 ml of ice cold Tris buffer pH 7.8 with Complete Mini-protease inhibitor and PhosStop phosphatase inhibitor cocktail (Roche Diagnostics). After a freeze/thaw the samples were centrifuged at 10,000 g for 15 minutes at 4°C. Protein concentrations were determined by Lowry method. PCNA Western blot analysis was carried out using a monoclonal anti-PCNA clone PC10 antibody (M0879, Dako) running both 10 and 70 μg of protein per lane on separate occasions, electrophoresed on 4-20% gradient SDS-polyacrylamide gels. Bands were detected using ECL Plus Western Blotting Detection System (GE Healthcare) with detection by either X-ray film and/or a Typhoon FLA 7000 fluorescent detection system. Band-integrated densities were determined with Image J software and averaged between the two gels. The γH2AX Western was performed following the same procedure described for PCNA but running on a 15% SDS-polyacrylamide gel using reaction with the polyclonal anti-phos-H2AX Ser 139 antibody (#07-164, Upstate Biotechnology).
Statistical analysis
The analysis of in vivo results focused on treatment group comparisons of tumor growth and survival. Regression analysis was used to model tumor growth as a non-linear function of follow-up time and to make treatment group comparisons. Within-animal correlation structures were included in the models to account for repeated measurement of tumor size over time. Plots of the estimated tumor means and their standard errors are provided in the results section. Estimates of survival were obtained with the methods of Kaplan-Meier and compared with log-rank tests. All associated statistical tests were two-sided and assessed for significance at the 5% level with the SAS statistical software (Cary, NC).
Results
Ketogenic diet enhances radio-chemo-therapy responses in mouse NSCLC xenografts
Athymic nude mice were injected with 2-3 × 106 H292 NSCLC cells in their right flank. When tumors reached approximately 4 mm in diameter, mice were treated with IR alone or IR combined with: ketogenic diet (KD), carboplatin, and/or standard fractionated IR doses (12 Gy in 6 × 2 Gy fractions). All animals consuming a KD ≥48 hours had serum ketone levels greater than 0.3 mEq/L (or 0.3 mmol/L) and were considered to be in ketosis as per the Emory Warner Clinical Laboratories of the University of Iowa Hospitals and Clinics. Consistent with previous studies (17), the mice eating the KD demonstrated blood βHB levels greater than 0.3 mEq/L (mean = 1.4±0.8 mEq/L) throughout the treatment period, while mice treated with standard mouse chow were consistently less than 0.3 mEq/L (mean 0.1±0.1 mEq/L).
Mice treated with KD + IR or KD + IR + carboplatin (12 Gy in 6 × 2 Gy fractions IR) demonstrated a significant decrease in tumor growth rate compared to mice treated with identical therapies on standard chow (p=0.0470 and p=0.0046 respectively) (Figure 1A). Furthermore, mice treated with KD + IR also demonstrated a significant survival advantage over mice treated with IR alone (p=0.0281); survival of mice treated with KD + IR + carboplatin versus IR + carboplatin was also prolonged (p = 0.0595) but did not reach the <0.05 significance level (Figure 1B). All treatments were well tolerated as demonstrated by a lack of weight loss as well as general condition and activity of the mice (Fig 1C). These data support the conclusion that KDs can effectively enhance responses to radio-chemo-therapy in animals bearing NSCLC xenografts without causing overt signs of toxicity.
Ketogenic diets enhance responses of lung cancer xenografts to a clinically relevant radiation dose fractionation scheme
In order to replicate the duration and dosing of common human lung cancer fractionated radiation therapy protocols, mice bearing H292 xenografts fed a KD or control diet were treated with a total dose of 61.2 Gy in 34 × 1.8 Gy fractions (Figure 2). Mice consuming a KD had achieved an average blood βHB level of 1.4 mM ± 0.4 by the first day of IR treatment. Due to the more protracted radiation exposure regimen, some mice lost a significant amount of weight (15% of starting weight) by the end of the third or fourth week of treatment. This weight loss was also associated with hyperkeratotic dermatitis likely caused by coryneform bacterium which is a common pathogen in athymic nude mice. The weight loss was managed by feeding the control mice with a diet supplement (2019 Teklad) consisting of approximately the same fat, carbohydrate and protein as the standard diet, but soaked in water. Mice on KD were supplemented with KetoCal 3:1 formula every other day for two weeks and the KetoCal 4:1 liquid formula. Ketone levels were measured prior to each IR dose to verify that the mice were maintained in ketosis. Mice were sacrificed once tumors reached 1.5 cm in diameter or the day following the completion of 34 IR fractions. None of the mice in the KD + IR group reached criteria to be sacrificed at the completion of IR treatment. Mice treated with the KD in combination with IR demonstrated significantly slower tumor growth (p=0.0210) (Figure. 2A) and prolonged survival (p=0.0041), relative to IR alone (Figure 2B). These results clearly demonstrate that a prolonged KD combined with a clinically relevant IR dose fractionation schedule is well-tolerated and enhances radiation response in NSCLC xenografts.
Ketogenic diets enhance responses of lung cancer xenografts to hypo-fractionated radiation
Hypo-fractionated radiation therapy (delivering higher doses in fewer fractions) has become of increasing interest with the recognition of a potential improvement in the therapeutic ratio between the tumor volume and normal anatomic structures. Clinically, hypofractionation allows for increased patient convenience and reduced costs without sacrificing efficacy and is used to treat early stage lung cancers (19). In addition, hypo-fractionated radiation shortens the duration of radiation therapy which would potentially increase patient compliance with the diet.
For hypo-fractionated radiation experiments H292 and A549 xenografts were grown in nude mice as in Figures 1 and 2. Mice were fed a KD for a total of 7 days starting 2 days before and continuing 2 days after 2 fractions of 6 Gy IR (12 Gy total dose). Both H292 and A549 xenograft bearing animals treated with KD in combination with hypo-fractionated IR demonstrated significantly slower tumor growth rates, relative to IR alone (p=0.0299 and p=0.0054, respectively) (Figures 3A and C). H292 xenografts treated with KD + IR also demonstrated a survival advantage compared to those treated with IR alone (p=0.0006) (Fig. 3B) but this was not seen in A549 xenografts (data not shown). All treatments were well tolerated as demonstrated by a lack of weight loss and general condition of the mice (supplemental Figure 1AB). These results demonstrate that a KD enhances responses of NSCLC xenografts to hypo-fractionated radiation therapy.
Ketogenic diets in combination with IR increase immuno-reactive 4HNE-modified proteins in tumor tissue
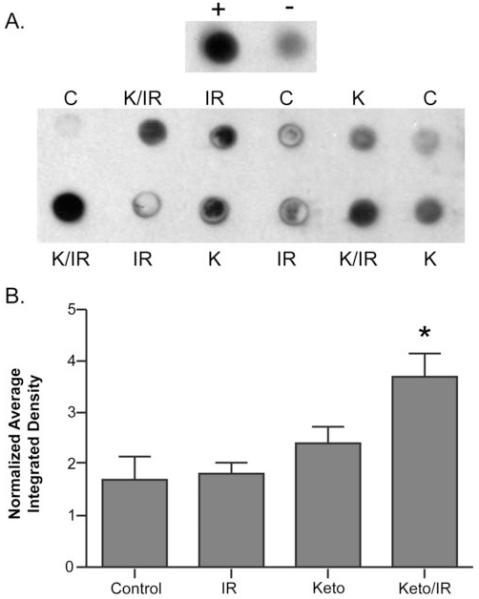
In order to assess oxidative stress in vivo, immunoreactive 4-hydroxy-2-nonenal (4HNE) modified protein was analyzed in tumors as a marker of oxidative protein damage caused by lipid peroxidation. Dot blot analysis of tumor samples harvested at the end of the hypofractionated-IR experiment shown in Figure 3AB demonstrated increased 4HNE-modified protein in H292 xenografts treated with KD + hypo-fractionated IR versus hypo-fractionated IR alone (p<0.05) (Figure 4AB). These data support the hypothesis that a KD combined with hypo-fractionated IR causes an increase in oxidative damage to proteins in tumor tissue resulting from lipid peroxidation-derived aldehydes.
Figure 4. KD combined with radiation increases 4HNE-modified proteins in H292 mouse lung cancer xenografts.
Equal protein from H292 xenograft tumor homogenates taken from animals treated as in Figure 3 (N=3 from each group) were blotted onto PVDF membranes and stained with polyclonal antibody against 4HNE-modified proteins. This analysis was repeated twice and a representative blot is shown in panel A. Positive (+) and negative (−) controls represent the immunoreativity derived from H292 cell homogenates treated with and without 100 uM genuine 4HNE for one hour at 37°C. Panel B shows quantification of dot blots by Image J analysis and samples were normalized to the background on each blot. Error bars represent ± 1SEM. One way ANOVA with Newman-Keuls Multiple Comparison Test demonstrated the K+IR was significantly greater than Control, IR, and KD alone (*p<0.05).
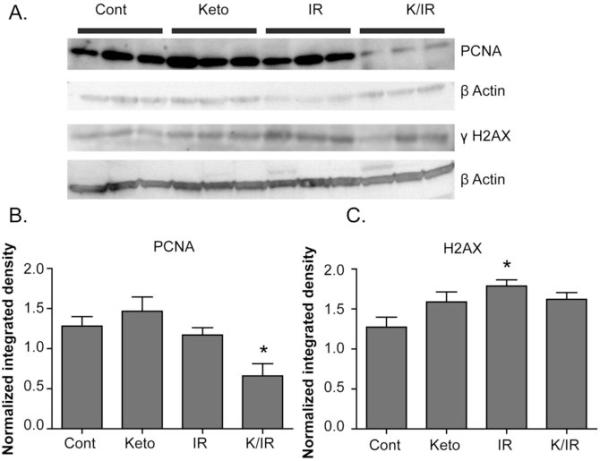
Ketogenic diets in combination with IR decrease immuno-reactive PCNA protein in tumor tissue following treatment with fractionated radiation
In order to further probe possible mechanisms responsible for the inhibition of tumor growth seen when the KD was combined with conventional fractionated radiation, tumors harvested at the end of treatment shown in Figure 2 were analyzed by Western blotting for markers of proliferation (using immuno-reactive proliferating nuclear antigen; PCNA) and DNA damage (using immuno-reactive phosphorylated histone γH2AX) (Figure 5). Consistent with the inhibition in tumor growth and prolongation of survival seen in Figure 2, tumors treated with KD + IR demonstrated significantly lower levels of immuno-reactive PCNA than mice in all other treatment groups (Figure 5AB). Phosphorylated histone γH2AX was significantly increased in tumors treated with radiation; however, mice treated with KD + IR did not demonstrate any further increase in levels of γH2AX indicating that enhanced radiation response does not appear to be related to increased levels of DNA damage in animals fed a KD (Figure 5C).
Figure 5. KD combined with fractionated radiation treatment results in decreased immuno-reactive PCNA in tumor tissue.
Mouse xenograft tumors treated as in Figure 2 were harvested 24 hours after the final 2 Gy radiation fraction. Tumor protein homogenates harvested from control, KD, IR, and KD + IR treated animals (3 from each group) were separated on denaturing gels, blotted onto membranes, and stained with anti-PCNA or anti-γH2AX antibodies (A). Quantification of dot blots was done by Image J analysis and samples were normalized to the background on each blot. Animals treated with K + IR had a significant reduction in PCNA staining, relative to IR animals (B). There was not a significant difference in γH2AX staining when IR and KD+IR were compared, suggesting that ketogenic diets do not increase DNA damage when combined with radiation (C). Error bars represent ± 1SEM. One way ANOVA with Newman-Keuls Multiple Comparison Test were used to determine which groups were significantly different from control (*p<0.05)
Ketones in cell culture media do not significantly enhance radiation response in human lung cancer cells in vitro
To mimic ketosis in an in vitro system, exponentially growing H292 (Supplemental Fig 2A) and A549 (Supplemental Fig 2B) human lung cancer cells were grown in tissue culture media containing 3 mM βHB and 1.6 mM AA and 70 mg/dl glucose for 24 hours. These concentrations of ketones and glucose mimic the concentrations and approximate ratios found in the blood of adult humans eating a KD (14). Glucose was utilized at a rate of ~35 mg/dL per day depending on the cell concentration and was replenished daily to 70 mg/dl. A549 cells were capable of metabolizing βHB as demonstrated by a decrease in βHB levels in the media during 5 days of growth (from 3 mM to 1.8 mM). The combination of ketones with radiation exposure (0-4 Gy) did not alter clonogenic survival in either H292 or A549 cells, relative to similarly treated cells in standard media (Supplemental Figure 2AB).
Discussion
The current results show that a ketogenic diet is an effective adjuvant capable of enhancing radio-chemo-therapy responses in xenograft models of human NSCLC. It is also important to note that the KD alone in our study did not result in any inhibition of tumor growth, relative to control. This finding is in agreement with two studies in orthotopic models of brain cancer (20, 21) where Maurer et. al. showed no improvement in survival when animals were fed a KD as well as Zhou et. al. who could only demonstrate a KD-induced tumor growth inhibition when the diet was combined with caloric restriction. However our findings in lung cancer xenografts differ from other investigations in prostate, gastric, and brain cancer models that show a positive therapeutic advantage using KD as a mono-therapy (22-24). These differences could be due to the different types of tumor and animal models utilized or the different fatty acid compositions of the KDs and control diets that were used. Our model utilizes a soy based fatty acid composition which is relatively high in oleic (18:1), linoleic (18:2), and linolenic (18:3) acids with standard mouse chow as the control. Since unsaturated fatty acids are substrates for lipid peroxidation that may govern the formation of aldehydes such as 4HNE this fatty acid composition may be influencing our results. Freedland et. al. used a high fat moderate high carbohydrate “Western” diet as the control diet in a prostate cancer model (23) while Otto et. al. used a KD supplemented with omega 3 fatty acids and medium-chain triglycerides (22). In addition, Stafford et. al. used a syngeneic intracranial mouse model of glioma demonstrating the potential role of tumor microenvironment in the biological effects of KDs (24).
The current study also demonstrated that KDs enhanced the responses of H292 and A549 NSCLC xenografts using two different radiation dosing schemes. In the conventional fractionation dosing scheme (61.2 Gy in 34 fractions with 1.8 Gy/fraction), significant increases in animal survival were achieved in the KD + IR group when compared to the IR alone treatment group (Fig 2). In a recent clinical trial studying the effects of a KD on the quality of life in 16 patients with advanced cancer, it was concluded that the KD was suitable for advanced cancer patients and resulted in no severe side effects (25). However it was also noted that 4 of the 16 patients stopped the diet before the end of a 7 week treatment time (25).
In order to shorten the time required to be maintained on a KD, xenograft responses to hypofractionated radiotherapy were determined. Clinically, hypo-fractionation allows for both increased patient convenience and reduced costs without sacrificing efficacy and is frequently used to treat early stage lung cancers (19, 26-28). KD combined with hypo-fractionated IR significantly slowed tumor growth rate and prolonged survival, when compared to radiation alone, in both the H292 and A549 lung cancer xenograft models (Figure 3AB). This finding in lung cancer is similar to a recent finding using a KD with hypo-fractionated radiation (2 × 4 Gy fractions) to treat transplantable mouse gliomas (29).
Elevated ketone levels have been implicated in the generation of cellular metabolic oxidative stress. Lim et. al. found elevated indices of lipid peroxidation in cultured human endothelial cells treated with AA (30). Stadler et. al. demonstrated an increase in protein oxidation and lipid peroxidation through a free radical dependent mechanism in rat livers of animals subjected to a model of acetone induced ketosis (31). Chronic exposure to βHB was also shown to increased reactive oxygen species (ROS) production in cardiomyocytes (32). Finally, Milder et. al. demonstrated an increase in H2O2 production and lipid peroxidation in the hippocampus of rats fed a KD (33). An excellent review addressing the effect of KD on redox biology in neuronal tissues was also recently published (34).
It has been hypothesized that cancer cells, relative to normal cells, exist in a condition of chronic metabolic oxidative stress mediated by increased steady-state levels of O2•− and H2O2, with a major site of pro-oxidant production being mitochondrial electron transport chain complexes. This increased level of ROS in cancer cells may be compensated for by increases in glucose metabolism though the pentose phosphate pathway resulting in the generation of NADPH to be utilized as a cofactor in hydroperoxide metabolism (3). Because KDs force cells to rely on mitochondrial oxidative metabolism for energy production while limiting glucose availability it, would be expected that a ketotic state would exacerbate metabolic oxidative stress in cancer cells, realtive to normal cells. Thus, a KD may selectively enhance radio-chemo-responses in cancer cells when combined with standard cancer therapeutics through a mechanism involving oxidative stress.
Consistent with this hypothesis, the current studies also demonstrated that tumors from mice treated with KD and radiation demonstrated significantly increased 4HNE-modified proteins (Figure 4). This observation supports the hypothesis that KD-induced enhancement of radiation response in vivo may involve increases in lipid peroxidation and protein damage that could contribute to growth inhibition as assayed by lower levels of immuno-reactive PCNA (Figure 5). The accumulation of 4HNE-modified proteins in tumors treated with the KD conbined with radiation are also consistent with previous results in breast cancer cells showing that exposure to a mixture of conjugated linoleic acid isomers could selectively inhibit breast cancer cell proliferation by increasing levels of 4HNE (35).
Interestingly, H292 and A549 cells exposed in vitro to ketones and IR doses ranging from 0.5 Gy to 4 Gy did not show enhanced radiation responses. Previous research has also demonstrated that radiation-responses in vitro do not necessarily correlate with radiation responses in vivo (36). Radiation response in vivo may be influenced by a variety of factors including oxygen effects, the presence of an immune system, anti-oxidant levels, DNA repair and the extracellular environment that are not adequately modeled in vitro. As an example, the in vitro clonogenic survival assays with ketones were performed in 21% oxygen which is significantly higher than the partial pressure of oxygen in solid tumors of anmimals fed KDs (37). Furthermore, therapuetic radiation doses also initiate a cascade of signalling events involving cytokines and oxidant producing pathways that further induce activation of the immune system and inflammation that contributes to tissue damage and radiation responses that is not adequately modeled in vitro (38). Our data suggests that when studying the effects of KDs on radiation responses that in vivo model systems may be more appropriate than in vitro model systems.
The current study supports the conclusion that KDs are well-tolerated by tumor bearing animals recieving concurrent radio-chemo-therapy. These results also support the conclusion that feeding a KD could serve as an easily implimented adjuvant for improving responses to radio-chemo-therapies in the treatment of lung cancer. Finally these data also support the hypothesis that a KD enhances radiation responses in lung cancer xenografts by a mechanism involving oxidative stress and inhibition of cancer cell proliferation in vivo.
Supplementary Material
Translational Relevance.
Ketogenic diets are high in fat, low carbohydrates, and are well-established as an alternative therapy for childhood epilepsy. This report demonstrates that a ketogenic diet enhances radio-chemo-therapy responses as well as enhancing oxidative stress in human lung cancer xenografts. Since ketogenic diets are an established therapy in humans, these studies may be rapidly translated into the clinical setting potentially allowing for improved cancer control without added normal tissue toxicity.
Acknowledgements
We thank Drs. Andrean Simons-Burnett, Daniel Berg, Thor Halfdanarson, Zita Sibenaller, James Jacobus, Alf Siochi as well as Sam Rodman, Tyler Ronnfeldt, Amamda Kalen, Ryan Beckenbaugh, Kellie Bodeker, Gareth Smith, Mindi TenNapel, and Starlette Sharpe for their technical assistance and helpful discussions. Funding was provided by the National Institutes of Health (CA139182, UL1RR024979, and CA133114), Radiological Society of North America (RR1020), the Carver Trust Research Program of Excellence in Redox Biology and Medicine, and a gift from Marie Foster, Nellie K. Spitz, and IBM. B.G.A is a B. Leonard Holman Pathway medical resident supported by the Department of Radiation Oncology at the University of Iowa Hospitals and Clinics.
Footnotes
There are no potential conflicts of interest to disclose.
References
- 1.de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer. 2007;55(1):79–87. doi: 10.1016/j.lungcan.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418(1):29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bize IB, Oberley LW, Morris HP. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Res. 1980;40(10):3686–93. [PubMed] [Google Scholar]
- 5.Springer EL. Comparative study of the cytoplasmic organelles of epithelial cell lines derived from human carcinomas and nonmalignant tissues. Cancer Res. 1980;40(3):803–17. [PubMed] [Google Scholar]
- 6.Parrella P, Xiao Y, Fliss M, Sanchez-Cespedes M, Mazzarelli P, Rinaldi M, et al. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 2001;61(20):7623–6. [PubMed] [Google Scholar]
- 7.Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132(1):1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fath MA, Diers AR, Aykin-Burns N, Simons AL, Hua L, Spitz DR. Mitochondrial electron transport chain blockers enhance 2-deoxy-D-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biol Ther. 2009;8(13):1228–36. doi: 10.4161/cbt.8.13.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem. 2012;68(1):141–51. doi: 10.1007/s13105-011-0112-4. [DOI] [PubMed] [Google Scholar]
- 10.Westman EC, Yancy WS, Jr., Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 2008;5:36. doi: 10.1186/1743-7075-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy WS, Jr., Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170(2):136–45. doi: 10.1001/archinternmed.2009.492. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Baldwin KM, Morris GS. Dietary effects on cardiac metabolic properties in rodents. J Mol Cell Cardiol. 1990;22(3):353–9. doi: 10.1016/0022-2828(90)91468-m. [DOI] [PubMed] [Google Scholar]
- 13.Brito SR, Moura MA, Kawashita NH, Brito MN, Kettelhut IC, Migliorini RH. Glucose uptake and glycolytic flux in adipose tissue from rats adapted to a high-protein, carbohydrate-free diet. Metabolism. 2001;50(10):1208–12. doi: 10.1053/meta.2001.25645. [DOI] [PubMed] [Google Scholar]
- 14.Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983;32(8):757–68. doi: 10.1016/0026-0495(83)90105-1. [DOI] [PubMed] [Google Scholar]
- 15.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res. 2011;17(19):6206–17. doi: 10.1158/1078-0432.CCR-11-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89(7):1375–82. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fath MA, Simons AL, Erickson J, Anderson ME, Spitz DR. Enhancement of cancer therapy using ketogenic diet. In: Spitz DR, Dornfeld KJ, Krishnan K, Guis D, editors. Oxidative Stress in Cancer Biology and Therapy. Humana Press; 2012. pp. 47–58. [Google Scholar]
- 18.Cohn JA, Tsai L, Friguet B, Szweda LI. Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress. Arch Biochem Biophys. 1996;328(1):158–64. doi: 10.1006/abbi.1996.0156. [DOI] [PubMed] [Google Scholar]
- 19.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68(1):11–9. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stafford P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutrition & Metabolism. 2010;7:74. doi: 10.1186/1743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutrition & Metabolism. 2011;8(1):54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmerman RD, Story M. Stereotactic body radiation therapy: a treatment in need of basic biological research. Cancer J. 2006;12(1):19–20. doi: 10.1097/00130404-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–31. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 28.Cheung PC, Yeung LT, Basrur V, Ung YC, Balogh J, Danjoux CE. Accelerated hypofractionation for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54(4):1014–23. doi: 10.1016/s0360-3016(02)03045-6. [DOI] [PubMed] [Google Scholar]
- 29.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7(5):e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. 1998;25(9):1083–8. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 31.Stadler K, Bonini MG, Dallas S, Duma D, Mason RP, Kadiiska MB. Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis. Am J Physiol Endocrinol Metab. 2008;295(2):E456–62. doi: 10.1152/ajpendo.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier A, Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2007;292(5):E1325–32. doi: 10.1152/ajpendo.00186.2006. [DOI] [PubMed] [Google Scholar]
- 33.Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40(1):238–244. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milder JB, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100(3):295–303. doi: 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albright CD, Klem E, Shah AA, Gallagher P. Breast cancer cell-targeted oxidative stress: enhancement of cancer cell uptake of conjugated linoleic acid, activation of p53, and inhibition of proliferation. Exp Mol Pathol. 2005;79:118–25. doi: 10.1016/j.yexmp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Malaise EP, Fertil B, Chavaudra N, Guichard M. Distribution of radiation sensitivities for human tumor cells of specific histological types: comparison of in vitro to in vivo data. Int J Radiat Oncol Biol Phys. 1986;12(4):617–24. doi: 10.1016/0360-3016(86)90071-4. [DOI] [PubMed] [Google Scholar]
- 37.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 38.Schaue, McBride Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173:406–417. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.