Abstract
Curcumin, a component of turmeric spice that imparts flavor and color to curry, is thought to possess anti-inflammatory and antioxidant properties in biological tissues. However, while such efficacies have been described in the context of carcinogenesis, the impact of curcumin on normal cell cycle regulation is poorly understood. Here, we provide evidence of curcumin toxicity in proliferating bovine aortic endothelial cells, at concentrations relevant to the diet and below those previously reported in cancer models. Upon confirming curcumin’s ability to upregulate hemeoxygenase-1 in a dose-dependent fashion, we found the minimally efficacious curcumin concentration to also inhibit endothelial cell DNA synthesis. Moreover, curcumin concentrations below the minimum 2 µM threshold required to induce hemeoxy-genase-1 bound tubulin protein in vitro and triggered hallmark evidence of mitotic catastrophe in vivo. Concentrations as low as 0.1 µM curcumin led to disproportionate DNA segregation, karyorrhexis, and micronucleation in proliferating endothelial cells. While suggesting a mechanism by which physiological curcumin concentrations inhibit cell cycle progression, these findings describe heretofore unappreciated curcumin toxicity with potential implications for endothelial growth, development, and tissue healing.
Keywords: Cell cycle, Curcumin, Endothelial, Tubulin
1. Introduction
Curcumin (diferuloylmethane) is a naturally occurring phenolic compound derived from the spice turmeric, the dried ground rhizome of the East Indian plant Curcuma longa Linn. In addition to imparting color and aroma to foods, turmeric powder (or “haldi” in Hindi) has been used since ancient times by the Indian culture as an herbal remedy thought to suppress inflammation (Aggarwal et al., 2003; Sharma et al., 2005). Recently, curcumin has also received attention in the scientific literature as a nutritional factor with efficacy to limit oxidative stress within biological tissues (Scapagnini et al., 2006). The mechanism of curcumin’s purported antioxidant role centers around stimulation of the Nrf-2/ARE pathway, leading to increased expression of Phase II detoxification enzymes (Lee and Surh, 2005). Specifically, curcumin has been shown to upregulate endothelial hemeoxygenase-1 (Motterlini et al., 2000), which catalyzes the degradation of extracellular free heme (Maines, 1997). Enzymatic degradation of heme by hemeoxygenase-1 yields carbon monoxide, iron, and biliverdin (Maines, 1997; Tenhunen et al., 1969). This newly formed biliverdin is then rapidly reduced, via biliverdin reductase, to the potent antioxidant bilirubin (Jansen et al., 2010; Stocker et al., 1987). While bilirubin that reaches the systemic circulation is subject to glucuronidation in hepatocytes (Hauser et al., 1984), an unconjugated bilirubin fraction likely persists and functions as a physiological antioxidant in both blood and tissues (Boon et al., 2012; Kim et al., 2012; Zelenka et al., 2012).
Concentrations of curcumin as low as 5 µM have been shown to induce hemeoxygenase-1 protein expression in cultures of confluent endothelial cells (Motterlini et al., 2000). It is unclear, however, whether dietary curcumin is sufficiently bioavailable to produce such efficacious curcumin concentrations within body fluids (Cheng et al., 2001). Moreover, curcumin concentrations found to stimulate hemeoxygenase-1 have not been evaluated for additional interactions with cellular constituents, such as tubulin protein, that could impact the growth of cycling (i.e., non-confluent) endothelial cells. Indeed, insights to potential curcumin toxicity have recently surfaced from studies reporting growth inhibition of cancerous cells treated with pharmacological curcumin doses (Sahu et al., 2009; Sun et al., 2012).
Substantial evidence now exists to support an antiproliferative mechanism by which curcumin induces G2/M cell cycle arrest and apoptosis-like death in a variety of cancer cell models (Chen et al., 1999; Dorai et al., 2000; Jiang et al., 1996; Moragoda et al., 2001). Curcumin also disrupts microtubule assembly and leads to mitotic cell cycle arrest in carcinoma cells from both breast and cervix (Gupta et al., 2006). More specifically, in MCF-7 breast cancer cells, curcumin has been shown to disrupt mitotic spindle structure, preclude normal anaphase movements, and lead to micronucleation (Holy, 2002). This cellular phenotype has been observed previously upon exposure to tubulin binding drugs with efficacy to suppress cancer cell growth. Once the cell enters mitosis, highly dynamic mitotic microtubules are responsible for orchestrating chromosomal positioning and movement. However, in the presence of a tubulin binding drug, the polymerization and/or dynamics of mitotic microtubules can become compromised and lead to M-phase arrest, incomplete congression of chromosomes at the metaphase plate, disproportionate chromosomal segregation, karyorrhexis (i.e., micronucleation), and ultimate death of the cell (Castedo et al., 2004; Jordan, 2002). While cellular death ensuing from disruption of mitotic microtubules yields some classical apoptosis biomarkers, it is a discrete form of programmed cell death, referred to previously as “mitotic catastrophe” (Castedo et al., 2004). A growing literature of antiproliferative agents, to include some naturally occurring dietary compounds, describes M-phase cell cycle arrest and mitotic catastrophe as a consequence of microtubule interactions. These microtubule disrupting, antiproliferative compounds continue to receive much attention as candidates for inclusion in chemotherapeutic and chemopreventive regimens (Jordan, 2002).
While growth inhibition of aberrantly cycling transformed cells is a desired clinical outcome, nutritional compounds having nonspecific antiproliferative mechanisms of action also impact normal cell growth (Gautam et al., 1998; Jackson et al., 2007). Maintaining functional viability of epithelial tissues (e.g., covering epithelia of the intestine, skin, and circulatory system) requires continuous cell division due to regular sloughing of cells with finite life spans (Junqueira et al., 1992). Also, actively dividing cells are integral to the normal physiology of growth, development, and wound healing (Hadley, 2000). These normal processes require new blood vessel formation (i.e., angiogenesis), and specifically the proliferation of endothelial cells in response to signaling by vascular endothelial growth factor (Ferrara, 2001) and nitric oxide (Morbidelli et al., 1996). It should be noted that previous reports of curcumin efficacy in arresting cell cycle progression provide no mechanistic evidence of specificity for cancerous cells. Moreover, disruption of microtubule assembly (Gupta et al., 2006) or mitotic spindle (Holy, 2002) in cancerous cells suggests a possible direct interaction of curcumin with tubulin protein. If such direct interaction exists, as with other naturally occurring antiproliferative and microtubule- disrupting agents (Jordan, 2002), curcumin may also pose a threat to normally dividing cell populations.
In the present study, we sought to determine whether concentrations of curcumin purported to induce efficacious hemeoxygenase-1, might also impact normal endothelial mitosis. As earlier work was conducted solely in confluent endothelial cells (Motterlini et al., 2000), we examined hemeoxygenase-1 expression in concurrent experiments using both confluent and nonconfluent cultures. Minimal threshold curcumin concentrations upregulating hemeoxygenase-1 were observed to fall within the range of previously reported curcumin concentrations measured in systemic circulation subsequent to oral intake (Cheng et al., 2001). Those minimal curcumin concentrations were, however, higher than concentrations found to disrupt normal mitosis in growing endothelial cells. Hence, this report provides cautionary evidence of the non-specific nature by which dietary curcumin could impact normal endothelial cell proliferation.
2. Materials and methods
2.1. Cell culture
Bovine aortic endothelial (BAE) cells, available from VEC Technologies (Rensselaer, NY), were used to investigate outcomes of exposing proliferating and non-proliferating cells to curcumin. Stock BAE cells were routinely cultured in M199 medium (with 0.016 g/L thymidine and 2.2 g/L sodium bicarbonate) containing 10% heat-inactivated fetal bovine serum and 5% bovine calf serum at 37 °C under a 5% CO2 atmosphere, and the medium was changed every 48 h. All experiments were conducted using stock cell passages 2–6, and curcumin (C8069, LKT Labs) or DMSO (vehicle, 0.1% final concentration across all samples) treatments were provided within fresh medium. Unless stated otherwise, proliferating BAE cells were treated 1 day after seeding, while cultures from the same passage were concurrently grown to confluence prior to the same curcumin or DMSO exposures (4 days after seeding).
2.2. Western blot analysis
BAE cells were seeded in 75 cm2 Corning tissue culture flasks (1.25 × 104 cells/cm2). Proliferating and non-proliferating cultures were exposed to increasing concentrations of curcumin (1–10 µM) or DMSO vehicle. Following treatments of 24 or 48 h, samples were washed twice with TBS, harvested in ice-cold lysis buffer (1% Triton X-100, 50 mmol/L Tris–HCl (pH 7.5), 100 mmol/L NaF, 15 mmol/L NaPPi, 1 mmol/L Na3VO4, 1.6 mg/L Aprotinin, 10 mg/L Leupeptin, 1 µmol/L Pepstatin), centrifuged (15,000g for 15 min at 4 °C), and assayed for total protein content. Aliquots of equal protein were fractionated by SDS–PAGE and transferred to nitrocellulose membranes. The membranes were blocked and then incubated with primary antibody to hemeoxygenase-1 (374087, EMD Chemicals, 4 µg/mL), α-tubulin (CP06, EMD Biosciences, 5 µg/mL), or actin (sc-1616, Santa Cruz Biotechnology, 1 µg/mL). After incubation with horseradish peroxidase-conjugated secondary antibody of the appropriate species (Santa Cruz Biotechnology), immunodetection was carried out using enhanced chemiluminescence (34080, Pierce Biotechnology). Exposed X-ray film was then scanned into Adobe Photoshop, and densitometric analysis performed on an Apple Macintosh G4 computer using the public domain NIH Image program [developed at the U.S. National Institutes of Health and available on the Internet (http://rsb.info.nih.gov/nih-image/)].
2.3. Cell growth assay
BAE cells were seeded at a density of 1.25 × 104 cells/cm2 in 25 cm2 Corning tissue culture flasks. One day after seeding, proliferating cultures (n = 4/group) were exposed to increasing concentrations of curcumin (5–30 µM) or DMSO vehicle for 48 h, prior to harvesting (via trypsinization) and cell counting (via hemocytometer) of both cells adherent to the culture flask and those floating in the culture medium.
2.4. [3H]thymidine incorporation assay of DNA synthesis
BAE cells were seeded at a density of 1.25 × 104 cells/cm2 in Costar 48-well plates. One day after seeding, proliferating cultures (n = 5/group) were exposed to increasing concentrations of curcumin (2–30 µM) or DMSO vehicle for up to 48 h, and together with [3H]thymidine (1 µCi) for the final 24 h. The medium was then removed and the cells assayed for tritium incorporation as previously described (Jackson and Singletary, 2004).
2.5. Cell cycle analysis and quantification of the mitotic index
BAE cells were seeded in 75 cm2 Corning tissue culture flasks (1.25 × 104 cells/cm2). One day after seeding, proliferating cultures (n = 3/group) were exposed to increasing concentrations of curcumin (5–15 µM) or DMSO vehicle and, after 24 h, harvested via trypsinization. The cells were prepared for analysis using a Becton Dickinson FACSCalibur Flow Cytometer as previously described (Jackson and Singletary, 2004).
In order to quantify cells in mitosis, BAE cells were seeded at a density of 1.25 × 104 cells/cm2 on 12 mm round glass coverslips and treated with increasing concentrations of curcumin (5–15 µM) or DMSO vehicle for 24 h. After washing once with PBS, cells were fixed, stained, and quantified using fluorescence microscopy as previously described (Jackson and Venema, 2006).
2.6. Immunofluorescence tubulin staining
BAE cells were sparsely plated on 12 mm round glass coverslips and exposed to increasing concentrations of curcumin (5–15 µM) or DMSO vehicle for 24 h. After washing once with PBS, cells were fixed with glutaraldehyde (1% in PBS, for 10 min at room temperature) followed by sodium borohydride (1 mg/mL in PBS, twice for 10 min each). Cells were then rinsed three times with PBS, permeabilized with wash buffer (0.1% Triton X-100, 1% BSA in TBS) for 10 min, and incubated with a monoclonal antibody to α-tubulin (CP06, EMD Biosciences, 5 µg/mL) for 30 min at room temperature. Following five washes with wash buffer, cells were incubated with a FITC-conjugated goat anti-mouse secondary antibody (sc-2078, Santa Cruz Biotechnology) and the DNA counterstained with DAPI (268298, Calbiochem, 10 µg/mL) for 30 min at room temperature. Finally, samples were washed again five times with wash buffer, washed once with dH2O, mounted on microscope slides with mounting medium (622701, MP Biomedicals), and analyzed by fluorescence microscopy.
2.7. Endothelial cytology
BAE cells were seeded in 75 cm2 Corning tissue culture flasks (1.25 × 104 cells/cm2). Proliferating and non-proliferating cultures were exposed to increasing concentrations of curcumin (0.01–1.00 µM) or DMSO vehicle and, after 24 or 48 h, harvested via trypsinization. Samples were then washed (with PBS), resuspended in PBS, and cytospun onto microscope slides. Cells were finally stained with Wright-Giemsa dye and examined under light microscopy for cytological evidence of mitotic catastrophe (aberrant mitotic figures, karyorrhexis, or micronucleation).
2.8. In vitro tubulin polymerization assay
Ice-cold bovine brain tubulin (>99% pure, kit BK006, Cytoskeleton) was incubated in the presence of 15 µM curcumin (C8069, LKT Labs) or DMSO vehicle for 30 min prior to transferring to a 96-well plate held at 37 °C. Absorbance readings (n = 4/group) were then immediately initiated at 340 nm using a Bio-Tek Synergy HT plate reader (one reading per minute, for 1 h according to kit instructions). The rate of tubulin polymerization (milli-OD340 units per min) was then calculated for each sample from the linear portion of the kinetics curve, and group mean rates were analyzed for statistical significance.
2.9. Gel filtration assay of in vitro curcumin-tubulin binding
Centrifugal gel filtration was used as previously described (Combeau et al., 2000), with modification, to assess the affinity of curcumin for purified tubulin protein. Bovine brain tubulin (>99% pure, kit BK006, Cytoskeleton) was first prepared as an aqueous solution in RB Buffer (100 mM MES–NaOH, pH 6.8, 1 mM EGTA, 0.5 mM MgCl2) at a concentration of 1 mg/mL. For the initial [3H]curcumin dose response, 100 µL aliquots of this tubulin solution were added to DMSO (12.5 µL, non-radiolabeled curcumin vehicle in the subsequent experiment) and incubated with either increasing concentrations of [3H]curcumin (12.5 µL, MT-1743, Moravek Biochemicals) or ethanol (12.5 µL, [3H]curcumin vehicle). Final concentrations of [3H]curcumin ranged from 0 to 10 µM. For the subsequent competition experiment, 100 µL aliquots of tubulin solution were incubated with 10 µM [3H]curcumin together with either non-radiolabeled curcumin (C8069, LKT Labs, 100 µM) or DMSO (vehicle). In both experiments, reaction mixtures (125 µL final volume) were allowed to incubate 2 h in a 28 °C water bath. Aliquots (100 µL) were then loaded on polyacrylamide spin columns (732–6232, Bio-Rad) and centrifuged (1000g for 4 min at 4 °C) prior to tritium scintillation counting and protein assay of the filtrates.
2.10. Statistical analysis
Data derived from experiments comparing only two means were analyzed by independent t-test and the accompanying test for homogeneity of variance. In the case of experiments requiring the comparison of more than two means, ANOVA was performed followed by Tukey’s Studentized Range (HSD) for the pair-wise comparisons. All statistical calculations were completed using JMP IN (SAS Institute, Cary, NC). Replicated experiments (each carried out at different times and with n ≥ 3 per group) were analyzed independently, and the data presented are those obtained in a single experiment. Statistically significant differences were established at P < 0.05.
3. Results
3.1. Curcumin induces hemeoxygenase-1 in both proliferating and non-proliferating endothelial cells
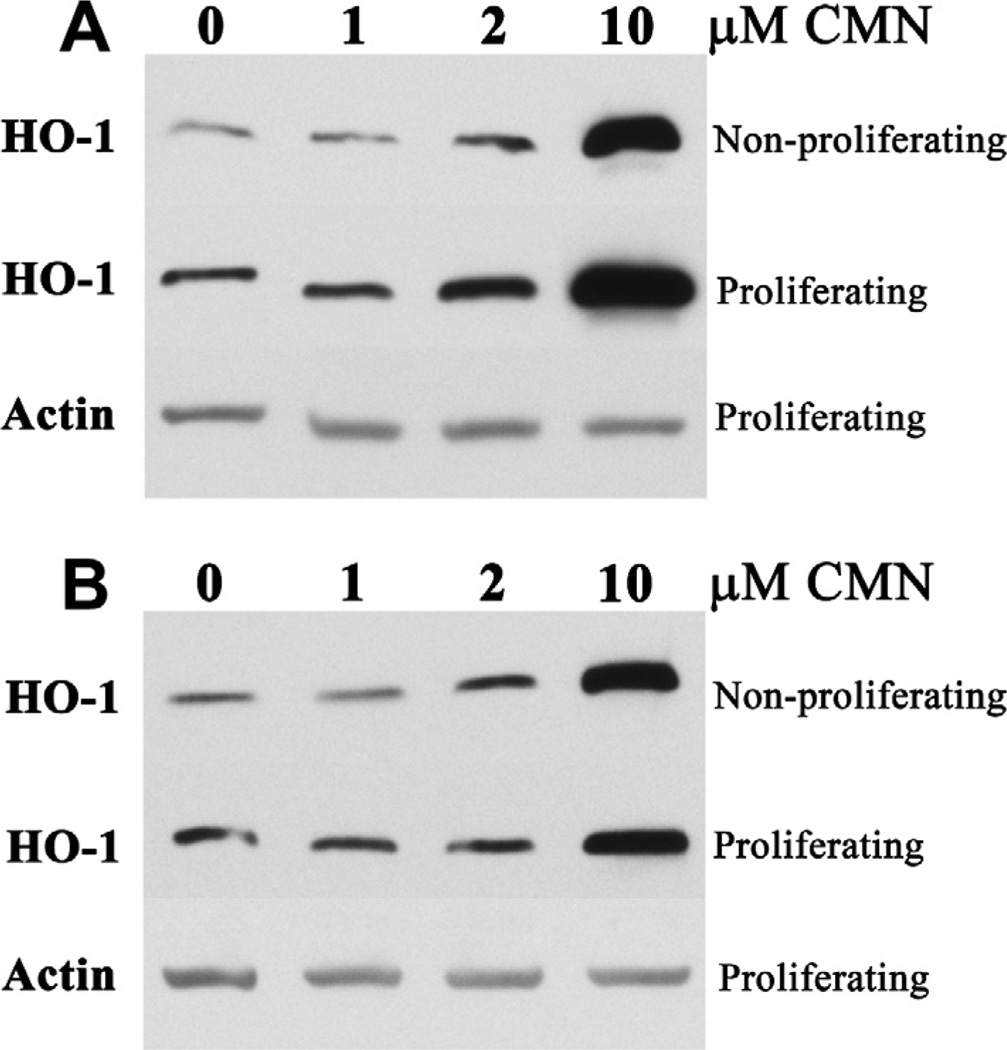
Curcumin stimulated hemeoxygenase-1 protein expression in both growing and growth-arrested BAE cell monolayer cultures. Hemeoxygenase-1 responded to curcumin concentrations as low as 2 µM within 24 h of a single curcumin exposure (Fig. 1A). This threshold 2 µM curcumin concentration increased hemeoxygenase-1 expression by ~50% in proliferating, as well as non-proliferating, BAE cells. Higher curcumin concentrations (e.g., 10 µM) led to the robust induction of hemeoxygenase-1 previously demonstrated in confluent, growth-arrested endothelial cells only (Motterlini et al., 2000). The efficacy of such higher curcumin concentrations persisted beyond 24 h, yet proliferating BAE cell hemeoxygenase-1 expression waned to control levels within 48 h of receiving 2 µM curcumin (Fig. 1B).
Fig. 1.
Curcumin induces hemeoxygenase-1 (HO-1) in both proliferating and non-proliferating BAE cells. Cultures were exposed to increasing concentrations of curcumin for 24 h (A), or 48 h (B), prior to Western blot analysis. Data are representative of 2 independent experiments.
3.2. Curcumin inhibits endothelial cell proliferation
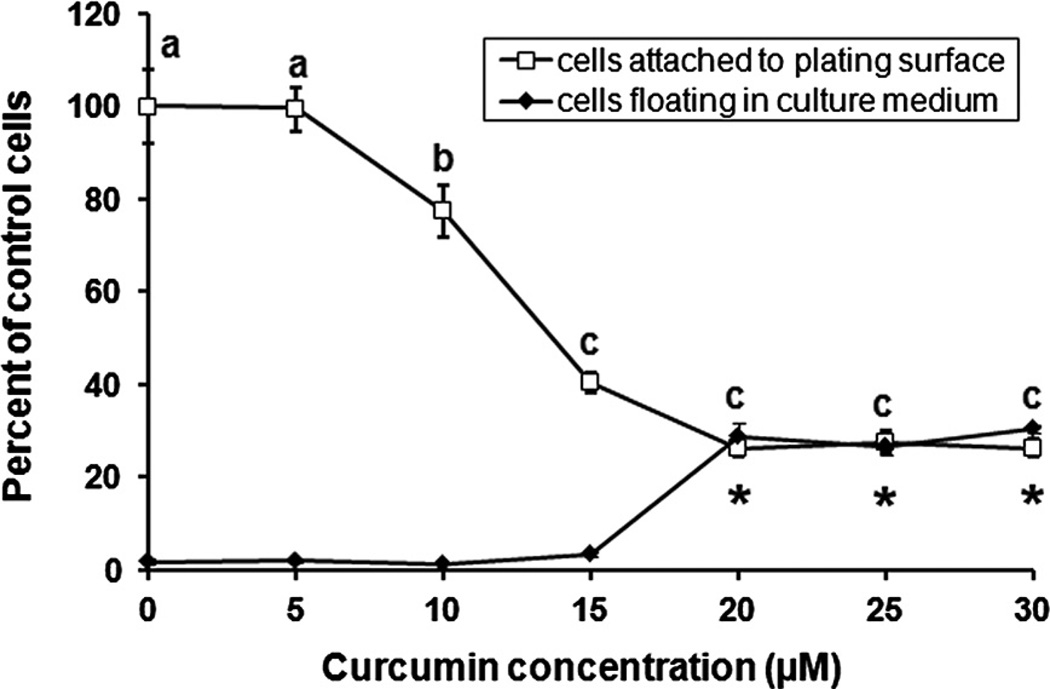
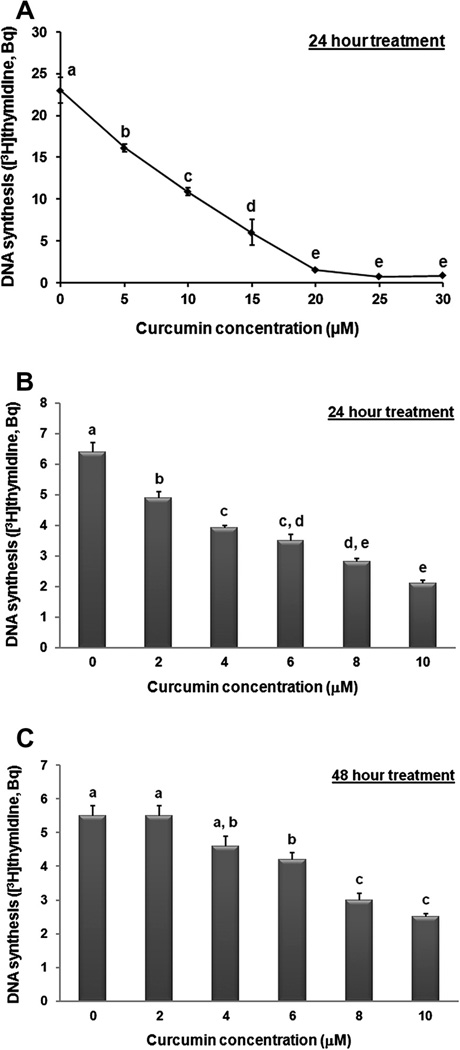
Curcumin inhibited BAE cell proliferation in a dose-dependent manner. Within 48 h, 10 µM curcumin led to a significant (P < 0.05) reduction in the number of BAE cells harvested from the plating surface, while higher curcumin concentrations also led to gross accumulation of cells floating in culture medium (Fig. 2). Curcumin also reduced DNA synthesis within 24 h (Fig. 3), with an IC50 value of ~10 µM. The inhibitory effect of curcumin on DNA synthesis was significant (P < 0.05) at 2 µM and greater concentrations (Fig. 3B), yet 20 µM curcumin was required to diminish [3H]thymidine incorporation to near background levels (Fig. 3A). Similar to the effect of curcumin on hemeoxygenase-1 expression in proliferating cells, DNA synthesis recovered to control levels within 48 h of initial exposure to the 2 µM threshold curcumin concentration (Fig. 3C).
Fig. 2.
Curcumin inhibits cell proliferation. Proliferating BAE cell cultures were exposed to increasing concentrations of curcumin for 48 h prior to counting of cells attached to the plating surface, as well as those floating in culture medium. Values are expressed as the percentage of control cells (*different from the vehicle control for floating cells, means representing attached cells that lack a common letter differ; P < 0.05). Values are means ± SEM, n = 4.
Fig. 3.
Curcumin inhibits DNA synthesis. Proliferating BAE cell cultures were exposed to increasing concentrations of curcumin for 24 h (A and B) or 48 h (C) prior to analysis of [3H]thymidine incorporation. Values are expressed as Becquerel (Bq) [3H]thymidine incorporation (means lacking a common letter differ, P < 0.05). Values are means ± SEM, n = 5 (A), n = 6 (B and C).
3.3. Curcumin induces endothelial cell cycle arrest
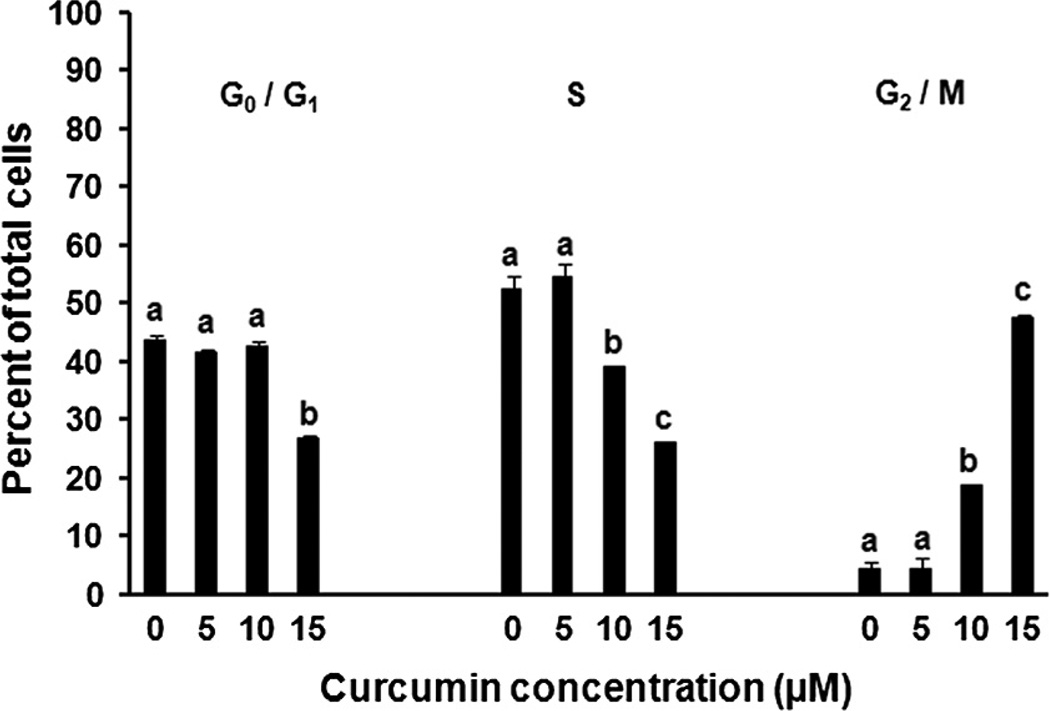
Asynchronous BAE cell cultures exposed to curcumin accumulated within the G2/M phase of the cell cycle in a dose-dependent fashion within 24 h. While this G2/M accumulation was clearly evident and statistically significant (P < 0.05) with 10 µM curcumin, the magnitude of the G2/M population of BAE cells exposed to 15 µM curcumin constituted an approximate 10-fold increase over that of the vehicle-administered controls (Fig. 4). In order to further characterize the impact of curcumin on BAE cell G2/M progression, we examined endothelial cell mitotic index in response to curcumin and observed accumulations of early, pre-metaphase mitotic figures (maximal response at 15 µM curcumin with the number of mitotic figures approximately three times that of control cultures, data not shown).
Fig. 4.
Curcumin induces G2/M cell cycle accumulation. Proliferating BAE cell cultures were exposed to increasing concentrations of curcumin for 24 h prior to analysis by flow cytometry. Values are expressed as the percentage of total cells in all phases of the cell cycle (means within each phase lacking a common letter differ, P < 0.05). Values are means ± SEM, n = 3.
3.4. Curcumin disrupts endothelial microtubules
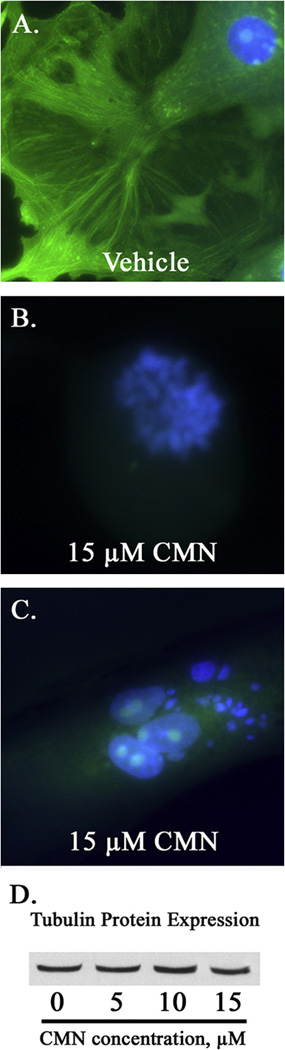
Tubulin staining of proliferating BAE cell cultures demonstrated curcumin’s ability to disrupt microtubule polymerization in vivo. While control cultures possessed abundant interphase cells with intact cytoplasmic microtubules (Fig. 5A), BAE cells exposed to 15 µM curcumin displayed only diffuse tubulin staining associated with either early mitotic figures (Fig. 5B) or aberrant interphase micronuclei (Fig. 5C). Curcumin (5–15 µM) did not alter tubulin protein expression (Fig. 5D), although 15 µM curcumin significantly (P < 0.05) impeded the rate of purified tubulin protein polymerization by ~18% compared to vehicle-treated controls in vitro (data not shown).
Fig. 5.
Curcumin disrupts microtubule polymerization in vivo. Microtubules (green) and DNA (blue) were stained within proliferating BAE cell cultures exposed to 15 µM curcumin (CMN) or DMSO (vehicle) for 24 h. The figure represents the abundance of early mitotic cells (B), as well as interphase cells possessing multiple micronuclei (C), lacking polymerized mitotic and cytoplasmic microtubule networks, respectively. Photomicrographs were taken at 400× magnification. Western blot analysis indicates that CMN treatment does not alter tubulin protein expression (D).
3.5. Curcumin binds tubulin protein and induces endothelial cell mitotic catastrophe
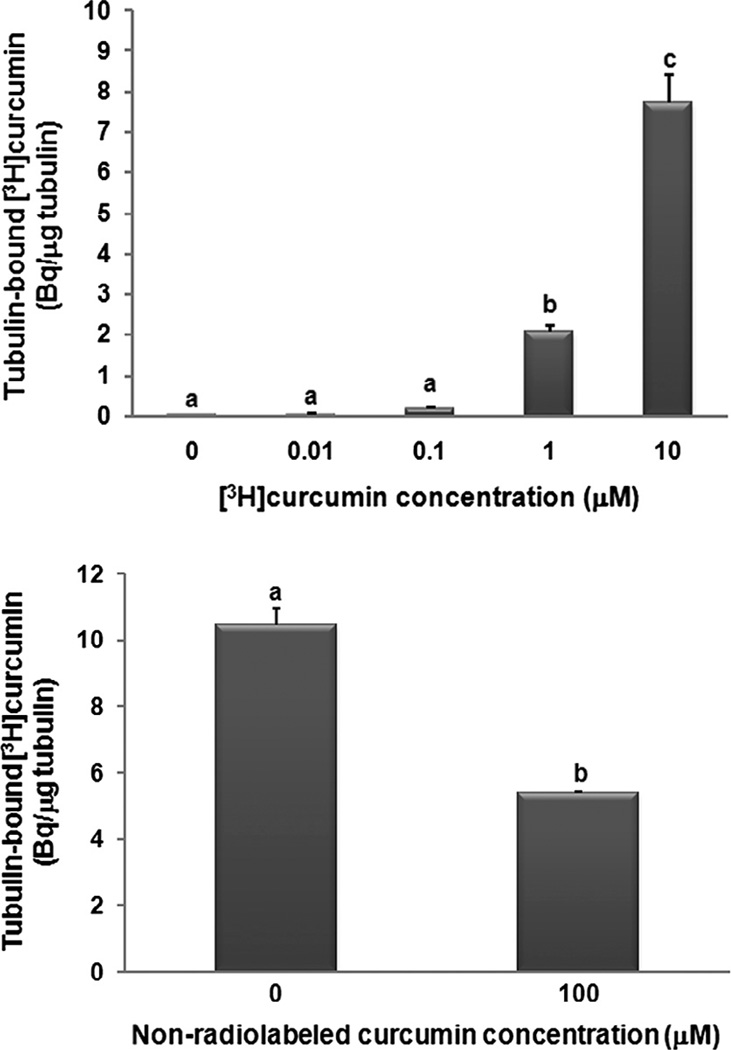
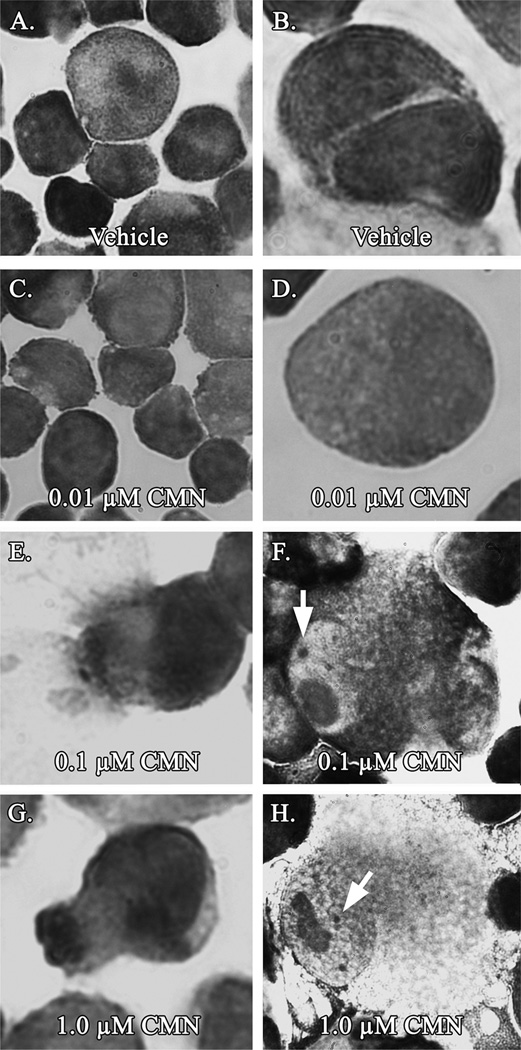
At concentrations as low as 1 µM, [3H]curcumin significantly (P < 0.05) bound to purified tubulin protein in vitro (Fig. 6A). In addition, pre-treatment of purified tubulin protein with a higher concentration of the same non-labeled curcumin used in our previous experiments significantly (P < 0.05) suppressed the affinity of [3H]curcumin for tubulin (Fig. 6B). Moreover, the concentration of curcumin triggering aberrant mitosis in vivo was an order of magnitude lower than that found to bind tubulin in vitro. At concentrations as low as 0.1 µM, curcumin induced hallmark evidence of mitotic catastrophe (disproportionate DNA segregation, karyorrhexis, and interphase cells displaying micronuclei), while exposure to lower curcumin concentrations yielded the normal endothelial phenotype characteristic of vehicle-administered controls (Fig. 7).
Fig. 6.
Curcumin binds tubulin protein in vitro. Purified bovine brain tubulin was incubated (A) in the presence of increasing concentrations of [3H]curcumin, or (B) in the presence of 10 µM [3H]curcumin together with either 100 µM non-labeled curcumin or DMSO (vehicle) at 28 °C for 2 h, prior to gel filtration chromatography. The [3H]curcumin bound tubulin protein (A and B), and this binding was suppressed by the higher (100 µM) concentration of non-labeled curcumin (B). Values are expressed as Becquerel (Bq) [3H]curcumin per microgram (µg) tubulin protein (means lacking a common letter differ, P < 0.05). Values are means ± SEM, n = 6 (A), n = 4 (B).
Fig. 7.
Curcumin triggers mitotic catastrophe in vivo. Proliferating BAE cell cultures were exposed to increasing concentrations of curcumin for 24 h prior to Wright–Giemsa staining and examination under light microscopy. Curcumin concentrations as low as 0.1–1.0 µM produced aberrant mitotic figures (E), karyorrhexis (see arrows, F and H), and micronucleation (G). Data are representative of 2 independent experiments.
4. Discussion
Our experiments have confirmed the endothelial bioactivity of curcumin, with hemeoxygnease-1 expression responding similarly in both proliferating and non-proliferating BAE cells. However, the minimal threshold curcumin concentration triggering hemeoxygenase-1 upregulation was also observed to suppress BAE cell proliferation. This finding was instrumental in driving our later experiments directed toward understanding the mechanism by which curcumin induces growth arrest of normal endothelial cells, a phenomenon previously described only within the context of limiting carcinogenesis (Meeran and Katiyar, 2008). Moreover, our findings suggest that relatively low curcumin concentrations, achievable in body fluids via the diet, could be toxic to normal endothelial tissues of individuals consuming curcumin for its other purported antioxidant and anti-inflammatory health benefits.
Curcumin concentrations in the 5–50 µM range have been reported to block cell cycle progression (Lee et al., 2009; Sun et al., 2012) and suppress proliferation (Anand et al., 2011) in various cancer cell lines. Our new findings indicate that normal primary endothelial cells are also susceptible to growth suppression by curcumin, yet at even lower doses. Within 24 h of initial exposure to 2 µM curcumin, BAE cell DNA synthesis was significantly reduced as compared with vehicle-treated controls (Fig. 3B). Interestingly, this same 2 µM curcumin concentration was just minimally efficacious in stimulating hemeoxygenase-1 expression in both proliferating and non-proliferating cell cultures (Fig. 1). BAE cells exposed to curcumin, therefore, appear to respond with not only hemeoygenase- 1 upregulation, but also suppressed proliferation. It is important to note that curcumin has been detected in human sera at approximately 1.7 µM concentration (Cheng et al., 2001), suggesting that 2 µM curcumin may be achievable following oral administration. Proliferation of BAE cells exposed to 2 µM curcumin did recover to control levels within 48 h of initial exposure (Fig. 3C), indicating that endothelial growth suppression could be a transient phenomenon at relatively low curcumin concentrations. Higher concentrations (>5 µM curcumin) were needed to yield significantly reduced DNA synthesis (Fig. 3C) and whole cell numbers (Fig. 2) following 48 h of treatment, further suggesting that some degree of growth recovery may be possible.
Insight to the mechanism by which curcumin inhibits endothelial cell proliferation was offered by a series of experiments examining the BAE cell cycle and potential curcumin interactions with microtubules and/or tubulin protein. We began with the observation that 10–15 µM curcumin suppressed only the growth of BAE cells adherent to the culture plating surface, with higher concentrations needed to trigger an accumulation of cells floating in culture medium (Fig. 2). Since 15 µMcurcumin is likely in excess of physiological concentrations achievable in body fluids as a result of dietary intake (Cheng et al., 2001), 15 µM curcumin was chosen as the maximum treatment in the subsequent cell cycle analysis. Similar to our findings with whole cell proliferation, concentrations of curcumin above 5 µM were needed to detect significant changes in flow cytometry histograms as compared with vehicleadministered controls (Fig. 4). These higher curcumin concentrations produced an accumulation of cells within the G2/M phase of the cell cycle, which led us to consider possible curcumin effects on mitotic progression that could explain the observed difference in sensitivity to curcumin dose between assays using flow cytometry (Fig. 4) and those of [3H]thymidine incorporation (Fig. 3A and B). Following 24 h of treatment, 2 µM curcumin significantly reduced incorporation of [3H]thymidine (Fig. 3B), yet 10 µM curcumin was required to observe G2/M phase accumulation (Fig. 4). While it is possible that a single mechanism may not explain curcumin’s antiproliferative effect, our findings suggest that physiological curcumin concentrations (i.e., at levels derived from the diet, ≤2 µM) may possess heretofore unappreciated toxic properties. Hence, we proceeded to further investigate a potential mechanism by which curcumin might impact normal mitosis, while allowing growth recovery at relatively low concentrations.
Reports from the cancer literature describe the efficacy of curcumin to block cell cycle progression (Meeran and Katiyar, 2008) and disrupt microtubule assembly (Gupta et al., 2006) in a variety of transformed cell types. Also, curcumin has recently been shown to bind with tubulin protein, at a location 32 Å away from the classical colchicine-binding site (Chakraborti et al., 2011). These previous reports, however, lack evidence of curcumin specificity for disrupting mitosis exclusively in cancerous cells. Moreover, while curcumin has been shown to induce hemeoxygenase-1 within confluent endothelial cell cultures (Motterlini et al., 2000), the literature provides no evidence of safety in populations of normally growing cells exposed to physiological curcumin concentrations. In the next step of our current study, we sought to determine whether curcumin could depolymerize normal endothelial cell microtubules.
Similar to the non-specific effect of colchicine, we found curcumin to completely abolish microtubule polymerization and lead to an accumulation of early mitotic figures, without affecting tubulin protein expression (Fig. 5). Also similar to earlier work with colchicine (Jordan, 2002), we observed [3H]curcumin binding directly with purified tubulin protein (Fig. 6A), and more importantly this occurred at a curcumin concentration (1 µM) achievable via the diet (Cheng et al., 2001). In order to further demonstrate an interaction of non-tritiated curcumin with tubulin, we also pre-treated purified tubulin protein with the same curcumin used in our previous experiments, which then suppressed the binding of [3H]curcumin (Fig. 6B). Therefore, these experiments provide the first evidence that curcumin binds tubulin protein at physiological concentrations, with higher concentrations necessary to achieve gross depolymerization of microtubules in vivo. Indeed, similar dose-dependent effects of colchicine and other anti-mitotic agents have been reported, whereby such compounds inhibit proliferation (via suppression of microtubule dynamics) at doses lower than those required to entirely preclude microtubule polymerization (Jordan, 2002).
Tubulin-binding agents such as colchicine do not drive cells into mitosis prematurely, but rather they preclude mitotic exodus and lead to an M-phase accumulation of asynchronous cells over time (Castedo et al., 2004; Jordan, 2002). Also, at relatively low molar concentrations with limited availability of molecules to bind soluble tubulin in proliferating cultures (Jordan, 2002), overall growth rates of those cells unaffected by curcumin treatments could ultimately recover to control levels. Our findings support a theory of curcumin bioactivity involving non-specific microtubule disruption, similar to that of colchicine and other potent anti-mitotic drugs used to combat uncontrolled cancerous cell growth. In contrast to colchicine, however, curcumin is both a dietary constituent and a nutraceutical receiving much attention for purported antioxidant and anti-inflammatory properties (Aggarwal et al., 2007). Due to the potential risk that dietary curcumin could pose to normal cell proliferation, our final experiments sought to determine a safe upper limit for endothelial tissue-level curcumin concentrations.
BAE cells exposed to 0.1 µM and greater curcumin concentrations displayed hallmark evidence of mitotic catastrophe upon examination under light microscopy (Fig. 7), lending further support to curcumin’s ability to disrupt mitotic microtubules in vivo. All BAE cells exposed to ≤1.0 µM curcumin remained adherent to the culture flask, and all were then captured for staining and subsequent microscopy. Due to cell cycle asynchrony, the portion of BAE cells detaching to the culture medium in response to higher curcumin concentrations in earlier experiments (Fig. 2) could be arrested within M-phase and not necessarily dead or apoptotic. Nevertheless, only concentrations of curcumin well in excess of those generated in plasma following controlled oral intakes (Cheng et al., 2001) lead to BAE cell detachment. On the other hand, physiological curcumin concentrations (e.g., 0.1 µM) an order of magnitude below that which significantly binds tubulin protein in vitro (Fig. 6A) may still disrupt normal endothelial cell mitosis in vivo (Fig. 7E and F). This approach, therefore, allowed for detection of aberrant mitosis at relatively low curcumin concentrations, and further demonstrated potential toxicity at concentrations far below the threshold needed to upregulate hemeoxygenase-1. In concurrent experiments, BAE cell cultures exposed to 0.01 µM curcumin (24 or 48 h treatments) consistently displayed no evidence of mitotic catastrophe (Fig. 7C and D), strongly indicating that this concentration is incapable of disturbing microtubule polymerization or dynamics at the endothelial tissue level.
In conclusion, the present study describes curcumin toxicity in proliferating endothelial cells at concentrations relevant to dietary curcumin intakes. The efficacy of curcumin to induce hemeoxygenase-1 is clearly overshadowed by its concurrent suppression of normal endothelial cell growth, and moreover by evidence of mitotic catastrophe at concentrations well below those shown to be bioavailable following oral consumption (Cheng et al., 2001). Unlike some other phytonutrients (e.g., the isothiocyanate sulforaphane, derived from broccoli), curcumin does not appear to potently stimulate the Nrf-2/ARE pathway at concentrations substantially below those which suppress endothelial cell proliferation (Gerhauser et al., 2003; Jackson et al., 2007; Zhang et al., 1992). Therefore, curcumin may not be the preferred dietary supplement or food additive for boosting Phase II detoxification enzymes in normal healthy individuals. Also, while many recent efforts have focused on developing curcumin analogs with greater efficacy to suppress the growth of cancer (Yadav et al., 2012), our findings suggest that curcumin itself may impact normal endothelial cell turn-over, and hence could be more toxic than previously considered (Goel and Aggarwal, 2010).
Abbreviations
- BAE
bovine aortic endothelial
- CMN
curcumin
- HO-1
hemeoxygenase-1
Footnotes
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or the Department of Defense.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Anand P, Sung B, Kunnumakkara AB, Rajasekharan KN, Aggarwal BB. Suppression of pro-inflammatory and proliferative pathways by diferuloylmethane (curcumin) and its analogues dibenzoylmethane, dibenzoylpropane, and dibenzylideneacetone: role of Michael acceptors and Michael donors. Biochem. Pharmacol. 2011;82(12):1901–1909. doi: 10.1016/j.bcp.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boon AC, Hawkins CL, Bisht K, Coombes JS, Bakrania B, Wagner KH, Bulmer AC. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 2012;52(10):2120–2127. doi: 10.1016/j.freeradbiomed.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23(16):2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Das L, Kapoor N, Das A, Dwivedi V, Poddar A, Chakraborti G, Janik M, Basu G, Panda D, Chakrabarti P, Surolia A, Bhattacharyya B. Curcumin recognizes a unique binding site of tubulin. J. Med. Chem. 2011;54(18):6183–6196. doi: 10.1021/jm2004046. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang ZS, Zhang YL, Zhou DY. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res. 1999;19(5A):3675–3680. [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- Combeau C, Provost J, Lancelin F, Tournoux Y, Prod’homme F, Herman F, Lavelle F, Leboul J, Vuilhorgne M. RPR112378 and RPR115781: two representatives of a new family of microtubule assembly inhibitors. Mol. Pharmacol. 2000;57(3):553–563. doi: 10.1124/mol.57.3.553. [DOI] [PubMed] [Google Scholar]
- Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer-I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3(2):84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- Gautam SC, Xu YX, Pindolia KR, Janakiraman N, Chapman RA. Nonselective inhibition of proliferation of transformed and nontransformed cells by the anticancer agent curcumin (diferuloylmethane) Biochem. Pharmacol. 1998;55(8):1333–1337. doi: 10.1016/s0006-2952(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Gerhauser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu G-Y, Sitthimonchai S, Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003:523–524. 163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer. 2010;62(7):919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. FEBS. J. 2006;273(23):5320–5332. doi: 10.1111/j.1742-4658.2006.05525.x. [DOI] [PubMed] [Google Scholar]
- Hadley ME. Endocrinology. fifth ed. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- Hauser SC, Ziurys JC, Gollan JL. Subcellular distribution and regulation of hepatic bilirubin UDP-glucuronyltransferase. J. Biol. Chem. 1984;259(7):4527–4533. [PubMed] [Google Scholar]
- Holy JM. Curcumin disrupts mitotic spindle structure and induces micronucleation in MCF-7 breast cancer cells. Mutat. Res. 2002;518(1):71–84. doi: 10.1016/s1383-5718(02)00076-1. [DOI] [PubMed] [Google Scholar]
- Jackson SJT, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25(2):219–227. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- Jackson SJT, Venema RC. Quercetin inhibits eNOS, microtubule polymerization, and mitotic progression in bovine aortic endothelial cells. J. Nutr. 2006;136:1178–1184. doi: 10.1093/jn/136.5.1178. [DOI] [PubMed] [Google Scholar]
- Jackson SJT, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascul. Pharmacol. 2007;46(2):77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Munzel T, Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1 – evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010;49(2):186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Yang-Yen HF, Yen JJ, Lin JK. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer. 1996;26(1):111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents. 2002;2(1):1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Carneiro J, Kelley RO. Basic Histology. seventh ed. Norwalk, CT: Appleton & Lange; 1992. [Google Scholar]
- Kim KM, Kim BT, Park SB, Cho DY, Je SH, Kim KN. Serum total bilirubin concentration is inversely correlated with Framingham risk score in Koreans. Arch. Med. Res. 2012;43(4):288–293. doi: 10.1016/j.arcmed.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29:5039–5044. [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Katiyar SK. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. 2008;13:2191–2202. doi: 10.2741/2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21(2A):873–878. [PubMed] [Google Scholar]
- Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28(8):1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer. 2009;100(9):1425–1433. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid. Redox Signal. 2006;8(3–4):395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur. J. Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sun SH, Huang HC, Huang C, Lin JK. Cycle arrest and apoptosis in MDAMB-231/Her2 cells by curcumin. Eur. J. Pharmacol. 2012;690(1–3):22–30. doi: 10.1016/j.ejphar.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969;244(23):6388–6394. [PubMed] [Google Scholar]
- Yadav B, Taurin S, Larsen L, Rosengren RJ. RL66 a second-generation curcumin analog has potent in vivo and in vitro anticancer activity in ER-negative breast cancer models. Int. J. Oncol. 2012;41(5):1723–1732. doi: 10.3892/ijo.2012.1625. [DOI] [PubMed] [Google Scholar]
- Zelenka J, Muchova L, Zelenkova M, Vanova K, Vreman HJ, Wong RJ, Vitek L. Intracellular accumulation of bilirubin as a defense mechanism against increased oxidative stress. Biochimie. 2012;94(8):1821–1827. doi: 10.1016/j.biochi.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho C-G, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]