Abstract
Objective
To examine the uptake of ART among pregnant women referred to an ART service and the associated rates and risk factors for vertical HIV transmission.
Method
Retrospective analysis of an observational cohort at a community ART clinic in Cape Town.
Results
Between 2002 and 2008, 367 treatment-naïve pregnant women accessed the clinic. The median age was 27.5 years, and median gestation at presentation was 28 weeks. The median baseline CD4 count and viral load were 134 cells/µl and 28 282 copies/ml. Two hundred and sixty-five women (72%) commenced ART before giving birth, 73 women (20%) were referred for prevention of mother-to-child transmission therapy (PMTCT), and 29 (8%) received no intervention. Among ART-eligible women, 13% were lost to follow-up. Of those starting ART, median duration of therapy prior to birth was 7.6 weeks (interquartile range (IQR) 4 – 11.9). The HIV transmission rate was 5.1% (95% confidence interval (CI) 2.8 – 9.0%). Factors associated with transmission were advanced maternal WHO disease stage (odds ratio (OR) 9.57, p=0.02), and follow-up viral load above 50 copies/ml (OR 3.64, p=0.03). Each additional week on ART reduced transmission by 20% (p=0.05). There was no HIV transmission among women who received more than 8 weeks’ therapy.
Conclusions
The rate of HIV transmission in this study was higher than reported in high-income countries. Prevention of vertical transmission with ART was hindered by women presenting late in pregnancy and with advanced stage of HIV disease. Interventions that facilitate earlier ART commencement and improve programmatic retention of pregnant women are required.
The South African national HIV prevalence among antenatal women aged 15 – 49 years in the 2008 antenatal care (ANC) survey was 29.3% (95% confidence interval (CI) 28.5 – 30.1%), and 16.1% (CI 12.6 – 20.2%) in the Western Cape, which is the least burdened province.1 From 2006 to 2007, 67.5% of HIV-positive women in the Western Cape received at least a single dose of nevirapine during labour.2
All HIV-positive pregnant women who meet South African national treatment guidelines may be referred to antiretroviral treatment clinics for antiretroviral therapy (ART) as per World Health Organization (WHO) guidelines.3 South Africa follows 2002 WHO guidelines with stage IV disease or a CD4 cell count of 200 cells/µl as a threshold for starting ART.
Perinatal transmission risk increases with low CD4 counts and high viral loads in the mother.4–6 Large cohort studies in the developed world have shown ART to be superior to AZT monotherapy,7,8 and the WHO recommends that all pregnant women with stage III or IV disease, or a CD4 cell count <350 cells/µl, should receive ART.9,10 While HIV transmission rates range from 0 – 2.9% for women in the developed world,7,8,11–13 the efficacy of ART in preventing vertical transmission has not been clearly documented in resource-limited settings. A study of 326 women in Côte d’Ivoire showed improved prevention of ART v. prevention of mother-to-child transmission therapy (PMTCT) (2.3% v. 16.1%, p<0.0001; where PMTCT was either AZT from 36 weeks or AZT and lamivudine from 32 weeks, with intrapartum nevirapine and 1 week’s AZT for the infant).14 Transmission rates from a hospital-based combined antenatal and antiretroviral clinic in Johannesburg were 5% for 689 patients on ART.15 Since these data come from small research cohorts, more programmatic data on ART initiation in pregnancy are needed.
We studied the uptake of ART and rates and risk factors for vertical HIV transmission among pregnant women referred for ART at a large community ART clinic, and the characteristics of mothers receiving ART compared with PMTCT or no intervention.
Methods
The ART service was based at the Gugulethu Community Health Centre in Nyanga, Cape Town.16 This peri-urban area is home to a predominantly black population of over 300 000, most of whom live in low socio-economic conditions. In 2005, the antenatal HIV seroprevalence was 29%.17 By September 2008, 3 407 patients had been started on ART at this clinic according to national guidelines, except that the commencement CD4 cell count threshold was extended from <200 cells/µl to <250 cells/µl for pregnant women in January 2006. Pregnant women were referred for ART from an onsite ANC service 130 m away.18
The ART service has regular laboratory monitoring and adherence support from community-based peer counsellors.16 Enrolment of pregnant patients was stratified by gestational age so that women <24 weeks pregnant would follow the standard protocol of ART commencement within 4 weeks; women between 24 and 34 weeks were fast-tracked to initiate therapy in 2 weeks; and those >34 weeks pregnant were aimed to start within a week. Drugs for fast-tracked patients were ordered on the day of their first attendance, and they were escorted to meet the counsellor team leader by their doctor. They could commence treatment even if they had not yet attended all 3 education sessions, although these had to be attended in due course.
Patients referred after 28 weeks were started on AZT monotherapy at the ANC service while awaiting ART. Zidovudine, lamivudine and nevirapine was the regimen of choice for pregnant women unless contraindicated, in which case an alternative regimen was constructed. ART-naïve women accessing the service between 1 September 2002 and 1 March 2008 were eligible for analysis if they were pregnant at first attendance. Singleton and multiple pregnancies were evaluable.
Women started on triple therapy before birth were categorised as the ‘ART’ group; women referred back to a maternity outpatient unit for PMTCT and not commenced on ART were categorised as the ‘PMTCT’ group; and women not started on ART prior to birth with no documented referral for PMTCT or having received AZT prior to birth were categorised as the ‘No intervention’ group.
Demographic data, WHO staging, treatment outcomes, laboratory results, ART regimens and regimen changes were recorded in a prospectively maintained database for all patients referred to the ART service since the start of the clinic in September 2002. Infant HIV status and age at polymerase chain reaction (PCR) testing were verified using the regional laboratory PCR test database. National guidelines are that PCR testing should take place at 6 weeks of age.3 Infant HIV status was followed up until September 2008.
Data analysis was by STATA/IC version 10. Fisher’s exact tests and Wilcoxon rank-sum tests were used to compare proportions and medians, respectively. Multiple logistic regression was used to examine the odds of vertical transmission according to maternal clinical characteristics. The upper bound of the rate of transmission was calculated according to Ghent working group formulae.19 For calculating the upper bound, only first-born twins were included as per the formula. For other calculations, both twins were included. All women provided informed consent. Data collection from this clinic for the study was approved by the University of Cape Town Research Ethics Committee.
Results
Maternal characteristics
Between 2002 and 2008, 2 350 ART-naïve women were referred for ART, and 367 were identified as pregnant at first clinic attendance. Their median age was 27.5 years (range 15 – 44); median gestational age 28 weeks (interquartile range (IQR) 24 – 32), and the median baseline CD4 cell count and viral load were 134/µl (IQR 88 – 179) and 28 282 copies/ml (IQR 8 103 – 74 859) respectively.
Intervention received
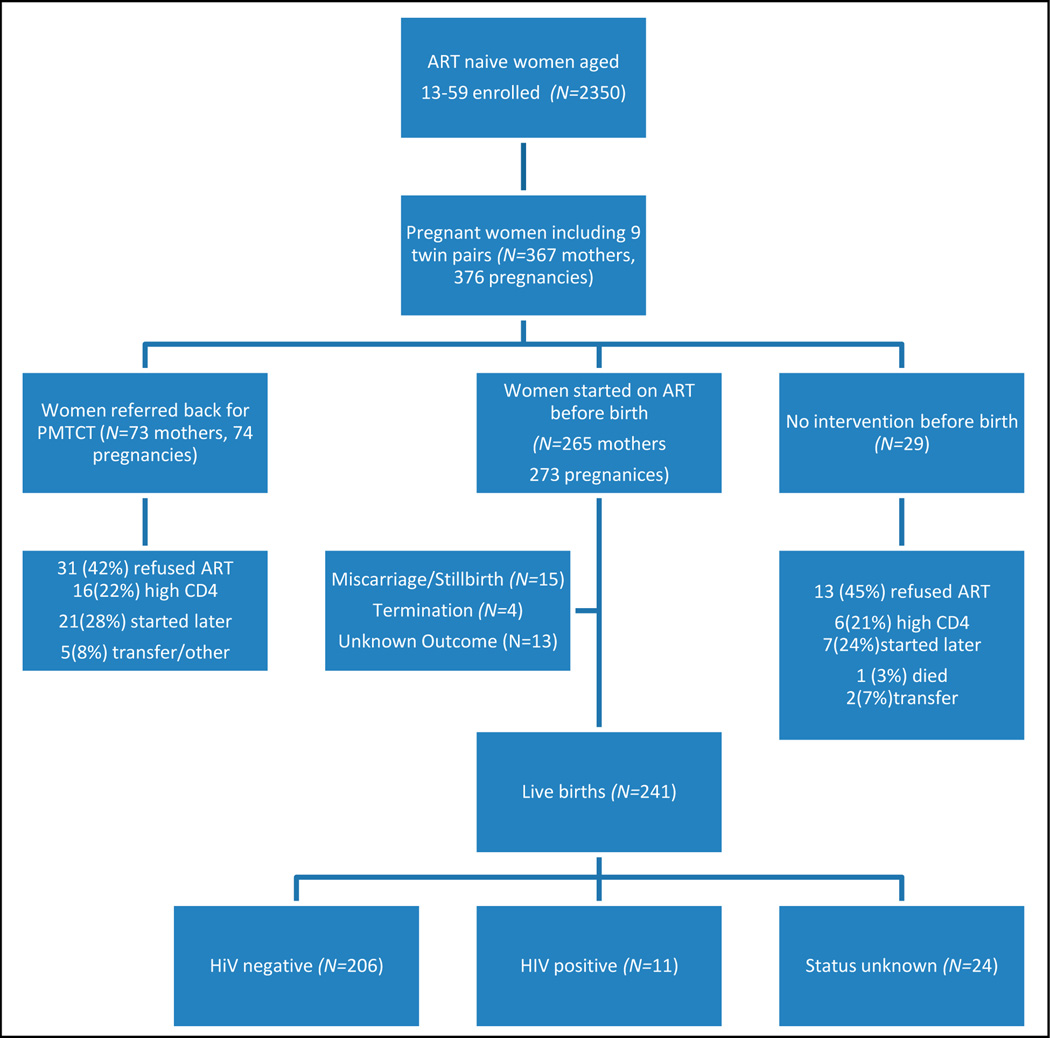
Two hundred and sixty-five mothers (73%) started ART prior to giving birth; 73 (20%) were referred back from the ART clinic to the midwife obstetric unit (MOU) for PMTCT, and 29 (8%) had no intervention (Fig. 1). After exclusion of 6 women who were confirmed to be ineligible for ART, 13 refused ART and 7 started ART later. Altogether, 44 out of 346 (12.7%, 95% CI 9.6 – 16.7%) eligible women were lost to follow-up or refused ART and therefore were not started on ART.
Fig. 1.
Flowchart of interventions received by pregnant women accessing ART clinic, and infant outcomes of those commencing ART.
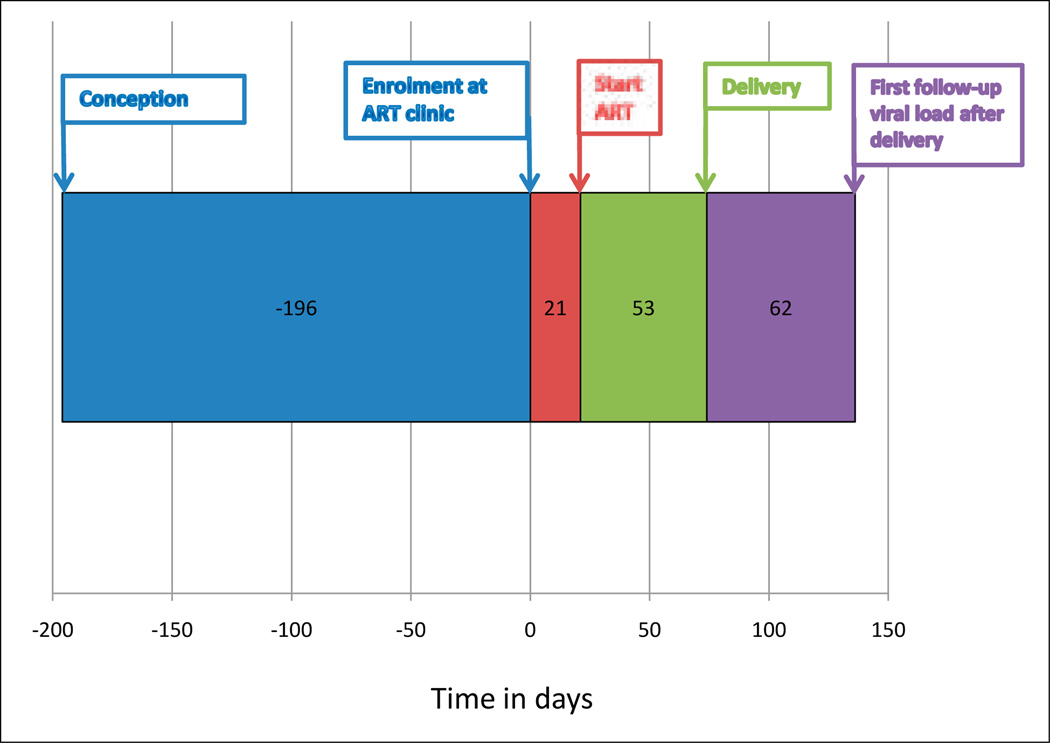
Women who commenced ART started a median of 21 days after enrolment (IQR 14 – 29); the median period on ART before birth was 7.6 weeks (IQR 4 – 11.9) (Fig. 2). The choice of ART regimen depended on maternal condition. Two hundred and forty-six (93%) started AZT, 3TC and NVP; 12 (5%) commenced d4T, 3TC and NVP owing to anaemia; 6 (2%) started d4T 3TC and EFV owing to TB and advanced pregnancy; and 1 (0.3%) started d4T 3TC and Kaletra. There were 8 regimen changes during pregnancy.
Fig. 2.
Timeline of ART initiation in women commencing therapy from conception until first follow-up viral load test after delivery using median values of days.
Comparison of PMTCT, ART and No Intervention Received
The demographics and outcomes of the groups of ART and PMTCT were compared (Table I). The PMTCT group were more advanced in their pregnancy, and had higher baseline CD4 cell counts and lower baseline viral loads (p<0.001, p<0.001, p=0.013 respectively). There was no difference in distribution of live births and stillbirths, or in HIV status of infants. The groups that received no intervention were significantly younger than those started on ART (p=0.02), and were more likely to have TB (p=0.015) and to have stillbirths (p=0.004). Both groups gave birth at earlier gestational age than the ART group.
Table I.
Maternal characteristics stratified by intervention received*
| Maternal characteristics and pregnancy outcomes |
ART (N=273) |
PMTCT (N=74) |
p-value ART v. PMTCT |
No intervention (N=29) |
p-value ART v. no intervention |
|---|---|---|---|---|---|
| Age | 27.8 (25–31) | 27 (23–32) | 0.547 | 24.5 (23.5–28.5) | 0.02 |
| WHO stage I and II | 179 (79%) | 55 (74%) | 0.146 | 15 (52%) | 0.296 |
| III | 78 (34%) | 16 (23%) | 13 (45%) | ||
| IV | 8 (3%) | 2 (3%) | 0 | ||
| Unknown | 0 | 0 | 1 (3%) | ||
| TB at enrolment | 23 (8%) | 5 (7%) | 0.425 | 7 (24%) | 0.015 |
| Gestation at enrolment | 27 (22–31) | 32 (29–34) | <0.001 | 26 (22–30) | 0.878 |
| CD4 at baseline | 127 (84–168) (N=268) |
166 (128–267) (N=67) |
<0.001 | 153 (75–247) (N=24) |
0.109 |
| Viral load at baseline | 30 721 (2 762–50 545) (N=267) |
17 794 (2 762–50 545) (N=67) |
0.013 | 40 812 (14 969–91 870) (N=24) |
0.262 |
| Gestation at birth | 38.3 (36–40) (N=252) |
36 (34–38) (N=30) |
0.002 | 34 (27–39) (N=8) |
0.028 |
| Live birth | 241 (88%) | 31 (42%) | <0.001 (live v. stillbirth, p=0.568) |
7 (24%) | <0.001 (live v. stillbirths, p=0.004) |
| Stillbirth/miscarriage | 14 (5%) | 2 (3%) | 4 (14%) | ||
| Termination/abortion | 4 (2%) | 0 | 0 | ||
| Unknown | 14 (5%) | 41 (55%) | 18 (62%) | ||
| Of live births: | |||||
| HIV-negative | 203 (84%) | 23 (74%) | 0.067 (excluding unknowns, p=0.317) |
3 (43%) | 0.011 (excluding unknowns, p=0.855) |
| HIV-positive | 11 (5%) | 0 | 0 | ||
| HIV status unknown | 27 (11%) | 8 (26%) | 4 (57%) | ||
| VL<400 at first follow-up | 203 (74%) | ||||
| 400<10 000 | 10 (4%) | ||||
| >10 000 | 20 (7%) | ||||
| Unknown | 40 (15%) | ||||
| Days after start | 115 (112–124) | ||||
| CD4 at first follow-up | 257 (165–335) (N=233) |
||||
| Days after start | 115 (112–124) |
For continuous variables, data represent medians (interquartile range).
Maternal and pregnancy outcomes
Of the women commenced on ART, 16 (6%) were lost to the programme prior to giving birth. Of these, 9 (3%) were lost to followup, 5 (2%) died and 2 were transferred to another ART clinic. Of the 5 deaths, 3 were due to TB and 1 to pneumonia, and the cause of the fifth was unknown. Overall, 34 (9.3%) pregnant women had TB.
There were 376 pregnancies among the 367 women including 9 sets of twins. There were 279 live births (74%), 21 stillbirths/miscarriages (6%), 4 terminations (1%) and 72 (19%) were of unknown outcome. Median estimated gestational age at birth or stillbirth was 38 weeks (IQR 36 – 40). There were 232 HIV-negative infants (83%), 11 HIV-positive (4%), and 36 (13%) infants of unknown HIV status. Median infant age at PCR was 13 weeks (IQR 10 – 17).
Twenty-one (5.6%) pregnancies ended in either stillbirth or miscarriage, 2 at 19 weeks’ gestation, 18 between 28 and 42 weeks, and 1 unrecorded. Stillbirths and miscarriages were analysed together. In multivariate analysis, longer duration of pregnancy at enrolment (OR 0.88, p=0.001) and commencing ART v. no intervention (OR 0.1, p=0.007) were protective. Higher baseline log viral load increased the risk (OR 2.46, p=0.07).
Nine infants died within 1 year of birth; the median age at death was 14 weeks (IQR 6 – 26). Three were HIV-positive, 2 negative and 4 unknown. Maternal TB was associated with infant death in crude analysis (p=0.042) but, in multivariate analysis, only HIV-positive status was independently associated with infant death (OR 30.5, p=0.001).
Risk factors for vertical transmission
The groups of HIV-negative, positive and status-unknown infants born to women commenced on ART were compared (Table II). There were 241 births in this group. Median age at PCR was 13 weeks (IQR 10 – 17). Two hundred and six infants were negative (85%), 11 (4.6%) positive, and 24 (10%) of unknown status (Fig. 1). Four of the infants of unknown HIV status died under the age of 17 weeks, and the remaining mothers of 20 infants had been transferred to another clinic, were lost to follow-up, or the infant had not been tested. The mother of infants of unknown status were younger (p=0.018).
Table II.
Table of maternal characteristics stratified by HIV status of infant*
| Maternal characteristics and pregnancy outcomes |
HIV-negative infants (N=206) |
HIV-positive infants (N=11) |
p -value HIV-negative v. HIV-positive |
Infants unknown HIV status (N=24) |
p-value HIV- negative v. unknown |
|---|---|---|---|---|---|
| Age | 28.2 (25–32) | 26.5 (22–30) | 0.138 | 24 (25–28) | 0.018 |
| WHO stage I and II | 145 (70%) | 3 (27%) | 0.01 | 23 (85%) | 0.637 |
| III | 55 (27%) | 6 (55%) | 4 (15%) | ||
| IV | 6 (3%) | 2 (18%) | 0 | ||
| TB at enrolment | 15 (7%) | 2 (18%) | 0.214 | 1 (4%) | 0.416 |
| Gestation at enrolment | 27 (23–31) (N=205) |
32 (28–34) (N=11) |
0.036 | 27 (23–31) (N=24) |
0.835 |
| CD4 at baseline | 125 (81–164) (N=202) |
174 (118–193) (N=11) |
0.063 | 144 (78–186) (N=23) |
0.18 |
| VL at baseline | 29 537 (8 969–75 758) (N=199) |
32 086 (14 409–67 656) (N=11) |
0.746 | 13 250 (1 800–40 176) (N=23) |
0.024 |
| Weeks on ART (median) | 7.6 (4–12) | 4.7 (2.7–7.6) | 0.029 | 8 (5–12) | 0.684 |
| Gestation at birth | 38 (36–40) (N=201) |
40 (37–41) (N=11) |
0.33 | 39 (38–41) (N=13) |
0.241 |
| Delivery mode: NVD | 68 (33%) | 2 (27%) | 9 (37%) | ||
| Caesarean | 54 (26%) | 3 (18%) | 0.43 | 6 (6%) | 0.488 |
| Unknown | 85 (41%) | 6 (55%) | 10 (41%) | ||
| First follow-up viral load | <50 (0) (N=192) |
54 (49–791) (N=11) |
0.018 | 49 (0) (N=15) |
0.387 |
| Start to follow-up VL (days) | 115 (112–123) | 129 (112–158) | 0.777 | 112 (112–116) | 0.271 |
| <400 at first follow-up | 169 (82%) | 8 (73%) | 14 (58%) | ||
| 400<10 000 | 7 (3%) | 1 (9%) | 0.235 | 0 | 1 |
| >10 000 | 16 (8%) | 2 (18%) | 1 (4%) | ||
| Unknown | 11 (5%) | 0 | 10 (42%) | ||
| CD4 at first follow-up | 248 (162–327) (N=196) |
337 (182–455) (N=11) |
0.207 | 247 (160–299) (N=15) |
0.869 |
| Start to follow-up CD4 (days) | 115 (112–123) | 119 (111–194) | 0.584 | 112 (112–116) | 0.384 |
| Breastfeeding | 6 (3%) | 0 | 0.722 | 1 (4%) | 0.312 |
| Not breastfeeding | 122 (58%) | 7 (64%) | 6 (26%) | ||
| Median age at PCR (days) | 93 (75–124) (N=122) |
87 (70–93) (N=7) |
For continuous variables, data represent medians (interquartile range).
After exclusion of unknowns, the transmission rate was 5.1% (95% CI 2.8 – 9.0%). The upper bound for the rate of transmission within the ART group estimated by the Ghent working group formulae was 7.83% (95% CI 4.9 – 12.2).19
Crude analysis showed duration of ART before birth to be significantly associated with transmission (p=0.029). All of the mothers who gave birth to HIV-positive infants had less than 8 weeks’ ART prior to delivery. Advanced maternal disease stage (stage III or IV compared with I or II, p=0.01), gestation at enrolment (p=0.036) and first follow-up viral load >50 copies/ml (p=0.018) were also significantly associated. No significant difference was found in HIV status of infant according to maternal ART regimen.
Multivariate analysis of the HIV-positive versus negative infants revealed duration of ART to be an independent predictor of transmission (Table III). Each week reduced risk of transmission by 20% (p=0.005). Additional independent predictor variables were maternal WHO stage at enrolment and viral load at first follow-up. Women with WHO stage 3 or 4 disease were 9.5 times more likely to transmit (p=0.02) than those at stage 1. Those with incompletely suppressed virus (viral load >50 copies/ml) at 16 weeks on ART had a transmission rate of 10% (95% CI 4 – 20%), whereas those with a viral load of <50 copies/ml had a transmission rate of 3% (95% CI 1 – 8%).
Table III.
Risk factors associated with vertical HIV transmission
| Odds ratio |
95% confidence interval |
p-value | ||
|---|---|---|---|---|
| Stage 1 v. stage 2 | 1.32 | 0.1 | 18.25 | 0.84 |
| Stage 1 v. stage 3 and 4 | 9.68 | 1.47 | 63.62 | 0.02 |
| Age at enrolment | 0.85 | 0.71 | 1.02 | 0.08 |
| Weeks on ART prior to birth | 0.8 | 0.64 | 0.1 | 0.05 |
| Follow-up viral load <50 v. >50 | 5.78 | 1.17 | 28.51 | 0.031 |
| Follow-up CD4 | 1 | 0.1 | 1.01 | 0.243 |
| Log baseline viral load | 0.73 | 0.24 | 2.23 | 0.57 |
| Baseline CD4 | 1 | 0.99 | 1.02 | 0.86 |
Discussion
The HIV transmission rate of those starting HAART during pregnancy was 5.1%, with an upper bound of 7.8% that was higher than rates reported in the developed world.7,8,11 Our study demonstrated that shorter duration of HAART in pregnancy was strongly associated with increased HIV transmission. The major factor limiting the potential duration of ART was advanced gestation at the time of presentation to the antenatal services. The period of ART was further shortened by systems delays in referral from the antenatal to the ART service severely restricting the time available to prepare women for ART.
The median gestational age at enrolment was 28 weeks. However, 25% of patients presented after 31 weeks of pregnancy. The potential duration of ART was further restricted because delivery tended to occur early at a median of 38 weeks with 25% of mothers giving birth before 36 weeks’ gestation. This limited window of opportunity for ART initiation resulted in a strategy of fast-tracking of women presenting in the third trimester of pregnancy. Fast-tracking may not be an optimal strategy but was considered necessary as an emergency intervention precipitated by late booking. Women accessing ART programmes during pregnancy are significantly more likely to be lost to follow-up than their non-pregnant peers.20,21 The reasons behind poor retention of pregnant women within ART programmes are uncertain; however, too little preparation prior to ART initiation could be a contributing factor.
Lack of treatment readiness was also an important constraint to implementation as 21% of eligible women either refused or delayed commencement of ART to after delivery. Such women had already successfully navigated their way through antenatal booking and referral before attending the ART clinic. As losses may occur at earlier stages of the entire cascade of antenatal clinic attendance, counselling and testing, referral for treatment, initiation of HAART and ongoing adherence to protocol, true losses from the programme are likely to have been even greater.
In the developed world, vertical HIV transmission rates of between 0% and 2.9% have been achieved for women on HAART.8,11–13 Triple therapy (HAART) is superior to AZT monotherapy in preventing mother-to-child transmission.7,8 Similarly, in this study, there were no HIV transmissions among women who received at least 8 weeks of therapy before delivery. The overall HIV transmission rate among our patients highlights that the effectiveness of HAART for vertical transmission is affected by the potency of the regimen used and also by other operational constraints. One contributing factor is that the present national ART treatment guidelines restrict HAART to those with AIDS or CD4 <200 cells/µl. Pregnant black women with high viral loads require several weeks of therapy to completely suppress viral load.12
Our data highlight that the effectiveness of HAART will be greatly increased if women can be encouraged to access antenatal services at an earlier stage of gestation. Antenatal services should also place emphasis on retaining women within care.
There were few adverse events related to use of HAART but there were multiple sequelae of untreated HIV. However, a significant proportion of women refused or delayed initiation of HAART. Social marketing of the benefits of HAART is urgently required to overcome the negative messages previously propagated by South Africa’s health authorities. The limited window of opportunity for initiating HAART in women presenting later in pregnancy has required fast-tracking them. Whether inadequate preparation due to fast-tracking plays a part in the poor retention of pregnant women in HAART programmes must be investigated.
We studied the effectiveness of HAART and did not address important parameters such as mode of delivery, breastfeeding and infant post-exposure prophylaxis. Additionally, pregnancy outcomes of the women referred back for PMTCT were unknown. Direct comparison of the effectiveness of PMTCT v. HAART in this study was therefore not possible. Interpreting the analysis of live v. stillbirths is complex, as women having miscarriages or early stillbirths would not attend the antenatal clinic, which builds in an ascertainment bias. Similarly, longer duration of pregnancy at enrolment emerged as protective, but this may have been because the more advanced the pregnancy, the less time there is for a stillbirth to occur.
The generalisability of the data to other parts of South Africa and the rest of sub-Saharan Africa is difficult to establish. This is one unique programme, and other public sector services may initiate ART more slowly. In other areas, antenatal booking may be later still, and treatment initiation may be further slowed by delays in CD4 enumeration. Further programmatic data are needed.
Summary
The rate of vertical HIV transmission (5.1%) among women commencing ART in this cohort was higher than that reported in the developed world, and was associated with advanced immunodeficiency at presentation and late initiation of HAART. No HIV transmissions occurred among women who received more than 8 weeks of HAART. However, 12.7% of women eligible for ART during pregnancy did not receive it and, in those starting HAART, the median length of therapy before delivery was less than 8 weeks. The effectiveness of HAART will be improved by earlier presentation to antenatal services and subsequent retention in care. Increased uptake of HAART will require social marketing of the considerable benefits of HAART in this patient population.
Acknowledgments
LGB, LM and RW were funded in part by the National Institutes of Health (NIH) through a CIPRA grant 1U19AI53217-01 and RW by an RO1 grant (A1058736-01A1). SDL was funded by the Wellcome Trust, London, UK. The authors gratefully acknowledge the dedicated staff of the Hannan Crusaid ART clinic, the Desmond Tutu HIV Centre, and Di Hardy for access to the NHLS database.
Contributor Information
Felicity C Fitzgerald, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, and Clinical Research Unit, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London.
Linda-Gail Bekker, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town.
Richard Kaplan, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town.
Landon Myer, Centre for Infectious Diseases and Epidemiology Research, School of Public Health & Family Medicine, University of Cape Town, and Department of Epidemiology, Mailman School of Public Health, Columbia University, USA.
Stephen D Lawn, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, and Clinical Research Unit, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London.
Robin Wood, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town.
References
- 1.UNAIDS. [accessed 14 October 2008];Progress Report on Declaration of Commitment on HIV and AIDS. Republic of South Africa. Reporting Period: January 2006 – December 2007. http://data.unaids.org/pub/Report/2008/south_africa_2008_country_progress_report_en.pdf.
- 2.Health Systems Trust. [accessed 5 November 2008];The District Health Barometer – Year 2006/07. http://www.hst.org.za/publications/717.
- 3.Policy and Guidelines for the Implementation of the PMTCT Programme. Pretoria: National Department of Health; [accessed 10 October 2008]. http://www.doh.gov.za/docs/policy/pmtct-f.html. [Google Scholar]
- 4.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of Human Immunodeficiency Virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 team. N Engl J Med. 1999;341(6):385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 5.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 6.European Collaborative Study. Risk factors for mother-to-child transmission of HIV-1. Lancet. 1992;339(8800):1007–1012. doi: 10.1016/0140-6736(92)90534-a. [DOI] [PubMed] [Google Scholar]
- 7.Cooper ER, Charurat M, Mofenson LM, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Sydnr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro D, Tuomala R, Pollack H, et al. Mother-to-child HIV transmission risk according to antiretroviral therapy, mode of delivery, and viral load in 2895 U.S. women (PACTG 367). 11th Conference on Retroviruses and Opportunistic Infections; February 2004; San Francisco, CA. [Abstract 99]. [Google Scholar]
- 9.World Health Organization. [accessed 10 October 2008];Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. 2006 revision. http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf. [PubMed]
- 10.World Health Organization. [accessed 30 November 2009];Rapid Advice: Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. http://www.who.int/hiv/pub/mtct/rapid_advice_mtct.pdf.
- 11.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland: 2000–2006. AIDS. 2008;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 12.European Collaborative Study. Patel D, Cortina-Borja M, Thorne C, Newell ML. Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnant women. Clin Infect Dis. 2007;44(12):1647–1656. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- 13.Warszawski J, Tubiana R, Le Chenadec J, et al. ANRS French Perinatal Cohort. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. AIDS. 2008;22(2):289–299. doi: 10.1097/QAD.0b013e3282f3d63c. [DOI] [PubMed] [Google Scholar]
- 14.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d’Ivoire. AIDS. 2008;22(14):1815–1820. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 15.Black V, Hoffman RM, Sugar CA, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;3:276–281. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96(4):315–320. [PubMed] [Google Scholar]
- 17.South African Government Information. [accessed 31 October 2008];HIV & AIDS and STI strategic plan for South Africa 2007–2011. http://www.info.gov.za/otherdocs/2007/aidsplan2007/executive_summary.pdf.
- 18.Stinson K, Boulle A, Coetzee D, Abrams E, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. doi: 10.1111/j.1365-3156.2010.02538.x. In press. [DOI] [PubMed] [Google Scholar]
- 19.Newell ML, Coovadia H, Cortina-Borja M, et al. Lancet. 2004;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–1681. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to followup in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]