Abstract
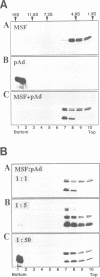
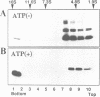
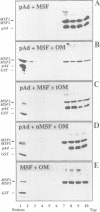
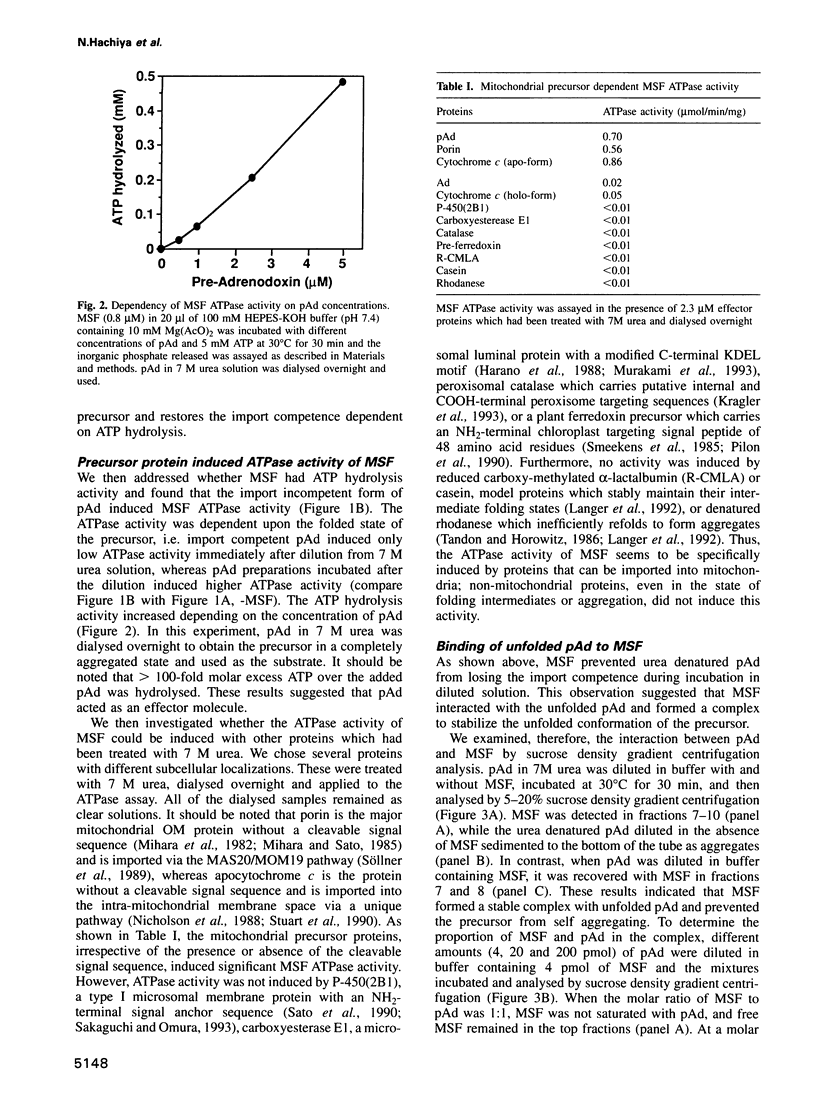
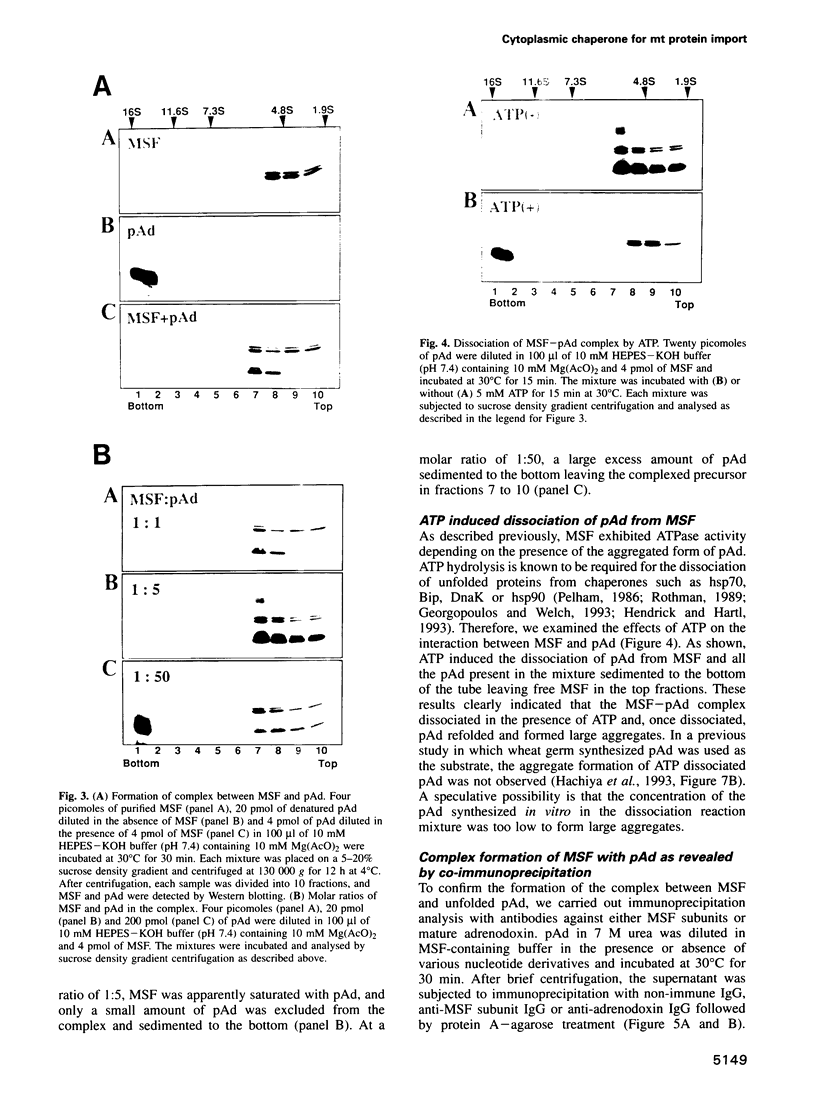
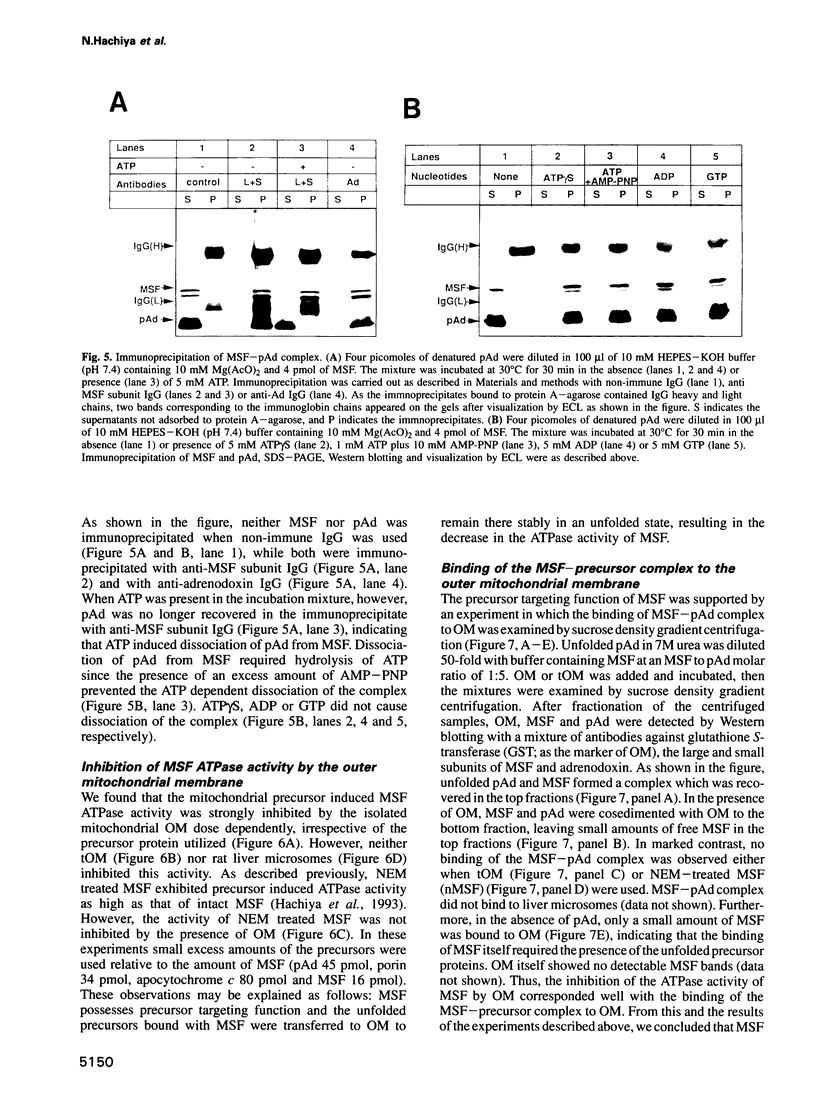
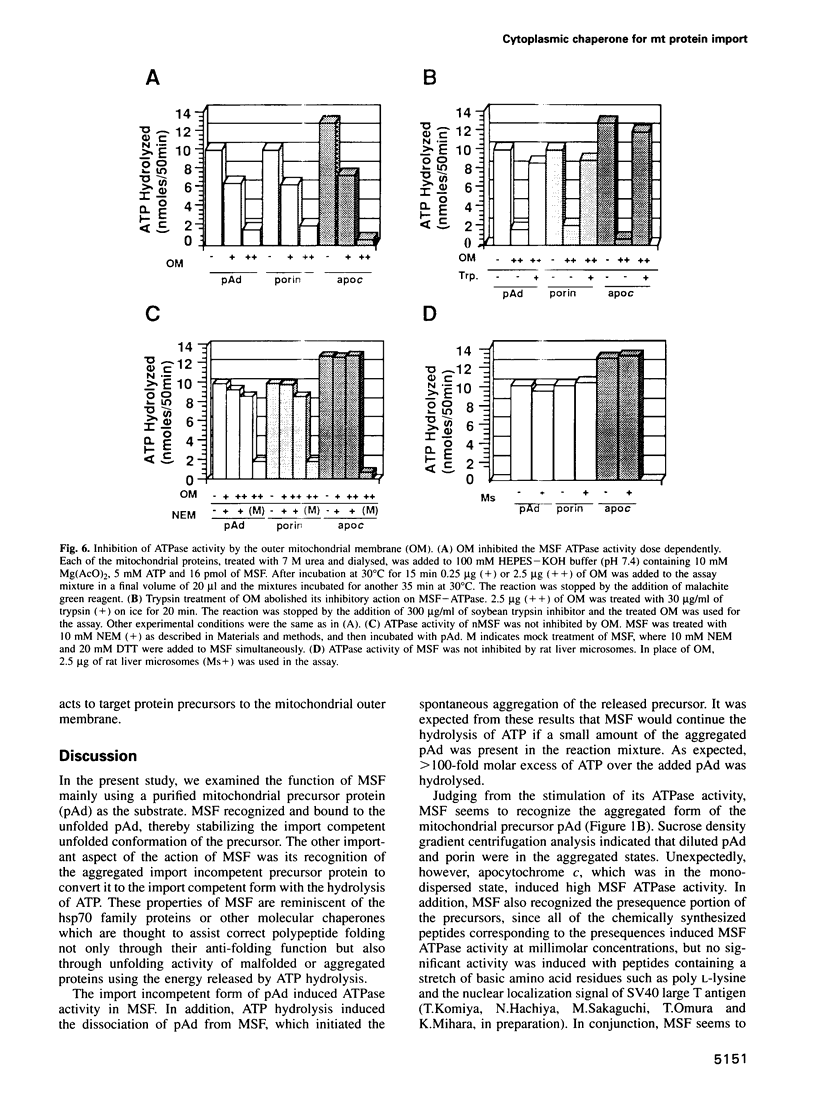
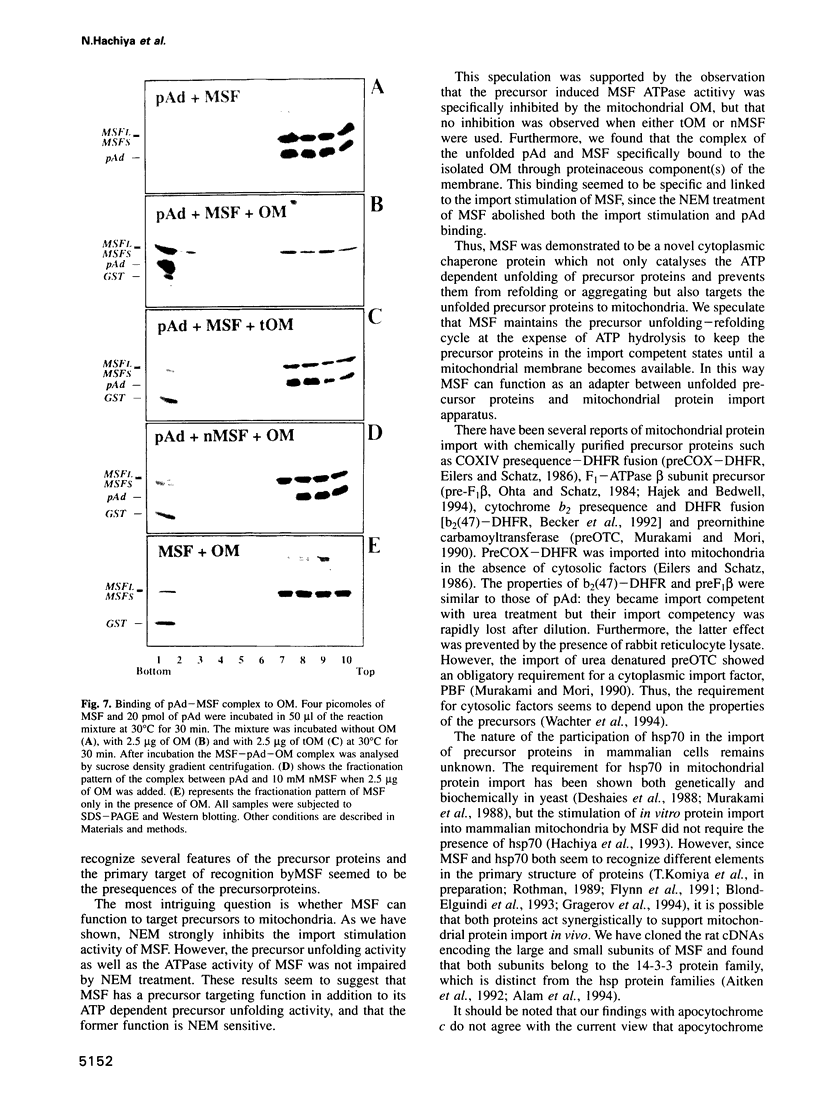
Mitochondrial import stimulation factor (MSF) unfolds wheat germ lysate synthesized aggregated mitochondrial precursor proteins and stimulates their mitochondrial import in an ATP dependent manner. Here we analysed the function of MSF mainly by utilizing chemically pure adrenodoxin precursor (pAd). MSF bound to the unfolded pAd and prevented it from losing import competence and also restored the import competence of the aggregated pAd dependent on ATP hydrolysis. The import incompetent aggregated mitochondrial precursors induced the ATPase activity of MSF and the activity was strongly inhibited by isolated mitochondrial outer membrane (OM) but not by trypsin treated outer membrane (tOM). The precursor induced ATPase activity of N-ethylmaleimide (NEM)-treated MSF was not inhibited by OM. In this context, the MSF-precursor complex specifically bound to OM and binding was abolished both by the treatment of OM with trypsin and by the treatment of MSF with NEM. These results show that MSF is a novel cytoplasmic chaperone protein with a mitochondrial precursor-targeting function.
Full text
PDF
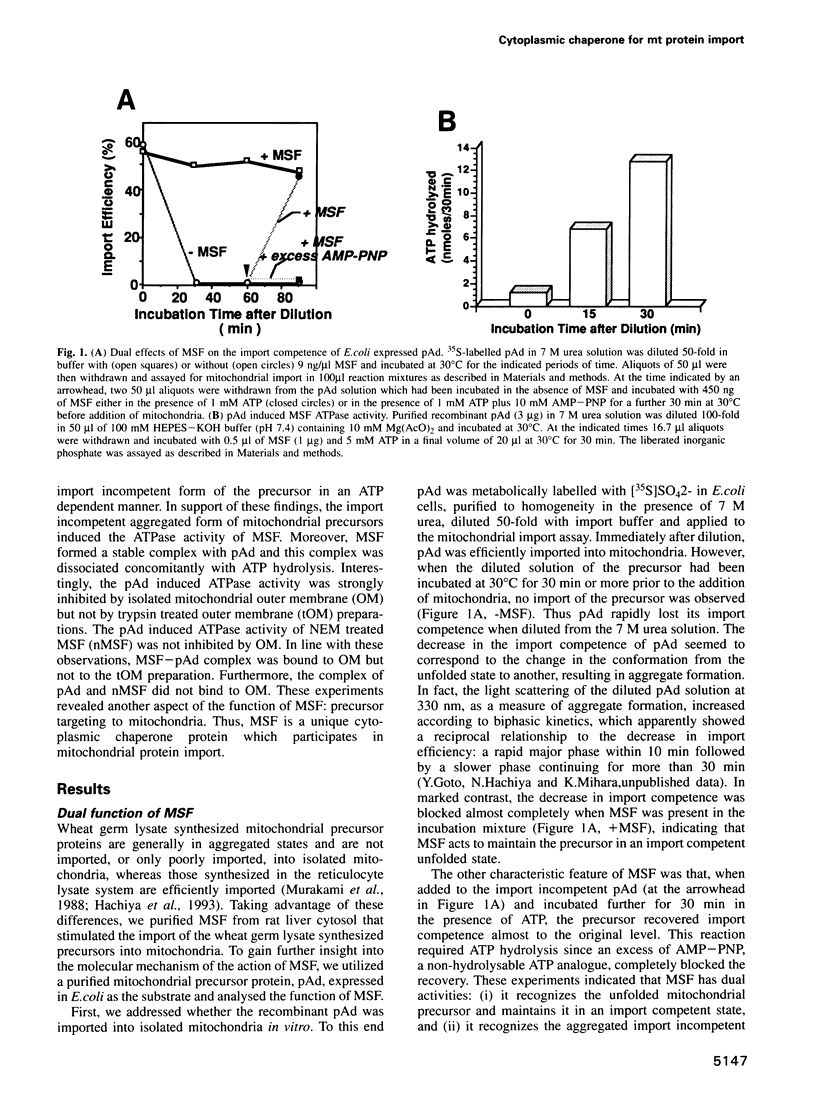


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Collinge D. B., van Heusden B. P., Isobe T., Roseboom P. H., Rosenfeld G., Soll J. 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci. 1992 Dec;17(12):498–501. doi: 10.1016/0968-0004(92)90339-b. [DOI] [PubMed] [Google Scholar]
- Alam R., Hachiya N., Sakaguchi M., Kawabata S., Iwanaga S., Kitajima M., Mihara K., Omura T. cDNA cloning and characterization of mitochondrial import stimulation factor (MSF) purified from rat liver cytosol. J Biochem. 1994 Aug;116(2):416–425. doi: 10.1093/oxfordjournals.jbchem.a124541. [DOI] [PubMed] [Google Scholar]
- Argan C., Lusty C. J., Shore G. C. Membrane and cytosolic components affecting transport of the precursor for ornithine carbamyltransferase into mitochondria. J Biol Chem. 1983 Jun 10;258(11):6667–6670. [PubMed] [Google Scholar]
- Becker K., Guiard B., Rassow J., Söllner T., Pfanner N. Targeting of a chemically pure preprotein to mitochondria does not require the addition of a cytosolic signal recognition factor. J Biol Chem. 1992 Mar 15;267(8):5637–5643. [PubMed] [Google Scholar]
- Bernstein H. D., Rapoport T. A., Walter P. Cytosolic protein translocation factors. Is SRP still unique? Cell. 1989 Sep 22;58(6):1017–1019. doi: 10.1016/0092-8674(89)90497-2. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993 Nov 19;75(4):717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Protein unfolding and the energetics of protein translocation across biological membranes. Cell. 1988 Feb 26;52(4):481–483. doi: 10.1016/0092-8674(88)90458-8. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991 Oct 24;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S. Are the cytosolic components of the nuclear, ER, and mitochondrial import apparatus functionally related? Cell. 1992 Jul 24;70(2):185–188. doi: 10.1016/0092-8674(92)90094-s. [DOI] [PubMed] [Google Scholar]
- Gragerov A., Zeng L., Zhao X., Burkholder W., Gottesman M. E. Specificity of DnaK-peptide binding. J Mol Biol. 1994 Jan 21;235(3):848–854. doi: 10.1006/jmbi.1994.1043. [DOI] [PubMed] [Google Scholar]
- Hachiya N., Alam R., Sakasegawa Y., Sakaguchi M., Mihara K., Omura T. A mitochondrial import factor purified from rat liver cytosol is an ATP-dependent conformational modulator for precursor proteins. EMBO J. 1993 Apr;12(4):1579–1586. doi: 10.1002/j.1460-2075.1993.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P., Bedwell D. M. Characterization of the mitochondrial binding and import properties of purified yeast F1-ATPase beta subunit precursor. Import requires external ATP. J Biol Chem. 1994 Mar 11;269(10):7192–7200. [PubMed] [Google Scholar]
- Harano T., Miyata T., Lee S., Aoyagi H., Omura T. Biosynthesis and localization of rat liver microsomal carboxyesterase E1. J Biochem. 1988 Jan;103(1):149–155. doi: 10.1093/oxfordjournals.jbchem.a122221. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Iwahashi J., Furuya S., Mihara K., Omura T. Characterization of adrenodoxin precursor expressed in Escherichia coli. J Biochem. 1992 Apr;111(4):451–455. doi: 10.1093/oxfordjournals.jbchem.a123778. [DOI] [PubMed] [Google Scholar]
- Kragler F., Langeder A., Raupachova J., Binder M., Hartig A. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J Cell Biol. 1993 Feb;120(3):665–673. doi: 10.1083/jcb.120.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Chen L., Fandl J., Tai P. C. Purification of the Escherichia coli secB gene product and demonstration of its activity in an in vitro protein translocation system. J Biol Chem. 1989 Feb 5;264(4):2242–2249. [PubMed] [Google Scholar]
- Kumamoto C. A. SecB protein: a cytosolic export factor that associates with nascent exported proteins. J Bioenerg Biomembr. 1990 Jun;22(3):337–351. doi: 10.1007/BF00763171. [DOI] [PubMed] [Google Scholar]
- Kuwahara S., Harada N., Yoshioka H., Miyata T., Omura T. Purification and characterization of four forms of cytochrome P-450 from liver microsomes of phenobarbital-treated and 3-methylcholanthrene-treated rats. J Biochem. 1984 Mar;95(3):703–714. doi: 10.1093/oxfordjournals.jbchem.a134660. [DOI] [PubMed] [Google Scholar]
- Landry S. J., Gierasch L. M. Recognition of nascent polypeptides for targeting and folding. Trends Biochem Sci. 1991 Apr;16(4):159–163. doi: 10.1016/0968-0004(91)90060-9. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Høj P. B., Hoogenraad N. J. Do cytosolic factors prevent promiscuity at the membrane surface? FEBS Lett. 1993 Aug 23;329(1-2):1–4. doi: 10.1016/0014-5793(93)80179-x. [DOI] [PubMed] [Google Scholar]
- Liu G., Topping T. B., Randall L. L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Meyer D. I. Protein translocation into the endoplasmic reticulum: a light at the end of the tunnel. Trends Cell Biol. 1991 Dec;1(6):154–159. doi: 10.1016/0962-8924(91)90016-3. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G., Sato R. In vitro synthesis and integration into mitochondria of porin, a major protein of the outer mitochondrial membrane of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7102–7106. doi: 10.1073/pnas.79.23.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Sato R. Molecular cloning and sequencing of cDNA for yeast porin, an outer mitochondrial membrane protein: a search for targeting signal in the primary structure. EMBO J. 1985 Mar;4(3):769–774. doi: 10.1002/j.1460-2075.1985.tb03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Mori M. Purified presequence binding factor (PBF) forms an import-competent complex with a purified mitochondrial precursor protein. EMBO J. 1990 Oct;9(10):3201–3208. doi: 10.1002/j.1460-2075.1990.tb07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Takagi Y., Mihara K., Omura T. An isozyme of microsomal carboxyesterases, carboxyesterase Sec, is secreted from rat liver into the blood. J Biochem. 1993 Jan;113(1):61–66. doi: 10.1093/oxfordjournals.jbchem.a124004. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Hergersberg C., Neupert W. Role of cytochrome c heme lyase in the import of cytochrome c into mitochondria. J Biol Chem. 1988 Dec 15;263(35):19034–19042. [PubMed] [Google Scholar]
- Nunnari J., Walter P. Protein targeting to and translocation across the membrane of the endoplasmic reticulum. Curr Opin Cell Biol. 1992 Aug;4(4):573–580. doi: 10.1016/0955-0674(92)90074-m. [DOI] [PubMed] [Google Scholar]
- Ohta S., Schatz G. A purified precursor polypeptide requires a cytosolic protein fraction for import into mitochondria. EMBO J. 1984 Mar;3(3):651–657. doi: 10.1002/j.1460-2075.1984.tb01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Tuboi S. Purification and identification of a cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. Arch Biochem Biophys. 1990 Aug 1;280(2):299–304. doi: 10.1016/0003-9861(90)90333-t. [DOI] [PubMed] [Google Scholar]
- Ono H., Tuboi S. The cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. J Biol Chem. 1988 Mar 5;263(7):3188–3193. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Pilon M., de Boer A. D., Knols S. L., Koppelman M. H., van der Graaf R. M., de Kruijff B., Weisbeek P. J. Expression in Escherichia coli and purification of a translocation-competent precursor of the chloroplast protein ferredoxin. J Biol Chem. 1990 Feb 25;265(6):3358–3361. [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Hachiya N., Mihara K., Omura T. Mitochondrial porin can be translocated across both endoplasmic reticulum and mitochondrial membranes. J Biochem. 1992 Aug;112(2):243–248. doi: 10.1093/oxfordjournals.jbchem.a123884. [DOI] [PubMed] [Google Scholar]
- Sato T., Sakaguchi M., Mihara K., Omura T. The amino-terminal structures that determine topological orientation of cytochrome P-450 in microsomal membrane. EMBO J. 1990 Aug;9(8):2391–2397. doi: 10.1002/j.1460-2075.1990.tb07414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. A., Nicholson D. W., Neupert W. Early steps in mitochondrial protein import: receptor functions can be substituted by the membrane insertion activity of apocytochrome c. Cell. 1990 Jan 12;60(1):31–43. doi: 10.1016/0092-8674(90)90713-o. [DOI] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Tandon S., Horowitz P. Detergent-assisted refolding of guanidinium chloride-denatured rhodanese. The effect of lauryl maltoside. J Biol Chem. 1986 Nov 25;261(33):15615–15618. [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Wachter C., Schatz G., Glick B. S. Protein import into mitochondria: the requirement for external ATP is precursor-specific whereas intramitochondrial ATP is universally needed for translocation into the matrix. Mol Biol Cell. 1994 Apr;5(4):465–474. doi: 10.1091/mbc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., Ray P. H., Bassford P. J., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]