Abstract
Purpose
Enzyme replacement therapy with rhGAA (Myozyme®) has lead to improved survival, which is largely attributable to improvements in cardiomyopathy and skeletal muscle function. However, crossreactive immunologic material-negative patients have a poor clinical response to enzyme replacement therapy secondary to high sustained antibody titers. Furthermore, although the majority of crossreactive immunologic material-positive patients tolerize or experience a downtrend in anti-rhGAA antibody titers, antibody response is variable with some crossreactive immunologic material-positive infants also mounting high sustained antibody titers.
Methods
We retrospectively analyzed 34 infants with Pompe disease: 11 crossreactive immunologic material-negative patients, nine high-titer crossreactive immunologic material-positive patients, and 14 low-titer crossreactive immunologic material-positive patients. Clinical outcome measures were overall survival, ventilator-free survival, left ventricular mass index, Alberta Infant Motor Scale score, and urine Glc4 levels.
Results
Clinical outcomes in the high-titer crossreactive immunologic material-positive group were poor across all areas evaluated relative to the low-titer crossreactive immunologic material-positive group. For the crossreactive immunologic material-negative and high-titer crossreactive immunologic material-positive groups, no statistically significant differences were observed for any outcome measures, and both patient groups did poorly.
Conclusions
Our data indicate that, irrespective of crossreactive immunologic material status, patients with infantile Pompe disease with high sustained antibody titer have an attenuated therapeutic response to enzyme replacement therapy. With the advent of immunomodulation therapies, identification of patients at risk for developing high sustained antibody titer is critical.
Keywords: Pompe disease, enzyme replacement therapy (ERT), crossreactive immunologic material (CRIM), CRIM negative (CN), high sustained antibody titers (HSAT), high-titer CRIM-positive (HTCP), low-titer CRIM-positive (LTCP), therapeutic proteins
Pompe disease (glycogen storage disease type II, acid maltase deficiency) is an autosomal recessive lysosomal storage disorder caused by a deficiency of acid α-glucosidase, resulting in accumulation of glycogen in multiple cell types including skeletal, cardiac, and smooth muscles.1 Glycogen accumulation in these tissues can occur rapidly and has been detected in fetuses as young as 16–18 weeks’ gestational age.2 The infantile form of Pompe disease is the most severe within the spectrum of Pompe phenotypes and is characterized by cardiomyopathy, hypotonia, and respiratory insufficiency. Death typically occurs secondary to cardiorespiratory failure within the first 1–2 years of life.1,3,4 Since the introduction of enzyme replacement therapy (ERT) with alglucosidase alfa (Myozyme®), many patients with infantile Pompe disease are living longer and are experiencing an enhanced quality of life.5–7 However, the response to ERT is very heterogeneous. Various factors known to impact the response to ERT include age on ERT initiation, extent of preexisting pathology, degree of muscle damage, muscle fiber type (i.e., type I versus type II), and defective autophagy.5,8–10 It has also been shown that baseline crossreactive immunologic material (CRIM) status is highly correlated with treatment response.11
Relative to most CRIM-positive (CP) patients, CRIM-negative (CN) patients tend to do poorly on ERT with death or invasive ventilation by age 27.1 months.11 In CN patients, there exist two deleterious GAA mutations, which lead to absence of native GAA enzyme production and, therefore, lack of exposure of the developing immune system to the GAA protein. Consequently, these patients are not immune tolerant to GAA and mount an antibody response to the native enzyme when given as ERT. Often these responses neutralize either enzyme uptake into cells or the catalytic activity of the enzyme. Thus, it is not surprising that high sustained antibody titers (HSAT) herald clinical decline in CN patients.11,12 In contrast, most CP patients produce some enzyme which, although nonfunctional or greatly reduced in function, establishes some degree of immune tolerance, and therefore, a robust antibody response is not elicited with exposure to the normal GAA enzyme. Consistent with this is the observation that anti-rhGAA antibody titers for most CP patients either remain relatively low or decline following a plateau at a modest titer (1:1200) with continued exposure to enzyme.11
However, as a subset of CP patients also develops high antibody titers,8,11,12 we were able to examine more critically the role of HSAT, as opposed to CRIM status per se, in the clinical course of Pompe disease.
METHODS
Study design
We retrospectively analyzed patients who met criteria for infantile Pompe disease. Inclusion criteria were confirmed diagnosis of Pompe disease, <1% of normal GAA activity (in skin fibroblasts and/or muscle biopsy), cardiomyopathy (left ventricular mass index [LVMI] ≥65 g/m2 by echocardiogram), and age at symptoms <12 months. Exclusion criteria included invasive ventilation at baseline and any major congenital anomaly. Thirty-four infantile patients, who included 23 CP and 11 CN patients, met the inclusion criteria. These 11 CN patients have been reported in our previous study.11 CP patients were further subdivided into two groups based on their antibody titers at different time points as shown in Figure 1.
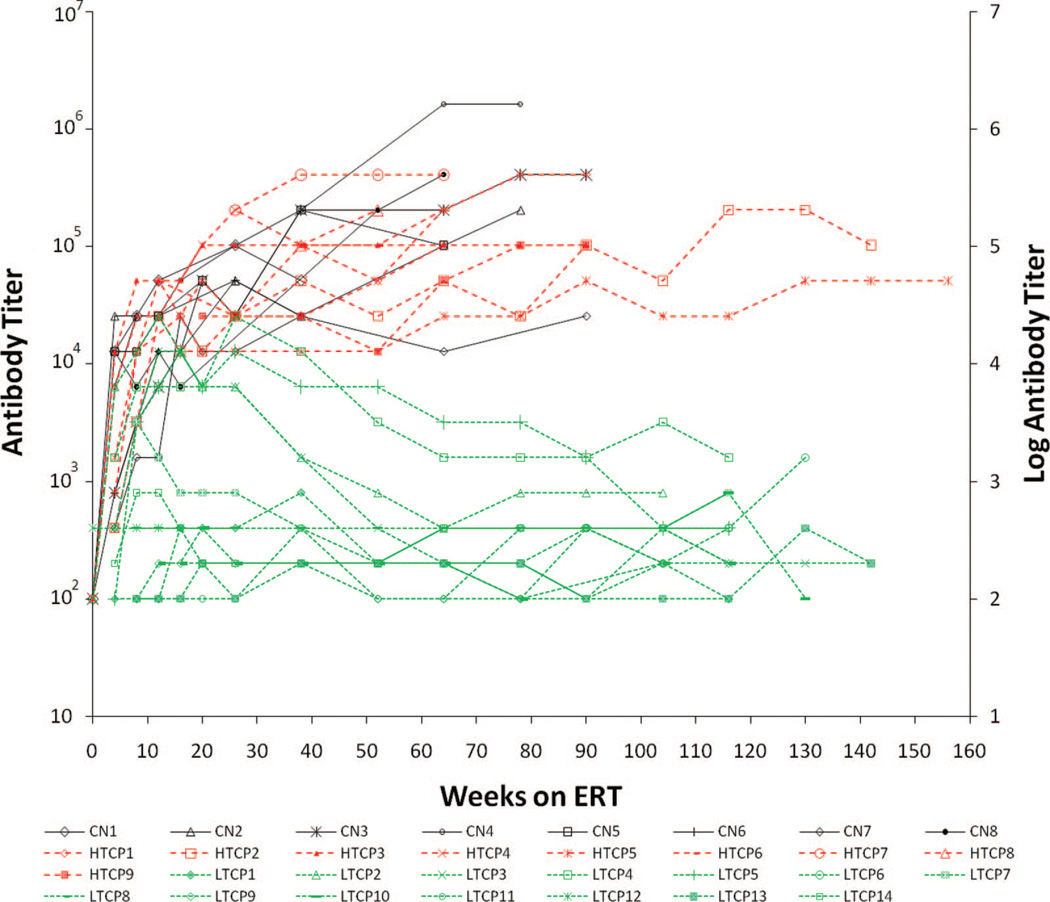
Fig. 1.
Antibody titers for CN (black), HTCP (red), and LTCP (green) at various time points. Primary y axis shows antibody titers, and secondary y axis shows corresponding log-transformed antibody titer value. CN, CRIM negative; HTCP, high-titer CRIM positive; LTCP, low-titer CRIM positive.
It was noted that CP patients who tolerized or showed a downward trend did not maintain titers at or above 1:51,200 for more than one time point. In contrast, the group that showed increasing titers had titers consistently above 1:51,200 (Fig. 1). This was the basis of selecting the antibody titer level of 1:51,200 as the cutoff value which was chosen conservatively. Patients with titers ≥1:51,200 on two or more occasions at or beyond 6 months (26 weeks) on ERT were defined as high-titer CP (HTCP) patients (red-dashed line in Fig. 1). CP patients who never had titers of 1:51,200 on two or more occasions at or beyond 26 weeks (6 months) on ERT and showed a downward trend/tolerized were defined as low-titer CP (LTCP) patients (green dashed line in Fig. 1). The study was approved by the institutional review board, and parental written informed consent was obtained. All patients received rhGAA (Myozyme®) supplied by Genzyme Corporation (Cambridge, MA) at a cumulative or total dose of 20 or 40 mg/kg every other week in accordance with previously published reports.5,6,8,12
Clinical outcomes
Clinical outcomes used were overall survival, ventilator-free survival, LVMI, Alberta Infant Motor Scale (AIMS), and urine Glc4 (Hex4), where available. The clinical response to ERT was evaluated across the three groups (11 CN, 9 HTCP, and 14 LTCP). Patients included in this analysis were followed up for at least 52 weeks since start of ERT or until death. We summarize these data and also present updated overall and ventilator-free survival data through December 2009. Two-dimensional, M-mode, and Doppler echocardiography were used to assess LVM index at baseline, at Weeks 26 and 52, and where possible beyond 52 weeks on ERT. Motor function evaluation was performed using the AIMS13 by an experienced physical therapist at baseline, at Weeks 26 and 52, and where possible beyond 52 weeks on ERT.
Laboratory methods
CRIM status was determined as described previously based on reactivity of a pool of monoclonal and polyclonal anti-GAA antibodies capable of recognizing both native and recombinant GAA.8,14 Anti-rhGAA IgG antibodies were assessed at baseline and at Weeks 4, 8, 12, 26, 38, 52, and 64. Moreover, in surviving infants, serotiters were also followed through Weeks 90, 104, and 130. Antibody status was ascertained using enzyme-linked immunosorbent assays and confirmed using radio-immunoprecipitation as described previously.8 Additional testing to determine the presence of inhibitory antibodies toward enzyme uptake or enzyme activity was performed in patients according to the respective clinical trial protocols and the requirements of the Genzyme Pharmaco-vigilance Department. An inhibitory antibody assay (enzyme activity) was used to measure inhibition of rhGAA enzyme activity by antibodies present in patient serum, and a flow cytometry-based assay (enzyme uptake) was used to evaluate whether patient antibodies interfere with uptake of rhGAA by human fibroblast cells in culture. Urine oligosaccharides were obtained to assess and follow Glc4 (Hex4), a biomarker for Pompe disease at baseline, at Weeks 26 and 52, and where possible beyond 52 weeks on ERT. Urine Glc4 was measured by HPLC–UV and tandem mass spectrometry, as described previously.15,16
Statistical analysis
Survival data were analyzed using the Kaplan–Meier method with two-tailed P values generated using the log-rank test.17 Other reported P values were generated by the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables, as well as Kruskal Wallis test for nonparametric analysis between the three groups. Analyses were performed with STATA version 11.0 (StataCorp LP, College Station, Texas). Because of the limited sample size, all group outcome variable data are presented as medians.
RESULTS
CRIM status stratification and baseline demographics
Of 23 CP patients, nine and 14 patients met the criteria for HTCP and LTCP, respectively. Baseline demographics and disease-related parameters (i.e., age, age at ERT, LVM index, AIMS score, and urine Glc4 levels) were comparable between the CN, HTCP, and LTCP groups (Table 1). Median age at start of ERT was 3.8 months (range: 2.1–7 months) for the CN group (n = 11), 6.9 months (range: 5.4 –13.0 months) for the HTCP group (n = 11), and 5.7 months (range: 1.9 –14.3 months) for the LTCP group (n = 14).
Table 1.
Baseline demographics
| CRIM negative (CN), n = 11 |
High-titer CRIM positive (HTCP), n = 9 |
Low-titer CRIM positive (LTCP), n = 14 |
|
|---|---|---|---|
| Gender | |||
| Male | 5 (45%) | 4 (44%) | 11 (79%) |
| Female | 6 (55%) | 5 (56%) | 3 (21%) |
| Race | |||
| White | 4 (36%) | 5 (56%) | 4 (29%) |
| Black | 3 (27%) | 1 (11%) | 3 (21%) |
| Hispanic | 1 (9%) | 1 (11%) | 2 (14%) |
| Asian | 1 (9%) | 1 (11%) | 3 (21%) |
| Other | 2 (18%) | 1 (11%) | 2 (14%) |
| Age at symptom onset (mo) | |||
| Mean (SD) | 1.2 (0.9) | 3.2 (2.0) | 2.2 (2.1) |
| Median | 1.45 | 3 | 1.8 |
| Min, Max | 0, 2.4 | 0, 6 | 0, 5.4 |
| Age at first ERT (mo) | |||
| Mean (SD) | 3.55 (1.93) | 7.7 (3.0) | 7 (3.8) |
| Median | 3 | 7 | 6.7 |
| Min, Max | 0.25, 7.0 | 5, 13 | 1.9, 15 |
| Adjusted at first ERT (mo) | |||
| Mean (SD) | 3.19 (1.81) | 7.5 (3.0) | 6.6 (3.9) |
| Median | 3.15 | 6.7 | 5.7 |
| Min, Max | 0, 6.1 | 4.8, 13 | 1.9, 15 |
Anti-rhGAA antibody titers
Titers for CN, HTCP, and LTCP patients at different time points are shown in Figure 1. Three patients in the CN group had different methods of antibody determination and were excluded from this analysis although they did exhibit high antibody titers by the other method. All patients (8 CN, 9 HTCP, and 14 LTCP) developed IgG antibodies to rhGAA. Although no apparent association was observed between the level of antibody titers and the dose of alglucosidase alfa (20 vs. 40 mg/kg/biweekly), the limited number of patients within each of the three groups receiving 40 mg/kg every other week did not permit statistical evaluation. In antibody titer analyses, the HTCP patients did not significantly differ from the CN patients, whereas the LTCP patients differed greatly from both HTCP and CN patients. All eight CN patients and six of seven HTCP patients seroconverted by 4 weeks of ERT, whereas only 4 of 14 LTCP patients did so: the median time to seroconversion after commencement of ERT in the CN and HTCP patients was 4 weeks, whereas it was 8 weeks for the LTCP patients (Fig. 1). Median antibody titers at 26 weeks were identical (1:51,200) between the CN (n = 7) and HTCP (n = 9) groups, whereas that for the LTCP group (1:400; n = 14) significantly differed from both (P = 0.0010) and remained significantly different at 52 weeks (median titer of 1:153,600 for CN [n = 6]; 1:51,200 for HTCP [n = 11]; and 1:200 for LTCP [n = 14] groups; P = 0.0010). Median peak titers were higher in the HTCP (n = 9) and CN (n = 8) patients, whose median peak titers were each 1:204,800 vs. a peak titer of 1:800 in LTCP (n = 14) patients, although several LTCP patients did have peak titers ≥1:12,800. However, the most significant difference between HTCP and CN patients versus the LTCP patients was in the persistence and trend of the response, with all LTCP patients exhibiting a downward trend beginning at approximately 26 weeks. Both the HTCP and CN groups showed a continued increase in serotiters, with a doubling of median titers (from 1:51,200 at 26 weeks to 1:102,400 at 64 weeks) for the HTCP and a tripling median titers (from 1:51,200 at 24 weeks to 1:153,600 at 52 weeks) for the CN. In marked contrast, of the 14 LTCP patients, 12 (85.7%) had titers ≤1:800 at 52 weeks and all subsequent time points, whereas two (14.3%) had titers of 1:3200 or 1:6400 at 52 weeks, with subsequent titers staying at or below 1:1600. No HTCP or CN patient showed a consistent decline in titer levels, and all HTCP patients developed titers of at least 1:51,200 and all CN patients developed titers of at least 1:25,600. Interestingly, no LTCP patient developed a titer >1:25,600.
Although HTCP and CN patients had highly similar patterns of response, the most marked difference between them regarded the development of neutralizing antibody (NAbs), which either abrogated rhGAA uptake into target cells or abrogated the catalytic activity of the enzyme. Such testing was performed on samples from most patients who developed anti-rhGAA antibodies and experienced infusion-associated reactions or showed signs of clinical decline. Twenty-two patients (five CN, eight HTCP, and nine LTCP) were tested for enzyme activity inhibition, and 21 patients (five CN, seven HTCP, and nine LTCP) were tested for uptake inhibition. Although uptake inhibition was detected in three of five CN patients, none of the HTCP or LTCP patients had detectable levels of uptake inhibition. With respect to inhibition of catalytic activity, two of five CN patients tested positive for activity inhibition, but only one of four HTCP patients and none of the LTCP patients tested showed detectable levels of activity inhibition. Thus, NAbs seem to develop commonly in CN patients but not in HTCP patients. The basis for this finding is not clear but may relate to the location of the mutations which, in CN, may be in epitopes centered in the catalytic or uptake domains. Alternatively, the development of NAbs may pertain to the intensity of the immune response, which may drive epitope spreading. Five CN, one HTCP patient, and five LTCP patients in this study were never tested for activity or uptake inhibition, and an additional one HTCP patient was never tested for uptake.
Survival
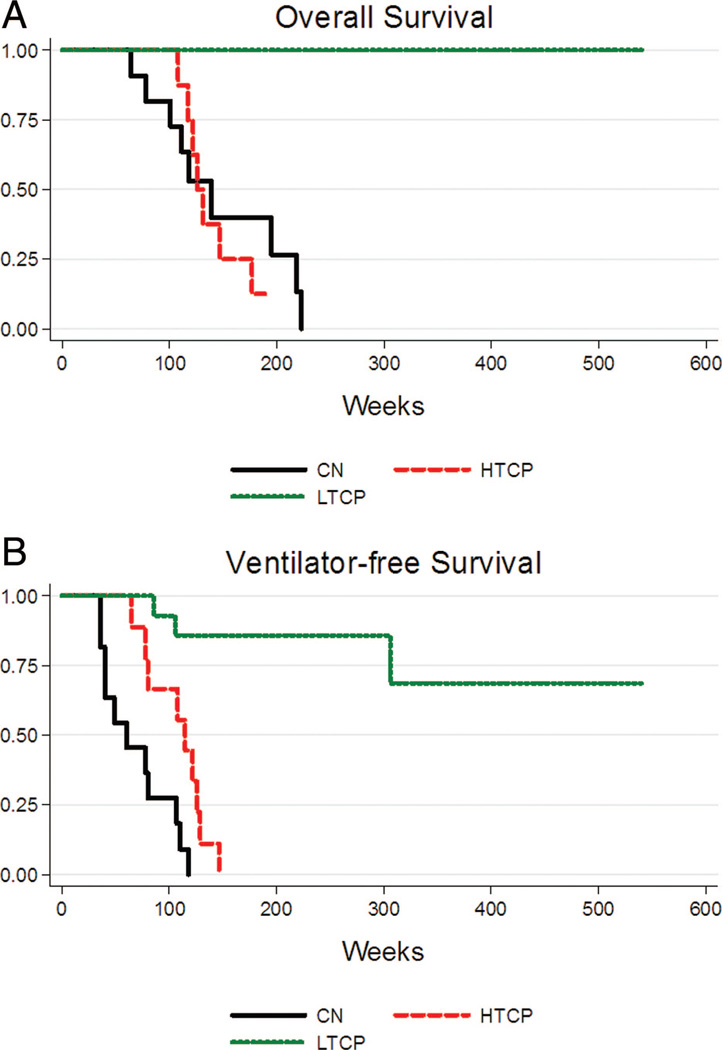
Figure 2, A and B shows overall and ventilator-free survival outcomes within the three groups (CN, HTCP, and LTCP). Overall survival in the HTCP group was highly similar to the CN patients (P = 0.84) but was dramatically different from the LTCP group (P < 0.0001; Fig. 2A). In terms of invasive ventilator-free survival, there was a statistically significant difference between the CN, HTCP, and LTCP groups (P < 0.0001; Fig. 2B). HTCP and CN groups mirrored one another in terms of overall and ventilator-free survival (Fig. 2, A and B). By Week 52, six of 11 (54.5%) CN patients were either deceased (n = 1) or invasively ventilated (n = 5), four of nine HTCP patients were deceased (n = 2) or invasively ventilated (n = 2), versus only one of 21 LTCP patient who was invasively ventilated (P < 0.0001). All 11 CN patients and all nine HTCP patients were either deceased (CN = 5 and HTCP = 6) or invasively ventilated (CN = 6 and HTCP = 3) by 118 weeks (27.1 months) and 147 weeks (33.8 months) of age, respectively, whereas no LTCP patients died and only three were invasively ventilated (median age of invasive ventilation = 97 weeks [24.4 months]; range = 19.7–70.3 months).
Fig. 2.
Kaplan-Meier curves for (A) overall survival and (B) ventilator-free survival for CN (black), HTCP (red), and LTCP (green). CN, CRIM negative; HTCP, high-titer CRIM positive; LTCP, low-titer CRIM positive.
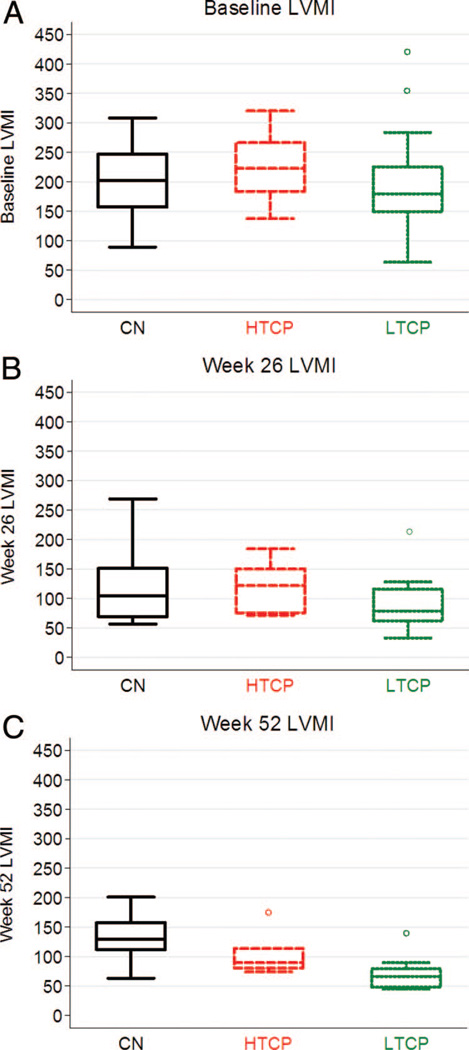
Cardiac function: LVMI
The upper limit of normal LVMI for infants is 64 g/m2 (≥2 SD higher than the age appropriate normal mean).18 In this study, all three groups showed similarly elevated LVMI at baseline (CN, 202.1 g/m2; HTCP, 207.5 g/m2; and LTCP, 179.42 g/m2; P = 0.60) (Fig. 3A). After 26 weeks on ERT, all three groups demonstrated a net decrease in median LVMI (CN, 126 g/m2 [n = 10]; HTCP, 121.9 g/m2 [n = 7]; and LTCP, 79.01 g/m2 [n = 12]) (Fig. 3B). There were no significant differences in LVMI at 26 weeks among CN, HTCP, and LTCP groups (P = 0.22). In marked contrast, at 52 weeks, the LTCP group (n = 13) demonstrated additional reduction in median LVMI to near-normal levels (LVMI = 66.2 g/m2), which was significantly different (P = 0.04) from the median LVMI in surviving CN (n = 9) and HTCP (n = 6) patients, which increased to 129 and 89.9 g/m2, respectively (Fig. 3C). No significant difference in median LVMI was observed between the HTCP and CN groups at Week 52 (P = 0.13), although the variance in this measure was much greater in the CN compared with the HTCP patients.
Fig. 3.
Left ventricular mass index (LVMI) in g/m2 at baseline (A), 26 weeks (B), and 52 weeks (C) of rhGAA treatment in CRIM negative (CN), high-titer CRIM-positive (HTCP) patients, and low-titer CRIM-positive (LTCP) patients.
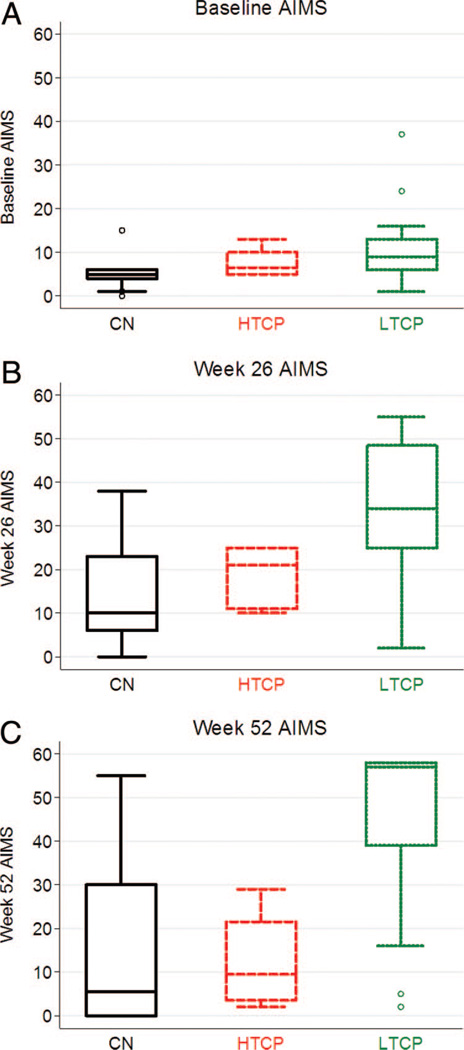
Gross motor development: AIMS
At baseline, gross motor development was delayed and essentially the same for all three groups. The average age of CN (n = 10), HTCP (n = 6), and LTCP (n = 14) patients on ERT initiation was 3.0, 7.0, and 6.7 months, respectively; the median AIMS score for CN, HTCP, and LTCP patients was 5.2 (age equivalent [AE] = 0.75 months), 6.5 (AE = 1.2 months), and 9 (AE = 2.3 months), respectively (Fig. 4A). After 26 weeks of ERT, gross motor function improved from baseline in all three groups. However, the median AIMS score for the CN (10; AE = 2.5 months; n = 9) and for the HTCP (21; AE = 5.1 months; n = 5) groups was less than that for the LTCP group (34; AE = 7.8 months; n = 12) (P = 0.041; Fig. 4B). Consistent with previous similarities, no significant difference in median AIMS score was observed between the HTCP and CN groups (P = 0.42) at Week 26. After 52 weeks of ERT, the median AIMS score for HTCP (14; AE = 3.9 months; n = 4) and for CN (5.5; AE = 0.75 months; n = 10) patients showed a remarkable decline and was significantly less than the median score for the LTCP (57; AE = 14.6 months; n = 13) group (Fig. 4C) (P = 0.03), who in contrast showed continued motor gains versus their baseline and Week 26 values. Although the HTCP patients maintained small gains in AIMS score at Week 52, when compared with baseline, CN patients showed a continued decline; this was not clinically or statistically significant (P = 0.83). Furthermore, at Week 52, the HTCP patients lost the small gains made at Week 26.
Fig. 4.
Alberta Infant Motor Scale (AIMS) scores at baseline (A), 26 weeks (B), and 52 weeks (C) of rhGAA treatment in CRIM negative (CN), high-titer CRIM-positive (HTCP) patients, and low-titer CRIM-positive (LTCP) patients.
Biomarker urinary Glc4 (Hex4)
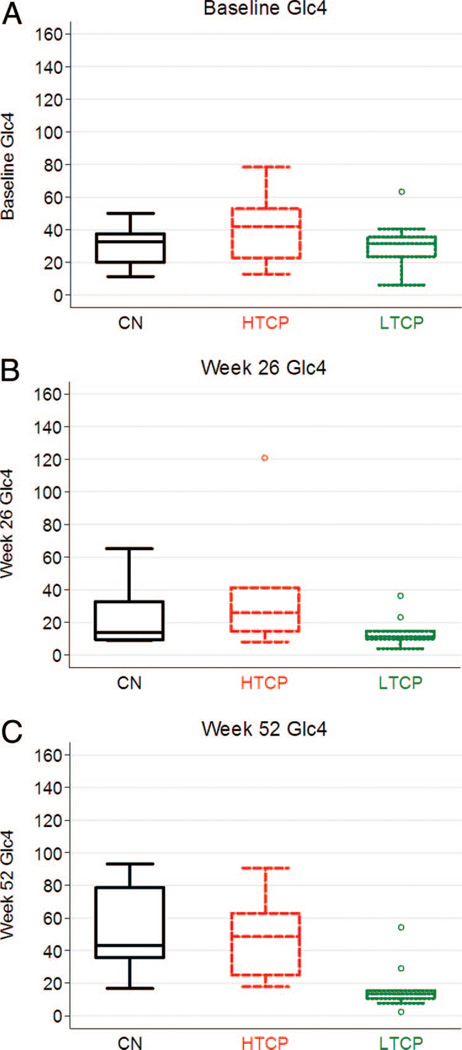
At baseline, no significant difference in urine Glc4 levels was observed among CN (n = 8), HTCP (n = 7), and LTCP (n = 11) groups (P = 0.31) (Fig. 5A). At 26 weeks, all groups showed an adequate response to ERT with no statistical difference among them (P = 0.28) (Fig. 5B). In marked contrast, at 52 weeks of ERT, median Glc4 levels in the CN (42.9 mmol/mol creatinine; n = 9) and HTCP (48.5 mmol/mol creatinine; n = 6) groups increased substantially, whereas Glc4 levels for LTCP patients remained low (13.4 mmol/mol creatinine; n = 9) (Fig. 5C). The difference in median Glc4 levels at Week 52 on ERT between the HTCP and LTCP as well as between the CN and LTCP groups achieved statistical significance (P = 0.01 and P = 0.0036, respectively). There was no significant difference (P = 0.91) in median Glc4 levels at Week 52 between the HTCP and CN groups.
Fig. 5.
Urine Glc4 in mmol/mol creatinine at baseline (A), 26 weeks (B), and 52 weeks (C) of rhGAA treatment in CRIM negative (CN), high-titer CRIM-positive (HTCP) patients, and low-titer CRIM-positive (LTCP) patients.
DISCUSSION
ERT with alglucosidase alfa has been shown to significantly improve survival, quality of life, and motor outcomes in most patients with infantile Pompe disease.5–7 A previous study by our group showed that CN status is a poor prognostic factor in patients with Pompe disease treated with ERT.11 CN patients develop HSAT after exposure to ERT, which is thought to be a key factor in the poor response to ERT. Some CP patients also develop HSAT, the impact of which on clinical outcome had not been evaluated. This study is the first to systematically evaluate the role of antibodies on clinical outcomes in CP patients with infantile Pompe disease and to see how they compare with CN patients. CP patients were divided into two groups: HTCP and LTCP based on antibody titers (see “Methods”). There were dichotomous outcomes among HTCP and LTCP patients on ERT in all parameters assessed. Similar to what has been observed in CN patients, HTCP patients showed a period of improvement in the first 6 months of enzyme replacement, after which they declined across all outcome measures. Furthermore, similar to CN patients, antibody titers persisted above at ≥1: 51,200 at or beyond 6 months post-ERT initiation, irrespective of initial levels. This persistence of high titer antibody significantly correlated with the observed clinical decline in HTCP patients. In contrast, LTCP patients, in whom antibody titer was either persistently low or declining by 26 weeks, had a more favorable clinical outcome. The antibody response observed in CN and HTCP patients which preceded clinical decline and persisted thereafter and the converse, the decline of antibody titers associated with a favorable outcome in LTCP patients, further substantiates the contention that the initial clinical response to treatment with the exogenous enzyme is terminated by the increased and persistent antibody response.
The major differences between HTCP and CN patients with respect to the antibody response pertained to the peak titer levels, which were higher in CN and to the presence of neutralizing activity. Neutralization of uptake or catalytic activity signifies that antibodies are directed to specific determinants of the enzyme in or close to the uptake and catalytic domains, respectively. Although CN patients demonstrated the presence of such antibodies, only one of four HTCP patients demonstrated neutralization of the catalytic domain but not uptake activity. However, high titers of binding antibodies, such as were present in the HTCP patients, although not specific for the uptake/catalytic domains, may also abrogate product efficacy by several mechanisms: diversion of enzyme uptake from intended muscle target into FcR positive cells, such as macrophages, by binding of the Fc portion of enzyme bound antibody and altered subcellular trafficking of enzyme within the cell, away from lysosomes and into early endosomes.19 Thus, the same poor clinical outcome experienced by CN and HTCP pertaining to sustained high titer immune responses may involve similar and some different antibody-mediated mechanisms.
One of two outliers for LVMI at baseline in the LTCP group (Fig. 3A) had a baseline LVMI of 417.6 g/m2, which is one of the highest LVMI values seen in an infant with Pompe disease. The same patient was an outlier at Week 26 (Fig. 3B) and Week 52 (Fig. 3C) with LVMI values of 211.6 and 139.6 g/m2. Although an outlier when compared with the LVMI values seen in other LTCP patients at baseline, Week 26, and Week 52, this patient ultimately had a decrease in LVMI value at Week 90 to an almost normal value (64 g/m2) of 65.1 g/m2 (data not shown). This patient was seronegative from Week 38 onward (LTCP 12 in Fig. 1). Although an outlier in LTCP group for LVMI values at different time points, this patient was not an outlier for Glc4 or AIMS score values at any time points. Two outliers with low AIMS score in LTCP group at Week 52 (AIMS scores, 2 and 6) started ERT at 9.8 and 5.9 months of age and became ventilator dependent at Week 26 (age 19.7 months) and Week 60 (age 70.3 months), respectively. One of these patients was also an outlier for Glc4 results at Week 52. Glc4 results for the other patient were not available for analysis. Age at start of ERT is a known prognostic factor of clinical outcome in infantile Pompe disease.20 Low AIMS score for these two patients could be due to starting ERT at a later age with irreversible muscle damage already having taken place.
The impact of the immune response on disease outcome is further substantiated in animal models of Pompe disease. Indeed, in GAA knock out mouse models, antibody formation to rhGAA reduced the efficacy of ERT.20 More recently, immune tolerance induction with an adeno-associated virus vector containing a liver-specific promoter in GAA knock out revealed enhanced target tissue penetration of enzyme compared with antibody positive counterparts.21 Moreover, clinical data, in which an ongoing immune response was abrogated,22 or immune responses were prevented coincident with onset of ERT in CN patients with Pompe disease, significantly improved outcomes (Priya S. Kishnani, Duke University Medical Center, personal communication, 2011). Clinically relevant inhibition of ERT by antibodies should be a consideration when there is evidence that a patient is responding poorly or deteriorates after an initial positive response to an expected therapeutic regimen.23
Antibodies to therapeutic proteins have been described in patients with late-onset Pompe disease as well other LSDs, such as Gaucher disease, Fabry disease, and mucopolysaccharidosis type I.24–30 In a canine model of mucopolysaccharidosis type I, the enzyme (alpha-l-iduronidase) was mistargeted to organs containing a high number of phagocytes, suggesting uptake of immune complexes by the macrophages.31 In Fabry disease, the variable nature and slowly progressive course of the disease and the limited efficacy of ERT in reversing established disease make it difficult to assess the influence on clinical outcome, but it is not unlikely that the antibodies do have a negative effect.26 Furthermore, beyond LSDs, antibodies have been shown to complicate treatment in other conditions where replacement proteins are administered, such as in the use of Factor VIII and IX for hemophilia A and B, respectively,32 erythropoietin for chronic kidney failure,33 beta-interferon in multiple sclerosis,34 insulin for diabetes,35 and growth hormone for short stature.36
Establishing relationships among genotype, phenotype, protein structure, immunogenicity, and clinical outcome is key to predicting which patients will require tolerance-inducing therapies before treatment with ERT so as to preclude the types of responses that sabotage efficacy. Although CN status can be easily identified by Western blot and mutation analysis, the current challenge is the early identification of CP patients at risk for developing HSAT. Rapid identification of at-risk patients is of paramount importance, so tolerance-inducing treatment can be started either before or at the same time as starting treatment with therapeutic protein. Abrogating the immune response will not only help in improving clinical outcomes but may also potentially minimize the amount of enzyme necessary to achieve acceptable clinical outcomes.
Although there are likely many factors contributing to the establishment of HSAT hitherto observed in a subset of CP patients, two very important considerations are (a) which sequences in rhGAA are seen as foreign by a given individual’s immune system (likely influenced by the underlying mutation(s) and any downstream three-dimensional structural impact on endogenously synthesized GAA) and (b) whether the individual has an major histocompatibility complex phenotype that can bind cognate peptide sequences derived from rhGAA with such affinity that presentation of peptide sequences to key players in the immune system is sufficient for recognition as “nonself.” The extent to which endogenously transcribed GAA (if any) is recognized as “self” depends in large part on the amount of protein produced and capacity for presentation of derived peptide sequences to immature T cells. In this setting, the sequence of GAA actually translated (if any) is likely to induce tolerance to the portion of GAA molecule it represents. These considerations have potential implications for both HTCP and LTCP patients in addition to CN patients. Indeed, although HTCP patients by definition produce some protein (being CP), the high titers observed may be explained, at least in part, by the possibility that the resulting protein product does not proceed to enter relevant endocytic pathways as a result of its instability and/or rapid degradation; as a result, the otherwise expected process of antigen presentation may not proceed to completion and thus preclude immune tolerization and in essence behaving similar to a CN patient. On subsequent initiation of ERT with recombinant enzyme, immature T cells (by their T-cell receptors) interact with processed rhGAA peptides on antigen-presenting cells; intracellular signaling pathways ultimately activate these T cells which in turn activates B cells. Antibodies complicating therapy are ultimately produced by the resulting transformation of immature B cells to antibody-producing plasma cells. This is in contrast to the likely scenario occurring in LTCP patients, whereby the GAA protein produced endogenously could be sufficiently stable to enter into endocytic pathways ultimately leading to presentation with major histocompatibility complex class II and tolerization of nascent T cells. In this situation, subsequent initiation of rhGAA ERT would not be as likely to induce a robust and potentially deleterious IgG immune response toward such exogenous GAA given the “familiarity” of the immune system to the complement of epitopes so derived.
Advancements in bioinformatics tools, most especially epitope prediction platforms, would provide further support for the merits of screening for Pompe disease (manuscript under preparation) and for other orphan diseases where successful therapeutic interventions are available for altering the disease trajectory. Although an important factor in predicting antibody formation to ERT, additional patient-related and treatment-related factors are known to potentially modulate antibody response in diseases treated with therapeutic protein.37 GAA mutations for CN patients have been described elsewhere.11 Table, Supplemental Digital Content 1, http://links.lww.com/GIM/A175 shows GAA mutations for HTCP and LTCP patients.
In summary, our study clearly shows the negative impact of HSAT on clinical outcomes in patients receiving a therapeutic protein, using infantile Pompe disease as a model. Most specifically, it identifies a subset of CP patients who, although typically having a good response to ERT, remain at high risk for development of immune responses which abrogate its efficacy. Indeed, there were no significant differences in clinical outcomes between CN and HTCP. Thus, identifying CN status and the CP patients at risk for development of HSAT through mutation and epitope analysis would represent a significant advancement in the field of therapeutic proteins. Furthermore, there remains a need to develop and implement additional immune tolerance-inducing strategies for both prophylactic purposes (to preclude antibody development in ERT) and for therapeutic purposes to treat after the development of antibodies. Although clinical trials of immunomodulation are currently underway, further emphasis on such studies is needed as the outcome is otherwise poor for CN and HTCP patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, their families and the health care providers who participated in different clinical studies of rhGAA whose outcomes are summarized here. This project was funded in part by the Lysosomal Disease Network, a part of NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by (1U54NS065768-01) from the NINDS and the NIH Office of Rare Diseases Research (ORDR). The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. The clinical studies from which patients were culled for this analysis were sponsored by Synpac, Inc. (Durham, NC) and Genzyme Corporation (Cambridge, MA). The clinical trials were supported by Genzyme Corporation and Grant 1UL1RR024128 from the Duke Clinical Research Unit Program, National Center for Research Resources and the National Institutes of Health. All data included in this paper were verified and analyzed independently by the authors. P.S. Kishnani had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Priya S. Kishnani has received research/grant support and honoraria from Genzyme Corporation. Priya S. Kishnani is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation. Y-T Chen has received honoraria from Genzyme Corporation. Alglucosidase alfa rhGAA, in the form of Genzyme’s product alglucosidase alfa, (Myozyme®/Lumizyme®) has been approved by the US FDA and the European Union as therapy for Pompe disease. Duke University and the inventors of the method of treatment and precursors of the cell lines used to generate the enzyme (rhGAA) used commercially have received royalties pursuant to the university’s policy on inventions, patents, and technology transfer.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.geneticsinmedicine.org).
A portion of the results submitted in this article was presented by Dr. S. G. Banugaria at the 33rd Annual Meeting of the Society of Inherited Metabolic Disorders and at the 2010 Annual Meeting of the American College of Medical Genetics (ACMG) in Albuquerque, New Mexico, March 2010.
Disclosure
This potential conflict for Duke University has been resolved through monetization. S. G. Banugaria, S. N. Prater, Y-K. Ng, J. A. Kobori, R.S. Finkel, R. L. Ladda and A. S. Rosenberg have no financial or proprietary interest in the materials presented herein.
REFERENCES
- 1.Hirschhorn R, Reuser AJJ. New York: McGraw-Hill; 2009. Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency [Internet Resource; Computer File Date of Entry: 20090331] Available at: http://genetics.accessmedicine.com/. [Google Scholar]
- 2.Hug G. Pre- and postnatal pathology, enzyme treatment, and unresolved issues in five lysosomal disorders. Pharmacol Rev. 1978;30:565–591. [PubMed] [Google Scholar]
- 3.van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe’s disease, 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- 4.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 6.Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- 7.Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66:329–335. doi: 10.1203/PDR.0b013e3181b24e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishnani PS, Nicolino M, Voit T, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawes ML, Kennedy W, O’Callaghan MW, Thurberg BL. Differential muscular glycogen clearance after enzyme replacement therapy in a mouse model of Pompe disease. Mol Genet Metab. 2007;91:343–351. doi: 10.1016/j.ymgme.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Raben N, Takikita S, Pittis MG, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546–552. doi: 10.4161/auto.4591. [DOI] [PubMed] [Google Scholar]
- 11.Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3:132–138. doi: 10.109700125817-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Piper M, Darrah J. Motor assessment of the developing infant. Philadelphia: WB Saunders, Co.; 1994. [Google Scholar]
- 14.Klinge L, Straub V, Neudorf U, et al. Safety and efficacy of recombinant acid alpha-glucosidase (rhGAA) in patients with classical infantile Pompe disease: results of a phase II clinical trial. Neuromuscul Disord. 2005;15:24–31. doi: 10.1016/j.nmd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.An Y, Young SP, Hillman SL, Van Hove JL, Chen YT, Millington DS. Liquid chromatographic assay for a glucose tetrasaccharide, a putative biomarker for the diagnosis of Pompe disease. Anal Biochem. 2000;287:136–143. doi: 10.1006/abio.2000.4838. [DOI] [PubMed] [Google Scholar]
- 16.Young SP, Stevens RD, An Y, Chen YT, Millington DS. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Anal Biochem. 2003;316:175–180. doi: 10.1016/s0003-2697(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Vogel M, Staller W, Buhlmeyer K. Left ventricular myocardial mass determined by cross-sectional echocardiography in normal newborns, infants, and children. Pediatr Cardiol. 1991;12:143–149. doi: 10.1007/BF02238520. [DOI] [PubMed] [Google Scholar]
- 19.Turner CT, Hopwood JJ, Brooks DA. Enzyme replacement therapy in mucopolysaccharidosis I: altered distribution and targeting of alpha-L-iduronidase in immunized rats. Mol Genet Metab. 2000;69:277–285. doi: 10.1006/mgme.2000.2979. [DOI] [PubMed] [Google Scholar]
- 20.Raben N, Nagaraju K, Lee A, et al. Induction of tolerance to a recombinant human enzyme, acid alpha-glucosidase, in enzyme deficient knockout mice. Transgenic Res. 2003;12:171–178. doi: 10.1023/a:1022998010833. [DOI] [PubMed] [Google Scholar]
- 21.Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- 23.Richards SM. Immunologic considerations for enzyme replacement therapy in the treatment of lysosomal storage disorders. Clin Appl Immunol Rev. 2002;2:241–253. [Google Scholar]
- 24.Porter S. Human immune response to recombinant human proteins. J Pharm Sci. 2001;90:1–11. doi: 10.1002/1520-6017(200101)90:1<1::aid-jps1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Vedder AC, Breunig F, Donker-Koopman WE, et al. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol Genet Metab. 2008;94:319–325. doi: 10.1016/j.ymgme.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Hollak CE, Linthorst GE. Immune response to enzyme replacement therapy in Fabry disease: impact on clinical outcome? Mol Genet Metab. 2009;96:1–3. doi: 10.1016/j.ymgme.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Richards SM, Olson TA, McPherson JM. Antibody response in patients with Gaucher disease after repeated infusion with macrophage-targeted glucoce-rebrosidase. Blood. 1993;82:1402–1409. [PubMed] [Google Scholar]
- 28.Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 29.van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 30.de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab. 2010;101:338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Dickson P, Peinovich M, McEntee M, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez NI, Hoots WK. Advances in hemophilia: experimental aspects and therapy. Pediatr Clin North Am. 2008;55:357–376. viii. doi: 10.1016/j.pcl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Casadevall N. Antibodies against rHuEPO: native and recombinant. Nephrol Dial Transplant. 2002;17(suppl 5):42–47. doi: 10.1093/ndt/17.suppl_5.42. [DOI] [PubMed] [Google Scholar]
- 34.Rudick RA, Simonian NA, Alam JA, et al. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG) Neurology. 1998;50:1266–1272. doi: 10.1212/wnl.50.5.1266. [DOI] [PubMed] [Google Scholar]
- 35.Marshall MO, Heding LG, Villumsen J, et al. Development of insulin antibodies, metabolic control and B-cell function in newly diagnosed insulin dependent diabetic children treated with monocomponent human insulin or monocomponent porcine insulin. Diabetes Res. 1988;9:169–175. [PubMed] [Google Scholar]
- 36.Rougeot C, Marchand P, Dray F, et al. Comparative study of biosynthetic human growth hormone immunogenicity in growth hormone deficient children. Horm Res. 1991;35:76–81. doi: 10.1159/000181877. [DOI] [PubMed] [Google Scholar]
- 37.Kempton CL, White GC., 2nd How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113:11–17. doi: 10.1182/blood-2008-06-160432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.