Abstract
Based on previous SAR studies on N-benzylindole and barbituric acid hybrid molecules, we have synthesized a series of aromatic substituted 5-((1-benzyl-1H-indol-3-yl)methylene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione analogs (3a–i) and evaluated them for their in vitro growth inhibition and cytotoxicity against a panel of 60 human tumor cell lines. Compounds 3c, 3d, 3f and 3g were identified as highly potent anti-proliferative compounds against ovarian, renal and breast cancer cell lines with GI50 values in low the nanomolar range. The 4-methoxy-N-benzyl analog (3d) was the most active compound with GI50 values of 20 nM and 40 nM against OVCAR-5 ovarian cancer cells and MDA-MB-468 breast cancer cells, respectively. Two other analogs, 3c (the 4-methyl-N-benzyl analog) and 3g (the 4-fluoro-N-benzyl analog) exhibited equimolar potency against MDA-MB-468 cells GI50 = 30 nM). Analog 3f (the 4-chloro-N-benzyl analog) exhibited a GI50 of 40 nM against renal cancer cell line A498. These results suggest that aromatic substituted N-benzylindole dimethylbarbituric acid hybrids may have potential for development as clinical candidates to treat a variety of solid tumors.
Keywords: N-Benzyl indole, Dimethylbarbituric acid, Percentage growth inhibition, Growth inhibitory activity (GI50), Lethal concentration (LC50)
Cancer is the second most life threatening disease after cardiovascular disease, affecting more than six million people per year worldwide.1 Drastic changes in life style during the end of the 19th century has increased the risk of humans developing different types of cancers. Also, considerable effort has been put into identifying molecules with anti-cancer properties from both natural and synthetic sources.
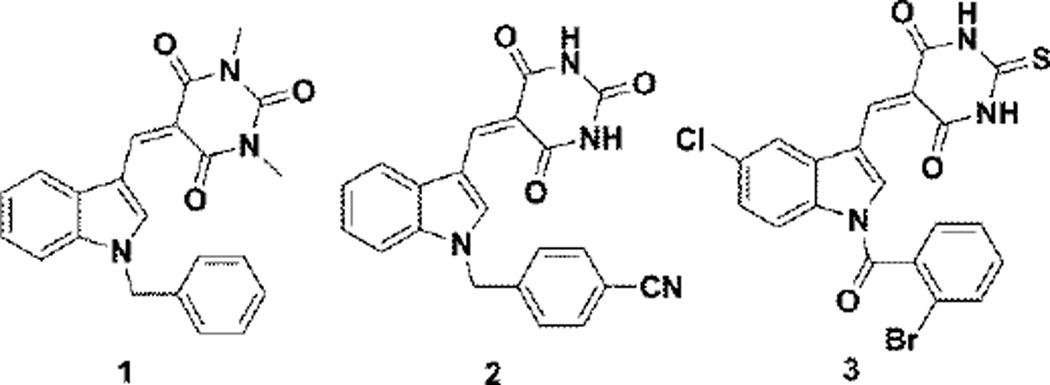
Indole and barbituric acids derivatives are known to have a wide range of beneficial biological activities such as anti-cancer,2 anti-inflammatory,3 anti-convulsant,4 anti-psychotic,5 anti-hypertensive,6 and anti-bacterial properties.7 Singh et al. have synthesized and evaluated some novel N-benzyl indole-barbituric acid hybrid molecules against a panel of 60 human tumor cell lines. They identified compound 1 (Fig. 1) as a promising lead compound with significant tumor growth inhibitory activity (GI50) against a variety of human cancer cell lines; the molecule also had good maximum tolerable dose (MTD) characteristics.8 Our laboratory has also reported on several novel indole barbiturates as anti-cancer and radio-sensitization agents9,10 (compound 2, Fig 1). Recently,11 we have reported that N-aroyl indole thiobarbituric acids (compound 3, Fig. 1) possess both anticancer and anti-inflammatory properties. Several of these analogs are also inhibitors of DNA repair and replication stress response polymerases.12
Figure 1.
In our continuing studies on improving the potencies of newly identified anti-cancer leads, we now report on the synthesis and antiproliferative properties of some aromatic substituted 5-(indolin-3-ylmethylene)-1,3-dimethylpyrimidine-2,4,6-triones as second generation indole barbituric acid hybrids.
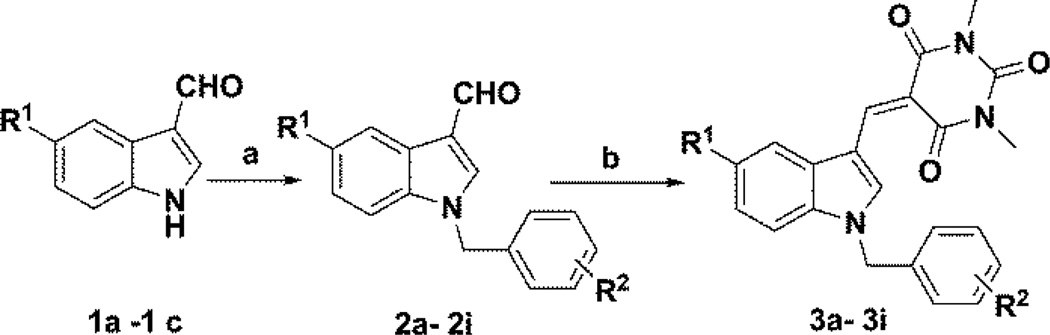
A series of N-benzylindole-3-carboxaldehydes (2a–i) were synthesized by reacting an appropriate indole carboxaldehyde (1a–c) with various aromatic substituted benzyl halides utilizing the phase transfer catalyst triethylbenzyl ammonium chloride (TEBAC) in a mixture of 50% w/v aq. NaOH solution and dichloromethane. The resulting N-benzyl products were obtained in 80–85% yield.5 The N-benzylindole-3-carboxaldehydes (2a–i) (1 mmol) were each then reacted with N,N-dimethylbarbituric acid (1.2 mmol) in methanol at room temperature to afford a series of 5-((1-benzyl-1H-indol-3-yl)methylene)-1,3-dimethyl-pyrimidine-2,4,6-(1H,3H,5H)-trione analogs (3a–i) (Scheme 1) in 75–90% yield. The synthesized compounds were fully characterized by 1H NMR and 13C NMR spectrometric analysis.13
Scheme 1.
Reagents and conditions: (a) appropriate benzyl halide, 50% NaOH, CH2Cl2, TEBAC, 2hrs; (b) dimethylbarbituric acid in methanol, room temp, 75–90% yield
In vitro screening of the above compounds was carried out against a panel of 60 human tumor cell lines utilizing the procedure described by Rubinstein et al.14 Compounds 3a–i were initially screened at 10−5 M to determine growth inhibition and cytotoxicity properties. Compounds 3c, 3d, 3f and 3g showed more than 60% growth inhibition in at least eight cell lines from the panel of sixty cell lines, and were selected for a complete dose response study at five different concentrations, viz. 10−4 M, 10−5 M, 10−6 M, 10−7 M and 10−8 M.
The growth inhibitory or cytotoxicity effect of the test compounds in the above cellular assay is measured by determining percentage cell growth (PG) inhibition. Optical density (OD) measurements of SRB-derived color just before exposing the cells to the test compound (ODtzero) and after 48hrs exposure to the test compound (ODtest) or the control vehicle (ODctrl) are recorded.15
Growth percentage is calculated utilizing one of the two formulas below. A negative growth percentage implies cytotoxicity.
The four compounds selected for full dose response studies were effective against lung cancer cell line NCI-H226, renal cancer cell line A498 and breast cancer cell line MDA-MB-468 in the single dose screen (Table 2). Activities of all four compounds against tumor cell line A498 was good, with ~ −90 percentage growth at 10µM. Although compounds 3h and 3e were not selected for complete dose-response studies, compound 3h was effective against the A498 cell line (−95 percentage growth), while compound 3e was active against the MDA-MB-468 cell line (−71 percentage growth) (Table 2).
Table 2.
Percentage growth inhibition of five human cancer cell lines by compounds (3a– 3i)a at 10 µM
| Cell line | Percentage Growth Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | |
| NCI-H226 | 98 | −32 | −63 | −55 | −72 | −82 | −78 | −79 | 88 |
| OVCAR-5 | 108 | 75 | −23 | −38 | 6 | −23 | −33 | −19 | 98 |
| A498 | 83 | 74 | −91 | −92 | 68 | −94 | −85 | −95 | 105 |
| TK-10 | 100 | 105 | −4 | −12 | 62 | −26 | −15 | 19 | 113 |
| MDA-MB-468 | 88 | 56 | −62 | −65 | −71 | −71 | −71 | −70 | 99 |
If (ODtest - ODtzero) ≥ 0. Then PG is shown as positive. If (ODtest - ODtzero) < 0, PG is shown as negative, implying cell death
Further evaluation of lead compounds 3c, 3d, 3f and 3g in the five dose screen showed that these compounds were very effective against five particular cancer cell lines: NCI-H460, OVCAR-5, A498, TK-10 and MDA-MB-468, with GI50 values in the nanomolar range. Breast cancer cell line MDA-MB-468 appeared to be the most sensitive to the growth inhibition effects of these compounds; 3c, 3d, 3f and 3g exhibited GI50 values of 30 nM, 40 nM, 60 nM, and 30 nM, respectively, with LC50 values of 620nM, 760nM, 700 nM, and 500 nM, respectively, against this cell type. Compounds 3c, 3d, 3f and 3g also exhibited good growth inhibition against renal cancer cell line A498, with GI50 values of 120nM, 60nM, 40nM, and 70 nM, respectively, and LC50 values of 690 nM, 527 nM, 640 nM, and 670 nM, respectively. All four compounds were active against renal cancer cell line TK-10 with GI50 values of 280 nM, 100 nM, 180 nM, and 590 nM, respectively, and also exhibited growth inhibitory effects against ovarian cancer cell line OVCAR-5 (GI50=70 nM, 20 nM, 160 nM, and 110 nM, respectively) and non-small cell lung cancer cell line NCI-H460 (GI50=910 nM, 810 nM, 400 nM, and 370 nM, respectively). Compound 3d also inhibited the growth of colon cancer cell line COLO 205 (GI50=630 nM) and melanoma cell line UACC-62 (GI50=900 nM).
In conclusion, a series of novel aromatic substituted 5-((1-benzyl-1H-indol-3-yl)-methylene)-1,3-dimethylpyrimidine-2,4,6-(1H,3H,5H)-trione analogs have been synthesized and evaluated for growth inhibition properties against a panel of 60 human cancer cell lines, and their GI50 and LC50 values have been determined. Four lead compounds (3c, 3d, 3f and 3g) have been identified with GI50’s in the nanomolar range against 5 different cell lines. All four compounds exhibited GI50 values in the range 30–60 nM and 40–120 nM against breast cancer MDA-MB-468 and renal cancer A49 cell lines, respectively; compounds 3c and 3d afforded GI50 values of 70 nM and 20 nM, respectively, against the ovarian cancer cell line OVCAR-5. The above four compounds generally have superior GI50 values compared to the previous lead compound 1 against most of the cell lines in the 60 tumor cell line panel. The biggest difference was the GI50 value of compound 1 against renal cancer cell line A49 (GI50=300 nM) compared to GI50 values over the range 40–120 nM for compounds 3c, 3d, 3f, and 3g. These novel aromatic substituted 5-((1-benzyl-1H-indol-3-yl)-methyl-ene)-1,3-dimethylpyrimidine-2,4,6-(1H,3H,5H)-triones represent promising new analogs that may have clinical potential in treating a variety of solid tumors.
Table 1.
5-((1-benzyl-1H-indol-3-yl)methylene)-1,3-dimethyl-pyrimidine-2,4,6(1H,3H,5H)-trione analogs (3a– 3i)
| Compound | R1 | R2 |
|---|---|---|
| 3a | COOCH3 | p-SO2Ph |
| 3b | H | o-Br |
| 3c | H | p-CH3 |
| 3d | H | p-OCH3 |
| 3e | H | p-CN |
| 3f | H | p-Cl |
| 3g | H | p-F |
| 3h | Cl | p-F |
| 3i | COOCH3 | p-COOCH3 |
If (ODtest - ODtzero) ≥ 0, then PG = 100 × (ODtest - ODtzero)/(ODctrl - ODtzero) and percentage growth is shown as positive.
If (ODtest - ODtzero) < 0, then PG = 100 × (ODtest - ODtzero)/ODtzero and percentage growth is shown as negative, which implies cell death.15
Table 3.
Growth inhibition (GI50/µM)a and cytotoxicity (LC50/µM)b data for compounds 3c, 3d, 3f and 3g against human cancer cells
| Panel/cell line | 3c | 3d | 3f | 3g | ||||
|---|---|---|---|---|---|---|---|---|
| GI50 | LC50 | GI50 | LC50 | GI50 | LC50 | GI50 | LC50 | |
| Leukemia | ||||||||
| K-562 | 2.23 | >100 | 2.18 | >100 | NA | >100 | NA | >100 |
| SR | 1.89 | >100 | 5.43 | >100 | 70.3 | >100 | >100 | >100 |
| Non-Small cell lung cancer | ||||||||
| HOP-62 | 7.44 | >100 | 6.22 | >100 | 10.7 | >100 | 7.70 | >100 |
| NCI-H226 | 1.95 | >100 | 1.44 | 53.9 | 1.26 | >100 | 2.56 | >100 |
| NCI-H23 | 8.19 | >100 | 4.67 | >100 | 10.2 | >100 | 51.5 | >100 |
| NCI-H460 | 0.91 | >100 | 0.81 | 82.8 | 0.40 | >100 | 0.37 | >100 |
| Colon cancer | ||||||||
| COLO 205 | 1.89 | >100 | 0.63 | 45.3 | 2.78 | 29.5 | 1.44 | >100 |
| HCT-116 | 5.45 | >100 | 5.01 | >100 | 4.47 | >100 | 6.26 | >100 |
| SW-620 | NA | >100 | 6.89 | >100 | >100 | >100 | >100 | >100 |
| CNS cancer | ||||||||
| SF-295 | 2.02 | >100 | 1.54 | >100 | 1.21 | >100 | 1.97 | >100 |
| SNB-19 | 26.8 | >100 | 15.8 | >100 | 18.3 | >100 | 26.7 | >100 |
| Melanoma | ||||||||
| LOX IMVI | 27.8 | >100 | 5.45 | >100 | 17.3 | >100 | >100 | >100 |
| MDA-MB-435 | NA | >100 | 3.29 | >100 | >100 | >100 | >100 | >100 |
| SK-MEL-2 | 3.36 | >100 | 2.78 | 6.25 | 2.84 | 75.8 | 6.35 | >100 |
| SK-MEL-5 | 9.17 | >100 | 5.08 | 64.4 | >100 | >100 | >100 | >100 |
| UACC-257 | 2.02 | >100 | 1.54 | 38.2 | 2.01 | >100 | 2.52 | >100 |
| UACC-62 | 2.42 | >100 | 0.90 | 68 | 2.06 | >100 | 2.89 | >100 |
| Ovarian cancer | ||||||||
| IGROV1 | 2.95 | >100 | 1.93 | >100 | 2.88 | >100 | 4.33 | >100 |
| OVCAR-5 | 0.07 | >100 | 0.02 | >100 | 0.16 | >100 | 0.11 | >100 |
| OVCAR-8 | >100 | >100 | 13.4 | 88.6 | 21.3 | >100 | >100 | >100 |
| NCI/ADR-RES | 6.23 | >100 | 3.59 | >100 | 16.3 | >100 | >100 | >100 |
| SK-OV-3 | 23.7 | >100 | 5.03 | >100 | 6.08 | >100 | 15.3 | >100 |
| Renal cancer | ||||||||
| 786-0 | 22.1 | >100 | 11.2 | >100 | 6.90 | >100 | 22.7 | >100 |
| A498 | 0.12 | 0.69 | 0.06 | 5.27 | 0.04 | 0.64 | 0.07 | 0.67 |
| RXF 393 | 15.2 | >100 | 14.8 | >100 | 12.5 | >100 | 14.9 | >100 |
| SN12C | >100 | >100 | 12.5 | >100 | 92.1 | >100 | >100 | >100 |
| TK-10 | 0.28 | >100 | 0.10 | 72.5 | 0.18 | 50.4 | 0.59 | >100 |
| Breast cancer | ||||||||
| MCF7 | 6.76 | >100 | 5.26 | >100 | >100 | >100 | >100 | >100 |
| MDA-MB-231/ATCC | 13.6 | >100 | 5.86 | >100 | 12.2 | >100 | 23.6 | >100 |
| BT-549 | 13.5 | >100 | 4.94 | >100 | 12.5 | >100 | 24.1 | >100 |
| T-47D | 5.00 | >100 | 3.53 | >100 | 3.53 | >100 | 5.38 | >100 |
| MDA-MB-468 | 0.03 | 0.62 | 0.04 | 0.76 | 0.06 | 0.70 | 0.03 | 0.50 |
NA: Not analyzed
GI50: 50% Growth inhibition, concentration of drug resulting in a 50% reduction in net protein increase compared with control cells.
LC50: Lethal concentration, concentration of drug lethal to 50% of cells
Acknowledgement
We thank Dr. Howard Hendrickson for providing the HRMS data. This research was supported by NIH/National Cancer Institute grant CA140409, and by an Arkansas Research Alliance grant. We are grateful to the National Cancer Institute for anticancer screening data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Parkin DM, Laara E, Muir CS. Int. J. Cancer. 1988;41:184. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- 2.Penthala NR, Yerramreddy TR, Madadi NR, Crooks PA. Bioorg. Med Chem. Lett. 2010;20:4468. doi: 10.1016/j.bmcl.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radwan MAA, Ragab EA, Sabry NM, El-Shenawy SM. Bioorg. Med. Chem. 2007;15:3832. doi: 10.1016/j.bmc.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. New Delhi: Mc Graw-Hill; 1991. pp. 358–360. [Google Scholar]
- 5.Madadi NR, Penthala NR, Brents LK, Ford BM, Prather PL, Crooks PA. Bioorg. Med Chem. Lett. 2013;23:2019. doi: 10.1016/j.bmcl.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min-Kyu P, Yun-Hee R, Hyo-Jung L, Eun-Ok L, Kwan-Hyun K, Min-Jong P, Byung-Hun J, Bum-Sang S, Chang-Hyun J, Seung-Hoon C, Kyoo-Seok A, Sung-Hoon K. Phytother. Res. 2008;22:58. [Google Scholar]
- 7.Kumar VG, Govindaraju K, Singaravelu G, Adhikesavalu D. Journal of Biopesticides. 2009;2:217. [Google Scholar]
- 8.Singh P, Kaur M, Verma P. Bioorg. Med Chem. Lett. 2009;19:3054. doi: 10.1016/j.bmcl.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Penthala NR, Yerramreddy TR, Crooks PA. Bioorg. Med Chem. Lett. 2011;21:1411. doi: 10.1016/j.bmcl.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayakumar NS, Reddy YT, Sekhar KR, Soumya S, Freeman ML, Crooks PA. Bioorg. Med. Chem. Lett. 2007;17:6821. doi: 10.1016/j.bmcl.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penthala NR, Purushothama RP, Vinod K, Crooks PA. Bioorg. Med Chem. Lett. 2013;23:1442. doi: 10.1016/j.bmcl.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coggins GE, Maddukuri L, Penthala NR, Hartman JR, Eddy S, Ketkar A, Crooks PA, Eoff RL. Chem. Biol. 2013;8:1722. doi: 10.1021/cb400305r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.General experimental procedure: In a 50ml round bottom flask the appropriate indole carboxaldehyde (1 mmol), benzyl halide (1.1 mmol), triethylbenzyl ammonium chloride (0.01 mmol) and 50% w/v aq NaOH were added to 5 volumes of DCM. The reaction mixture was stirred at room temperature and monitored by TLC. When the reaction was completed, water was added and the mixture extracted into DCM. The organic layer was concentrated under reduced pressure at 40°C and the residue was purified by flash chromatography using methanol/DCM as mobile phase to afford the corresponding N-benzylindole 3-carboxaldehydes in 80–85% yield. The N-benzylindole-3-carboxaldehyde (1 mmol) and N,N-dimethylbarbituric acid (1.2 mmol) were added to 10 volumes of methanol and the resulting mixture stirred at room temperature. The final product, the appropriate 5-((1-benzyl-1-H-indol-3-yl)methylene)-1,3-dimethyl-pyrimidine-2,4,6(1H, 3H,5H)-trione crashed out of the solution once the reaction was complete (1–2 hrs). The final product was filtered and recrystallized from methanol to afford the 5-((1-benzyl-1-H-indol-3-yl)methylene)-1,3-dimethyl-pyrimidine-2,4,6-(1H,3H 5H)-trione in 75–90% yield. Analytical data for compound 3d: Yellow solid; Yield 90%: mp >300 °C, 1H NMR (400 MHz, DMSO-d6): δ 3.36 (s, 6H N-CH3, 3.70 (s, 3H, -OCH3), 5.45 (s, 2H, -CH2), 6.89–6.91 (d, J =8.8 Hz, 2H, ArH), 7.23–7.31 (m, J =30 Hz, 4H, ArH), 7.61–7.63 (d, J =7.6 Hz, 1H, ArH), 8.09–8.12 (d, J =7.6 Hz, 1H, ArH), 8.45 (s, 1H, ArH), 9.93 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6): δ 49.69, 55.44, 111.85, 111.96 114.52, 117.74, 121.46, 121.53, 122.97, 124.01, 125.23, 128.99, 129.45, 137.31, 141.21, 159.31, 185.09. HRMS (ESI): m/z calcd for C23H22N3O4 [M-H] 404. 1610; found 404.1606. Compound 3g: Yellow solid; Yield 85%; mp >300 °C, 1H NMR (400 MHz, DMSO-d6): δ 3.24 (s, 6H, -N-CH3), 5.70 (s, 2H, -CH2), 7.17–7.22 (t, J =17.6 Hz 2H, ArH), 7.34–7.40 (m, J =25.6 Hz 4H, ArH), 7.67–7.69 (d, J =8Hz 1H, ArH), 7.87–7.89 (d, J =8.4Hz 1H, ArH), 8.75 (s, 1H, ArH), 9.66 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6): 28.22, 28.85, 50.02, 109.33, 111.43, 112.61, 118.34, 118.43, 123.63, 124.47, 129.26, 129.65, 130.42, 133.00, 135.87, 136.77, 142.42, 144.15, 151.66, 162.03, 163.38. HRMS (ESI): m/z calcd for C22H19N3O3F [M-H] 392. 1410; found 392.1389. 3f: Yellow solid; Yield 90% mp >300 °C, 1H NMR (400 MHz, DMSO-d6): δ 3.24–3.25 (d, J =3.6 Hz 6H, N-CH3), 5.71 (s, 2H, -CH2), 7.31–7.35 (m, J =16.8 Hz, 4H, ArH), 7.41–7.43 (d, J =8 Hz, 2H, ArH), 7.63–7.65 (d, J =8 Hz, 1H, ArH), 7.87–7.89 (d, J =6.4 Hz, 1H, ArH), 8.75 (s, 1H, ArH), 9.66 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6): δ 28.22, 28.27, 28.85, 28.90, 50.02, 109.34, 111.43, 112.61, 118.35, 118.44, 123.63, 124.47, 129.26, 129.66, 130.43, 133.00, 135.88, 136.77, 142.42, 144.16, 151.66, 162.03, 163.39. HRMS (ESI): m/z calcd for C22H19N3O3Cl [M-H] 408. 1115; found 408.1122. 3c: Yellow solid; Yield 90%; mp >300 °C, 1H NMR (400 MHz, DMSO-d6): δ 2.24 (s, 3H, -CH3), 3.23–3.24 (d, J =3.2 Hz, 6H, N-CH3), 5.62 (s, 2H, -CH2), 7.14–7.16 (d, J =7.6 Hz, 2H, ArH), 7.20–7.22 (d, J =7.6 Hz, 2H, ArH), 7.32–7.33 (t, J =5.6 Hz, 2H, ArH), 7.63–7.65 (d, J =7.6 Hz, 1H, ArH), 7.85–7.86 (d, J =6.8 Hz, 1H, ArH), 8.73 (s, 1H, ArH), 9.64 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6): δ 28.21, 28.84, 109.16, 111.34, 118.47, 123.46, 123.75, 127.68, 127.92, 129.72, 129.82, 129.90, 130.47, 133.78, 136.90, 137.67, 142.38, 142.56, 144.32, 151.72, 162.09, 163.47. HRMS (ESI): m/z calcd for C23H22N3O3 [M-H] 388. 1661 found 388.1636.
- 14.Rubinstein LV, Shoemaker RH, Paull KD, Simo RM, Tosini S, Skehan P, Scudiero PA, Monks A, Boyd MR. J. Natl. Cancer Inst. 1990;82:1113. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 15.NCI screening services. www.dtp.nci.nih.gov.