Abstract
Methemoglobinemia is a rare cause of tissue hypoxia that can quickly become fatal without immediate recognition and prompt treatment. It refers to an increase in methemoglobin in the red blood cells, which can be due to genetic deficiency of the enzymes responsible for reducing hemoglobin or can develop after exposure to oxidizing agents or xenobiotics. Local anesthetics, particularly benzocaine, have long been implicated in the formation of methemoglobin. Benzocaine is used for teething pain as well as before invasive procedures such as intubation and transesophageal echocardiogram. In this case report, we describe a patient with acute appendicitis who developed severe methemoglobinemia following use of benzocaine during an emergent intubation. Our objective is to increase awareness of this rare but potentially fatal complication associated with the use of this anesthetic.
Methemoglobin is found in small quantities in red blood cells (RBCs) in the normal physiologic state. The reductive capacity of the RBCs can be compromised when exposed to overwhelming oxidative stress. Typical symptoms of methemoglobinemia range from confusion and dizziness to arrhythmias, coma, and death. Methylene blue is the recommended treatment for severe cases of methemoglobinemia. However, for this treatment to be effective, it must be given immediately, which requires prompt recognition of the condition. We describe a patient who developed severe methemoglobinemia from benzocaine, which was used during intubation in preparation for surgery. As of 2011, the Food and Drug Administration had reported 319 cases of methemoglobinemia related to benzocaine, including seven cases of death and 32 cases categorized as life-threatening (1).
CASE DESCRIPTION
A 56-year-old white woman with known gluten and lactose intolerance was admitted with severe right-sided abdominal pain, nausea, vomiting, and fever. Appendicitis was diagnosed and an uneventful laparoscopic appendectomy was performed. On the second postoperative day, the patient became hypotensive and tachypneic and developed bilateral pleural effusions followed by profound respiratory failure requiring emergent intubation, which was traumatic and caused significant damage to the oral mucosa. Benzocaine was used during the intubation procedure. The patient developed significant cyanosis immediately after intubation. Blood drawn for arterial blood gas (ABG) tests was chocolate brown. Methemoglobinemia was diagnosed, and methylene blue was promptly administered. Prior to administration of methylene blue, the methemoglobin concentration was 45%, with an increased anion gap metabolic acidosis (Tables 1 and 2). The cyanosis and methemoglobinemia resolved over 2.5 hours following methylene blue administration. The patient was then taken to the operating room for emergent exploratory laparotomy. A small amount of murky fluid was collected in the abdomen and sent for culture. No evidence of intestinal leak or bleeding was found.
Table 1.
Arterial blood gas results after emergent intubation
| Test | Result |
|---|---|
| pH | 7.32 |
| Partial pressure of carbon dioxide (mm Hg) | 25 |
| Partial pressure of oxygen (mm Hg) | 116.7 |
| Bicarbonate (mEq/L) | 12.7 |
| Carbon monoxide (%) | 0 |
| Methemoglobin (%) | 44.9% |
| Oxygen (%) | 54.6% |
Table 2.
Laboratory workup done at admission
| Blood test | Results |
|---|---|
| White blood cells (K/μL) | 13.8 |
| Hemoglobin (g/dL) | 14.9 |
| Hematocrit (%) | 40.9 |
| Platelets (per μl) | 48 |
| Sodium (mEq/L) | 138 |
| Potassium (mEq/L) | 4.4 |
| Chloride (mEq/L) | 98 |
| Bicarbonate (mEq/L) | 13 |
| Blood urea nitrogen (mg/dL) | 14 |
| Creatinine (mg/dL) | 1.4 |
| Anion gap (mEq/L) | 27 |
| Glucose (mg/dL) | 32 |
Her postoperative course was complicated by multiorgan failure with respiratory and hepatic failure, lactic acidosis, rhabdomyolysis, and hemodynamic instability requiring norepinephrine. Continued hypotension led to acute kidney injury requiring continuous veno-venous hemodialysis. Her pulmonary function continued to worsen, and she was switched to high-frequency oscillator ventilation to maintain oxygenation. Chest tubes were also placed for bilateral pulmonary effusions. She developed sepsis and was placed on broad-spectrum antibiotics. On hospital day 7, the patient was weaned from vasopressors, but she remained unresponsive and had severely impaired neurologic function. An electroencephalogram confirmed anoxic brain injury, and the patient died.
DISCUSSION
Red blood cells are under constant oxidative stress by being exposed to drugs and oxygen, as well as byproducts of intracellular metabolism. In a normal physiologic state, there is roughly 1% methemoglobin in the RBCs. This amount is kept in check by reducing enzymes within the erythrocytes (2, 3).
The human body has three main mechanisms for reducing methemoglobin. The nicotinamide adenine dinucleotide (NADH) pathway, catalyzed by cytochrome b5 reductase, is the most important. It is responsible for reduction of up to 95% of the methemoglobin (4). This mechanism efficiently reduces the ferric atom in methemoglobin by transferring an additional electron from NADH to methemoglobin. Another reductive mechanism utilizes G6P dehydrogenase and its capability to produce nicotine adenine dinucleotide phosphate (NADPH), which can lead to the reduction of methemoglobin (3, 4) (Figure 1). A third minor and nonenzymatic pathway that works to reduce methemoglobin involves reduced glutathione, ascorbic acid, and cysteine.
Figure 1.
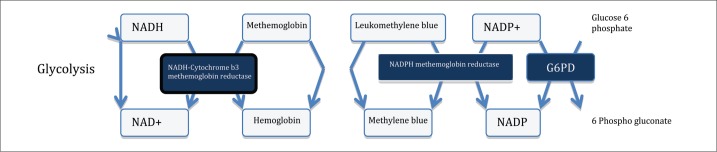
The mechanism of methemoglobin reduction including the methylene blue rescue pathway.
Methemoglobin becomes clinically relevant when the oxidative burden overwhelms the cellular capability for reduction. Patients usually become symptomatic when the level of methemoglobin exceeds 15%. Symptoms tend to positively correlate with the methemoglobin level.
Causes of methemoglobinemia can be divided into inherited defects of the oxidizing enzymes and acquired forms. The most common inherited forms are autosomal recessive conditions. Patients have decreased levels of NADH-cytochrome-b5 reductase. There is no reason to suspect that our patient had any genetic susceptibility to develop this condition.
A myriad of oxidizing compounds have been identified that can quickly overwhelm the reducing capabilities of the RBC. The list includes industrial dyes, nitrates, chlorates, herbicides, antibiotics such as dapsone and sulfonamides, as well as local anesthetics, notably benzocaine and prilocaine (2).
The diagnosis of methemoglobinemia is based on clinical symptoms and requires a high index of suspicion in patients with a known exposure. Patients will present with cyanosis out of proportion to the respiratory status, in the presence of normal arterial oxygen content (partial pressure of oxygen > 60 mm Hg) (2). No symptomatic improvement is seen with oxygen administration. The arterial blood draw is chocolate brown due to the oxidized hemoglobin. ABG will reveal an oxygen saturation gap (2). Methemoglobinemia should be suspected when the oxygen saturation from the ABG is higher than the oxygen saturation reported by pulse oximetry. A saturation gap of over 5% strongly suggests methemoglobinemia (5). The differential diagnosis of a saturation gap includes carbon monoxide poisoning as well as sulfhemoglobinemia (2).
A pulse oximeter emits monochromatic light at wavelengths of 660 (red) and 940 (infrared) nm. As light travels through the tissues, the pulsation of the arteries converts this light into an alternating pattern. This alternating light reaches a photodetector and is amplified. When light travels through a nonpulsatile tissue such as a vein, it is not changed to alternating and therefore not amplified by the photodetector. This allows pulse oximeters to detect only the hemoglobin in arteries (Figure 2).
Figure 2.
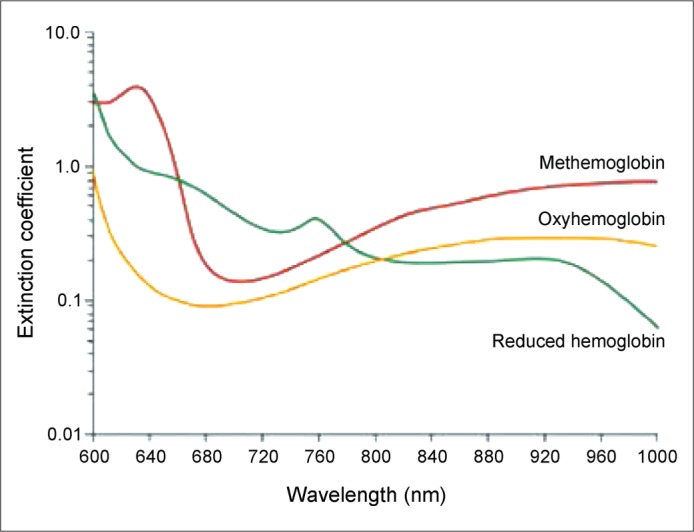
Hemoglobin extinction curves. Pulse oximetry uses the 660 and 940 nm wavelengths. As carboxyhemoglobin and oxyhemoglobin absorb equally at 660 nm, they both read the same oxygen saturation on a conventional pulse oximeter. From Huford W, Kratz A (2). Copyright © 2004 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Different hemoglobin species absorb light differently at both wavelengths, and the ratio of red to infrared absorption is calculated. When the two major hemoglobin molecules are oxygenated hemoglobin (oxyhemoglobin) and nonoxygenated hemoglobin (reduced hemoglobin), the ratio of absorbance at these two wavelengths can be converted to oxygen saturation. Methemoglobin absorbs light equally at these two wavelengths and therefore always has a ratio of absorption of 1, which equates to an oxygen saturation of 85% (5). Therefore, the pulse oximeter will display an oxygen saturation of approximately 85% regardless of the patient's real oxygenation status.
Newer oximeters called co-oximeters measure absorption at multiple wavelengths, which circumvents the issue seen with traditional pulse oximeters. Co-oximeters will determine the percentage of hemoglobin converted to methemoglobin and carboxyhemoglobin and also will provide an accurate estimate of the true oxygen saturation state of hemoglobin. However, these co-oximeters are not widely used (5).
Most cases of methemoglobinemia will resolve within 24 to 36 hours. In severe cases, particularly when the methemoglobin blood level is above 30%, prompt treatment with methylene blue is advised. Methylene blue is quickly reduced by NADPH methemoglobin reductase to leukomethylene blue, which then reduces methemoglobin to hemoglobin. This is called the methylene blue rescue pathway. This rescue pathway relies on an intact glucose-6-phosphate dehydrogenase (G6PD) system. In the rare event that the patient's status worsens after methylene blue administration, G6PD deficiency should be strongly suspected.
Benzocaine is still a widely used anesthetic. There are several different formulations, the spray form being the most commonly used. Benzocaine sprays are marketed under different brand names such as Hurricaine, Cetacaine, Exactacain, and Topex (1). The benzocaine concentration in these formulations ranges from 14% to 20%. The benzocaine dosage that can produce methemoglobinemia in adults has been estimated to be approximately 300 mg, with initial onset of symptoms within 20 to 60 minutes.
The patient presented was exposed to benzocaine during the second emergent intubation. It is unknown whether the anesthetic utilized in the first intubation included benzocaine. However, the benzocaine was administered to a mucosa that was likely traumatized during the first intubation, potentially increasing its absorption and causing a greater oxidative burden.
This patient likely had a gastrointestinal perforation from her prior episode of acute appendicitis. After the second intubation, it was difficult to wean her off the ventilator. ABG analysis showed her methemoglobin level rising to 45%. The methemoglobin level quickly declined over a period of 2.5 hours after receiving methylene blue. Unfortunately, the patient developed significant hemodynamic instability likely due to multiple factors, ultimately developed multiorgan failure and anoxic brain injury, and subsequently died.
References
- 1.US Food and Drug Administration. FDA continues to receive reports of a rare, but serious and potentially fatal adverse effect with the use of benzocaine sprays for medical procedures [Safety announcement, April 7, 2011] Available at http://www.fda.gov/Drugs/DrugSafety/ucm250040.htm.
- 2.Hurford WE, Kratz A. Case 23-2004. A 50-year-old woman with low oxygen saturation. N Engl J Med. 2004;351(4):380–387. doi: 10.1056/NEJMcpc049013. [DOI] [PubMed] [Google Scholar]
- 3.Chung NY, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: a case report. J Emerg Med. 2010;38(5):601–606. doi: 10.1016/j.jemermed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Janssen WJ, Dhaliwal G, Collard HR, Saint S. Clinical problem-solving. Why “why” matters. N Engl J Med. 2004;351(23):2429–2434. doi: 10.1056/NEJMcps040669. [DOI] [PubMed] [Google Scholar]
- 5.El-Husseini A, Azarov N. Is threshold for treatment of methemoglobinemia the same for all? A case report and literature review. Am J Emerg Med. 2010;28(6):748.e5–748.e10. doi: 10.1016/j.ajem.2009.10.014. [DOI] [PubMed] [Google Scholar]


