Abstract
VEGF-targeted therapy increases both the progression-free (PFS) and overall survival (OS) of patients with metastasized renal cell cancer (mRCC). Identification of molecular phenotypes of RCC could improve risk-stratification and the prediction of the clinical disease course. We investigated whether gene-specific DNA hypermethylation can predict PFS and OS among patients undergoing anti-VEGF-based therapy. Primary tumor tissues from 18 patients receiving targeted therapy were examined retrospectively using quantitative methylation-specific PCR analysis of CST6, LAD1, hsa-miR-124-3, and hsa-miR-9-1 CpG islands. PFS and OS were analyzed for first-line and sequential antiangiogenic therapies using the log rank statistics. Sensitivity and specificity were determined for predicting first-line therapy failure. Hypermethylation of CST6 and LAD1 was associated with both a shortened PFS (log rank p = 0.009 and p = 0.004) and OS (p = 0.011 and p = 0.043). The median PFS observed for the high and low methylation groups of CST6 and LAD1 was 2.0 vs.11.4 months. LAD1 methylation had a specificity of 1.0 (95% CI 0.65–1.0) and a sensitivity of 0.73 (95% CI 0.43–0.90) for the prediction of first-line therapy. CST6 and LAD1 methylation are candidate epigenetic biomarkers showing unprecedented association with PFS and OS as well as specificity for the prediction of the response to therapy. DNA methylation markers should be considered for the prospective evaluation of larger patient cohorts in future studies.
Introduction
Renal cell cancer (RCC) is one of the top ten causes of cancer deaths in industrial countries [1]. Though recent improvements in targeted therapy have resulted in prolonged survival of patients with metastatic RCC (mRCC), the overall outcome is still poor [2], [3].
Due to different available compounds and a growing number of new agents affecting molecularly targeted structures, such as vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) signaling [4] an optimal sequence of targeted therapies might exist for patients, potentially increasing survival with mRCC treatment. Although prognostic models, such as the MSKCC (Memorial Sloan Kettering Cancer Center) and Heng scoring systems, have been reported to be independent predictors of clinical outcome, [5], [6] discrimination between outcomes is still limited. Tumor-specific biologically based parameters have been suggested to improve these issues [7].
Most RCCs have clear cell (ccRCC) histology and exhibit functional inactivation of the von Hippel-Lindau (VHL) gene due to mutations or epigenetic silencing in approximately 80% of tumors [8], [9]. However, the progression-free (PFS) and overall survival (OS) of patients with mRCC are independent of the loss of VHL function [10]. In contrast, blood-based analysis of single nucleotide polymorphisms (SNPs) potentially affecting sunitinib target genes and ligands identified two polymorphisms in VEGFR3 that are associated with the PFS, but not OS, of patients undergoing targeted therapy [11]. Measurement of serum carbonic anhydrase IX (CA9) protein levels in metastatic ccRCC patients revealed significantly decreased OS among patients with higher CA9 serum concentrations [12]. An individual advantage of tissue- or blood-based measurements for patients undergoing therapy is perceptible, but the accuracy, sensitivity, and specificity of these methods have not yet been reported or validated [13].
Although large patient cohorts have been subjected to exom-wide mutational analyses, only a limited number of genes other than VHL and polybromo 1 (PBRM1) have been identified to have mutations in RCC, and most with low frequency [14]. Therefore, the limited number of frequently mutated genes reduces the probability of identifying mutation-based predictors with appropriate sensitivity and specificity. DNA methylation of CpG islands (CGIs) in a substantial number of regulatory or tumor suppressor genes has been identified as a functional surrogate of mutations and has been reported to be a frequent event in RCC [15]. Moreover, functional loss of VHL has been found to associate with broadened appearance of epigenetic alterations [16], and all of the second-frequent mutations are related to altered chromatin/histone stabilization or modification, mechanisms linked to CGI methylation and gene expression [17]. Therefore, the frequent detection of epigenetic alterations in RCC differentiates RCC tumor biology and provides candidates for novel diagnostic, prognostic, or predictive markers [18]. CGI methylation in several genes has already been identified as candidate prognosticators independent from clinicopathological parameters [19]–[21]. However, epigenetic biomarkers predicting the clinical course of mRCC patients subjected to targeted therapy, have, to the best of our knowledge, not been reported.
We hypothesized that CGI methylation is related to the response to therapy, as well as the survival of mRCC patients undergoing antiangiogenic therapy. We investigated four candidate genes, three of which, cystatin E/M (CST6) and the micro RNA genes miR-9-1 and miR-124-3, were identified recently with tumor-specific CGI hypermethylation and a possible association with the prognosis of RCC patients [19], [21], [22]. The Ladinin 1 (LAD1) gene was identified recently by our group as a new candidate methylation marker in RCC showing univariate association with adverse clinicopathological parameters such as tumor grade, lymph node metastasis, status of distinct metastasis and advanced disease (unpublished data).
LAD1 encodes an anchoring filament protein, a component of the basement membrane that likely contributes to the stability of the epithelial-mesenchymal interaction [23].
The present study investigated whether a DNA methylation mark can predict the response of targeted antiangiogenic therapy of mRCC patients and describes the identification of two DNA methylation markers in the CST6 and LAD1 CGIs as candidate epigenetic predictors of the PFS and OS of mRCC patients undergoing targeted therapy.
Materials and Methods
Ethics Statement
Informed consent was obtained from each patient, and the local ethics committee (Ethic Committee; Prof. H. D. Tröger, Hannover Medical School, Carl-Neuberg-Str. 1, Hannover, Germany; Study_No: 1213–2011) specifically approved this study. Patients agreed in a written form for utilization of tissue specimen for basic research. A written statement of our ethic committee for this study was obtained.
Patient Characteristics and Treatment Regimens
Clinicopathological data, corresponding tissues, and follow-up data including the PFS and OS of patients with mRCC who were treated with first-line VEGF-targeted therapy were collected between November 2005 and October 2011 in the Clinic of Hematology and the Department of Urology and Urologic Oncology at Hannover Medical School (Table 1). The MSKCC score or ECOG performance status were not available. Patients received the following treatment regimens in the first-line setting: sunitinib (n = 12, 67%), sorafenib (n = 4, 22%), axitinib (n = 1, 5.5%), and bevacizumab (n = 1, 5.5%).
Table 1. Patient characteristics.
| Patient No. | Age (years) | Sex | RCC type | iTNM status | First-line treatment | PFS (months) | OS (months) | *Response |
| 1 | 68 | F | clear cell | T1bNxMx | Sunitinib | 1.34 | 2.47 | NE |
| 2 | 66 | M | clear cell | T4N1Mx | Sunitinib | 1.77 | 1.77 | PD |
| 3 | 57 | M | clear cell | T3bNxMx | Sunitinib | 2.76 | 3.62 | PD |
| 4 | 59 | M | chromophobe | T4N2Mx | Sunitinib | 1.70 | 9.76 | PD |
| 5 | 72 | F | clear cell | T3bN0Mx | Sorafenib | 5.52 | 23.64 | SD |
| 6 | 48 | F | clear cell | T3NxMx | Sunitinib | 2.26 | 2.99 | PD |
| 7 | 62 | M | clear cell | T2aNxMx | Sunitinib | 2.63 | 3.25 | PD |
| 8 | 80 | F | clear cell | n.a. | Sunitinib | 0.88 | 26.14 | SD |
| 9 | 50 | M | clear cell | T2NxMx | Sorafenib | 11.86 | 13.68 | SD |
| 10 | 71 | F | clear cell | T1bN1M1 | Axitinib | 13.70 | 19.04 | SD |
| 11 | 69 | F | clear cell | T1bNxMx | Bevacizumab | 6.21 | 11.07 | SD |
| 12 | 57 | M | clear cell | T3N0Mx | Sunitinib | 11.50 | 29.77 | SD |
| 13 | 49 | F | clear cell | T3aNxM1 | Sunitinib | 11.27 | 26.86 | PR |
| 14 | 60 | M | clear cell | T3bNxMx | Sunitinib | 0.42 | 0.76 | NE |
| 15 | 54 | M | clear cell | T2aNxMx | Sorafenib | 3.03 | 13.05 | PD |
| 16 | 53 | M | clear cell | T1NxMx | Sorafenib | 43.66 | 59.28 | SD |
| 17 | 64 | M | clear cell | n.a. | Sunitinib | 30.31 | 59.24 | CR |
| 18 | 51 | M | papillary | T1aNxMx | Sunitinib | 1.08 | 3.42 | PD |
Note:
*Response: according to RECIST 1.1 criteria.
Sex: Male, Female.
NE: not evaluable du e to RECIST 1.1 criteria.
CR: complete response.
PR: partial response.
SD: stable disease.
PD: progressive disease.
Age: At the beginning of first-line therapy.
n.a: not available.
iTNM: initial TNM status of primary RCC.
PFS was defined as the time from the beginning of the first day of systemic therapy to the detection of a progressive event according to RECIST 1.1 criteria on a computer tomography (CT) scan [24]. OS was defined as the period from the first day of systemic therapy until the patient’s death or censored at the last follow-up. The terminus “not evaluable” in Table 1 describes patients with a PFS <2 months due to an early cessation of therapy caused by toxicity or early death before the first recommended CT scan after therapy was initiated. The initial TNM classification of primary tumors was evaluated according to the Union for International Cancer Control 2002 classification [25]. Patient follow-up included up to three sequence changes in the therapy regimen.
The terms ´prognostic` and ´predictivè were used according to the definition by the National Cancer Institute [26].
Tissue Specimens, Isolation, and Bisulfite Conversion of Tumor DNA
Independent control of histopathology, tumor cell content of routine pathological specimens, and the selection of tissue areas for tissue extraction were determined by the pathologist. Subsequently, cylinders 1.5 mm in length and 2 mm in height were stamped out from the formalin-fixed and paraffin-embedded (FFPE) tissue blocks using an 18 Charrière core stamp and subjected to DNA isolation. Genomic DNA was extracted using the automated MagNA Pure LC 2.0 system and MagNA Pure LC DNA isolation kit II - tissue (Roche Diagnostics Deutschland, Roche Applied Science, Mannheim, Germany). The quality of extracted DNA was assessed using spectrophotometry, gel electrophoresis, and quantitative PCR, which characterized the yield, purity, and grade of degradation of isolated DNA. Bisulfite conversion of DNA was carried out using the EZ DNA Methylation-Gold Kit (Zymo Research Corporation, Irvine CA, USA) and 1 µg of isolated DNA. Fully methylated and converted DNA, as well as unmethylated bisulfite-converted DNA controls, were used as reported previously [21].
Quantitative Methylation-specific Real-time PCR Analysis
Quantitative real-time fluorimetric 5′ exonuclease PCR (qMSP) assays were performed to quantify the CGI methylation levels of CST6, LAD1, hsa-miR-124-3, and hsa-miR-9-1. The methylation analysis of hsa-miR-124-3 was carried out as described previously [21]. qMSP systems were established for CST6, LAD1, and hsa-miR-9-1 using Beacon Designer software (PREMIER Biosoft, Palo Alto CA, USA). The base positions of investigated CGI sites for CST6, LAD1, hsa-miR-124-3, and hsa-miR-9-1 are presented in Table 2. The base positions refer to the USCS Genome Browser [27]. The qMSP systems were characterized as described for the hsa-miR-124-3 methylation measurements [21]. Duplicate real-time PCRs were performed on an ABI 7900HT (Life technologies, Foster City, USA) in 384-well plates as described previously [21]. Experimenters were blinded to the histopathological and clinical status of the samples. Relative methylation levels were calculated as an analogue of the ΔΔCt method by normalizing the difference in CGI methylation determined by real-time detection and independent internal control measurements to the corresponding difference in the fully methylated DNA control samples as described previously [21], [28].
Table 2. Gene informations.
| CST6 | LAD1 | miR-9-1 | miR-124-3 | |
| Chromosome | 11q13 | 1q32.1 | 1q22 | 20q13.33 |
| Name | Cystatin E/M | Ladinin 1 | micro RNA9-1 | micro RNA124-3 |
| GeneID | 1474 | 3898 | 407046 | 406909 |
| CpG Island | ||||
| # number of CpG sites | 59 | 54 | 99 | 424 |
| # base position (bp) | 65779312–65777967 | 201368561–201369032 | 156390404–156391581 | 61806255–61810867 |
| bp of CpG sitesinvestigated byqMSP | 65779535, ∼541, ∼600, ∼604,∼612, ∼620, ∼630, ∼640,∼644, ∼647 | 201368651, ∼669, ∼672, ∼689,∼693, ∼696, ∼700, ∼704, ∼713,∼725, ∼733 | 156390684, ∼701, ∼745,∼747, ∼753, ∼758,∼764, ∼783 | 61809002, ∼007, ∼026,∼035, ∼044, ∼059,∼065, ∼072 |
Note: Gene informations according to the USCS Genome Browser [27].
Statistical Analysis of Survival
Kaplan-Meier plots were used to present relative survival in the PFS and OS analyses following dichotomization of tumors into high and low methylation phenotypes. The median survival and corresponding 95% confidence intervals (CIs) were reported. Differences in PFS and OS were tested using log-rank statistics and median survival ratios calculated. P-values <0.05 were considered significant. To allow a comparison with the literature, univariate Cox regression analyses were performed to estimate hazard ratios (HRs). To calculate sensitivity and specificity for therapy failure, a PFS cutoff value of 6 months was used for dichotomization [29] into therapy responders and non-responders.
The heat map and receiver operating characteristic curves were constructed using the heatmap2 and ROCR function in the R package (version 2.11.0.1) with a default clustering algorithm and gplot package [30].
Results
Bimodal Distribution of Relative Methylation Levels in the CGIs of Candidate Genes
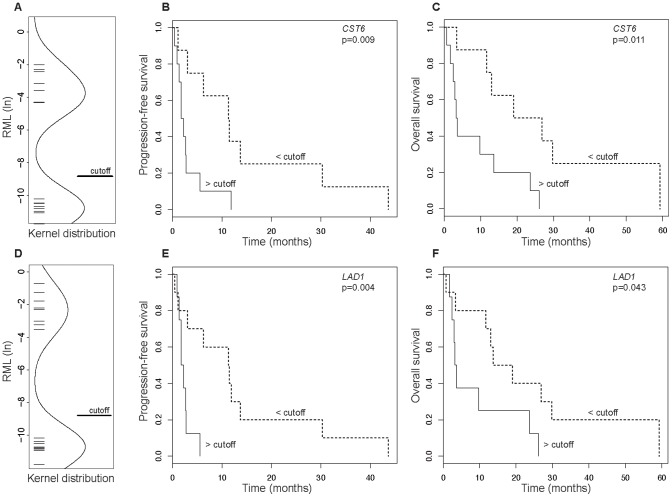
Quantitative methylation analyses of the CGIs of CST6, LAD1, hsa-miR-124-3, and hsa-miR-9-1 revealed the presence of a bimodal distribution of relative methylation values (Figure 1A and D; data not shown for hsa-miR-124-3 and hsa-miR-9-1). Applying a single cutoff value of 0.02% (corresponding to −8.75 in the ln-scale used for Kernel density plots in Figure 1A and D) for relative methylation, high and low methylated epigenotypes were uniformly distinguished for all of the analyzed genes and used for consistent dichotomization in survival analyses.
Figure 1. Survival analyses.
A and D. istribution of the relative methylation values of CST6 (A) and LAD1 (D) in mRCC patients. A cutoff value is presented for dichotomization. B and E. Kaplan-Meier plots of the progression-free survival of mRCC patients dichotomized by high and low methylation of CST6 (B) and LAD1 (E). C and F Kaplan-Meier plots of the overall survival of mRCC patients dichotomized by high and low methylation of CST6 (C) and LAD1 (F).
Analysis of PFS
Kaplan-Meier and log rank analysis of PFS in high and low methylated tumors demonstrated a significant difference for both CST6 and LAD1. High methylation was associated with a median survival of 2.0 months, compared to 11.4 months among patients with low methylation (p = 0.009 and p = 0.004, Table 3A). In contrast, neither miR-124-3 nor miR-9-1 demonstrated a statistical relationship with PFS (p = 0.339 and p = 0.319).
Table 3. Survival analyses.
| A) | PFS | Median survival (months, 95% CI) | Median survival ratio (high/low) | ||
| p-value* | low methylation | high methylation | HR (95% CI)** | ||
| CST6 | 0.009 | 11.4 (6.2–NE) | 2.0 (1.3–NE) | 0.175 | 4.1 (1.3–12.6) |
| LAD1 | 0.004 | 11.4 (3.0–NE) | 2.0 (1.7–NE) | 0.175 | 6.4 (1.6–26.0) |
| miR-124-3 | 0.339 | 11.9 (6.2–NE) | 2.6 (1.7–11.5) | 0.218 | 1.8 (0.5–6.6) |
| miR-9-1 | 0.319 | 4.6 (1.3–NE) | 2.7 (1.8–NE) | 0.587 | 1.7 (0.6–4.7) |
| B) | OS | Median survival (months, 95% CI) | Median survival ratio (high/low) | ||
| p-value * | low methylation | high methylation | HR (95% CI) ** | ||
| CST6 | 0.011 | 22.9 (13.1–NE) | 3.4 (2.5–NE) | 0.148 | 4.1 (13.0–13.4) |
| LAD1 | 0.043 | 16.4 (11.7–NE) | 3.4 (3.0–NE) | 0.207 | 2.9 (1.0–8.6) |
| miR-124-3 | 0.786 | 13.7 (11.7–NE) | 9.8 (3.2–29.8) | 0.715 | 0.8 (0.2–3.1) |
| miR-9-1 | 0.624 | 12.4 (3.4–NE) | 14.4 (3.2–NE) | 1.161 | 1.3 (0.5–3.6) |
Abbreviations:
PFS: Progression-free survival.
OS: Overall survival.
NE: not estimable.
HR: Hazard ratio.
CI: Confidence interval.
*: log-rank statistical analysis.
**: Univariate Cox regression for purpose of comparision.
low methylation cutoff <8.75.
high methylation cutoff ≥8.75.
Analysis of OS
Kaplan-Meier analysis and log rank statistics revealed that high methylation of CST6 and LAD1 was associated with impaired OS. A median OS of 22.9 and 3.4 months (p = 0.011, Table 3B) was obtained for low and high CST6 methylation, respectively. A median OS of 16.4 and 3.4 months (p = 0.043, Table 3B) was obtained for low and high LAD1 methylation.
Analysis of Sensitivity and Specificity
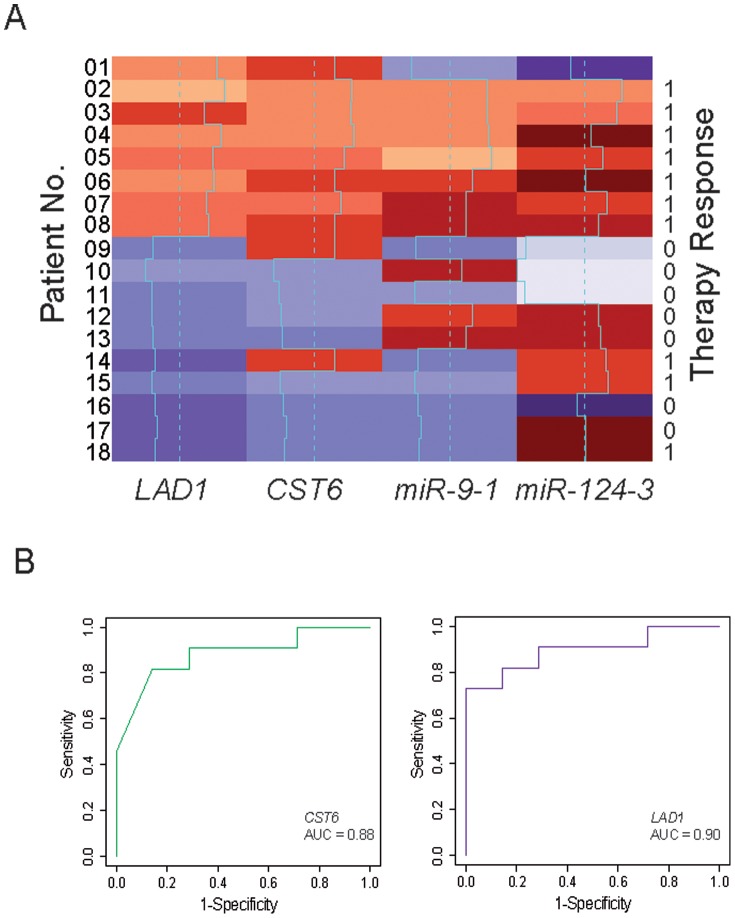
To determine the sensitivity and specificity of CST6 and LAD1 methylation analyses for predicting first-line therapy failure, methylation values were dichotomized into low and high methylation phenotypes using the same cutoff value of 0.02% as specified above. PFS values were dichotomized using a cutoff of 6 months, a parameter that was previously suggested to better distinguish between therapy responders and non-responders [29]. High methylation of LAD1 and CST6 was a characteristic of failed therapy (Figure 2A). In the case of LAD1, all eight patients with high methylation were non-responders. The specificity was 1.0 (95% CI 0.65–1.0) and sensitivity 0.73 (95% CI 0.43–0.90) for the detection of therapy failure using LAD1 methylation (Table 4), whereas the specificity was 0.86 (95% CI 0.49–0.97) and sensitivity 0.82 (95% CI 0.52–0.95) for CST6 methylation (Table 4).
Figure 2. Heat map illustration of therapy response and Receiver Operating Characteristic Curves for CST6 and LAD1.
A. Heat map of normalized relative methylation values (natural logarithm) detected in LAD1, CST6, miR-9-1, and miR-124-3 CGIs for each patient. Low to high methylation values are encoded as violet (low) to red (high) hues. The dashed and solid lines describe the median and individual methylation values, respectively. Patient numbers given on the left correspond to the numbering presented in Table 1. Therapy response (0) and therapy failure (1) are indicated for each patient on the right. Notably, all of the patients (no. 1–8) exhibiting high methylation of LAD1 and 9 of 10 patients (no. 1–9, 14) exhibiting high methylation (red colored) of CST6 were part of the non-responder (1) group. B. The receiver operating characteristics (ROC) curves illustrated the discrimination of methylation measurements and the area under the curve (AUC) shows that even with our small patient cohort, a robust result for the accuracy of both methylation markers (AUC CST6 = 0.88 and AUC LAD1 = 0.90) can be detected. The sensitivity (true positive rate) is plotted against 1-specificity (false positive rate).
Table 4. Sensitivity and specificity.
| Sensitivity | 95% CI | Specificity | 95% CI | p-value* | |
| CST6 | 0.818 | 0.52–0.95 | 0.857 | 0.49–0.97 | <0.001 |
| LAD1 | 0.727 | 0.43–0.90 | 1.000 | 0.65–1.00 | 0.004 |
| miR-124-3 | 1.000 | 0.74–1.00 | 0.429 | 0.16–0.75 | 0.339 |
| miR-9-1 | 0.636 | 0.35–0.85 | 0.571 | 0.25–0.84 | 0.319 |
CI: Confidence interval.
*log-rank test.
Discussion
The clinical outcomes of patients with mRCC have improved since VEGF-targeted therapies and mTOR inhibitors were made available [2], [3]. However, the stratification of patients using biomarkers could allow a better understanding of drug resistance and identify an optimized patient-specific sequence of antiangiogenic therapies, improving individual survival [7]. Moreover, the side effects of anti-VEGF-based regimens, such as diarrhea, rash, hand-foot syndrome, hypertension, and asthenia, which often severely impair quality of life during treatment can be minimized.
We found that DNA hypermethylation of CST6 and LAD1 in primary RCC tumor tissue is significantly associated with the PFS of patients receiving anti-VEGF-based medication as a first-line therapy and also the OS of patients sequentially treated with anti-VEGF targeted drugs and mTOR inhibitors in second- and third-line therapy. Our methylation markers predicted therapy failure with high specificity and good sensitivity.
To the best of our knowledge, these findings are unprecedented in several respects, as previous studies were not tissue based and either provided no significant association with therapy response [12] or reported only limited statistical power [11]. While serum measurements of CA9 levels revealed no significant differences between therapy responders and non-responders [12] the analysis of genetic variants, possibly interacting with the sunitinib pathway, identified two VEGFR3 SNPs to be associated with therapy response and PFS, but not with OS [11]. This study shows that individual biological variables may affect the response to therapy. On the other hand, our methylation-based candidate predictors go beyond the measurement of gene variants in several important aspects. First, the potential LAD1 and CST6 DNA methylation-based markers were measured in tumor cells, which directly exhibit tumor characteristics that may represent drivers of resistance and biological aggressiveness. Hypermethylation of CST6 and LAD1 exhibited prognostic and predictive value in our study and is a putative biomarker for patient selection. Based on the clinical outcomes in our study, different therapeutic strategies for hypermethylated tumors will be required. After the network of epigenetic alterations and biological behaviors has been untangled, additional novel targets of therapeutic interventions may be identified.
Whether the difference in epigenetic tissue- and genetic blood-based measurements accounts for both epigenetic markers being related to the PFS and OS of mRCC patients is an interesting question. Gene variants were only associated with PFS, a surrogate endpoint for survival measurements in mRCC that has possible limitations [31]. To the best of our knowledge, a tissue-based molecular marker has not previously been associated with OS. From a statistical point of view, our epigenetic study delivered higher HRs in survival analyses and provided a more balanced classification into responders and non-responders than the study by Garcia-Donas et al. [11], and therefore together contributing to a higher power of this study. Considering that a much smaller patient cohort was available for our measurements, our findings indicate that a strong effect has possibly been identified. Moreover, bearing in mind that a growing number of agents can be used for the treatment of mRCC, future identification of an optimum therapy regimen could be facilitated by epigenetic markers that allow good separation of patients into responders and non-responders.
Interestingly, the methylation levels of all candidate markers clearly decayed into easily distinguishable high and low methylation groups, eliminating the need to arbitrarily define cutoff points for dichotomization. Therefore, virtually no overlap existed between the responders and non-responders in the present study. Thus our LAD1 and CST6 methylation analyses yielded high specificities of 1.0 and 0.86 for the detection of therapy failure, underlining the possible relevance of these markers in mRCC.
This study may also answer whether DNA methylation-based prognosticators represent appropriate predictors of disease. Both miR-9-1 and miR-124-3 [21], [22] failed as predictors because no association was found with the PFS or OS of patients undergoing therapy. This finding might be explained by the fact that mRCC patients generally face a poor prognosis, and many tumors exhibit high methylation for the miR genes as expected for candidate prognosticators.
The independence from clinical or laboratory parameters could not be determined in the present study because the low sample numbers prevented multivariate analysis. Correspondingly, the relevant questions whether markers could be combined to optimize the predictive power or whether markers exhibit redundant information can only be answered in future studies by use of enlarged study cohorts.
However, the HRs observed for clinical parameters for patient outcome were lower with limited accuracy/discriminatory power. Our results require confirmation in an independent validation study including the consideration of clinical scoring systems as confounders.
In conclusion, our study identified LAD1 and CST6 CGI methylation as two epigenetic markers that are associated with the PFS and OS of mRCC patients undergoing antiangiogenic therapy. We have also shown the potential to improve the molecular prediction of the response to therapy. Our results further stress the notion that epigenetically altered RCCs exist, and novel specific strategies may be required to treat patients with such tumors.
Acknowledgments
We thank Margrit Hepke and Christel Reese for technical assistance.
Funding Statement
The authors have no support or funding to report.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin. 2011/02/08 ed. [DOI] [PubMed]
- 2. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, et al. (2013) A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. European journal of cancer 49: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 4. Skolarikos AA, Papatsoris AG, Alivizatos G, Deliveliotis C (2006) Molecular pathogenetics of renal cancer. Am J Nephrol 26: 218–231. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, et al. (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22: 454–463. [DOI] [PubMed] [Google Scholar]
- 6. Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, et al. (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The lancet oncology 14: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galsky MD (2013) A prognostic model for metastatic renal-cell carcinoma. Lancet Oncol 14: 102–103. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson P (2008) The impact of immune gene polymorphisms in kidney and liver transplantation. Clinics in laboratory medicine 28: 455–468, vii. [DOI] [PubMed]
- 9. Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, et al. (2010) Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463: 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, et al. (2008) von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180: 860–865 discussion 865–866. [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Donas J, Esteban E, Leandro-Garcia LJ, Castellano DE, del Alba AG, et al. (2011) Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 12: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 12. Gigante M, Li G, Ferlay C, Perol D, Blanc E, et al. (2012) Prognostic value of serum CA9 in patients with metastatic clear cell renal cell carcinoma under targeted therapy. Anticancer Res 32: 5447–5451. [PubMed] [Google Scholar]
- 13. Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, et al. (2011) Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 60: 644–661. [DOI] [PubMed] [Google Scholar]
- 14. Varela I, Tarpey P, Raine K, Huang D, Ong CK, et al. (2011) Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baldewijns MM, van Vlodrop IJ, Schouten LJ, Soetekouw PM, de Bruine AP, et al. (2008) Genetics and epigenetics of renal cell cancer. Biochim Biophys Acta 1785: 133–155. [DOI] [PubMed] [Google Scholar]
- 16. Vanharanta S, Shu W, Brenet F, Hakimi AA, Heguy A, et al. (2013) Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat Med 19: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkin J, Goh XY, Vetter M, Pickering L, Swanton C (2012) Epigenetic regulation in RCC: opportunities for therapeutic intervention? Nat Rev Urol 9: 147–155. [DOI] [PubMed] [Google Scholar]
- 18. Morris MR, Hesson LB, Wagner KJ, Morgan NV, Astuti D, et al. (2003) Multigene methylation analysis of Wilms’ tumour and adult renal cell carcinoma. Oncogene 22: 6794–6801. [DOI] [PubMed] [Google Scholar]
- 19. Morris MR, Ricketts C, Gentle D, Abdulrahman M, Clarke N, et al. (2010) Identification of candidate tumour suppressor genes frequently methylated in renal cell carcinoma. Oncogene 30: 1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Vlodrop IJ, Baldewijns MM, Smits KM, Schouten LJ, van Neste L, et al. (2010) Prognostic significance of Gremlin1 (GREM1) promoter CpG island hypermethylation in clear cell renal cell carcinoma. Am J Pathol 176: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gebauer K, Peters I, Dubrowinskaja N, Hennenlotter J, Abbas M, et al. (2013) Hsa-mir-124–3 CpG island methylation is associated with advanced tumours and disease recurrence of patients with clear cell renal cell carcinoma. Br J Cancer 108: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W, et al. (2010) Hsa-miR-9 methylation status is associated with cancer development and metastatic recurrence in patients with clear cell renal cell carcinoma. Oncogene 29: 5724–5728. [DOI] [PubMed] [Google Scholar]
- 23. Motoki K, Megahed M, LaForgia S, Uitto J (1997) Cloning and chromosomal mapping of mouse ladinin, a novel basement membrane zone component. Genomics 39: 323–330. [DOI] [PubMed] [Google Scholar]
- 24. Sohaib A (2012) RECIST rules. Cancer imaging : the official publication of the International Cancer Imaging Society 12: 345–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobin LH, Compton CC (2010) TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 116: 5336–5339. [DOI] [PubMed] [Google Scholar]
- 26.NCI National Cancer Institute Dictionary of Cancer Terms.
- 27. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. (2002) The human genome browser at UCSC. Genome Res 12: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, et al. (2005) Analysis of repetitive element DNA methylation by MethyLight. Nucleic acids research 33: 6823–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seidel C, Busch J, Weikert S, Steffens S, Fenner M, et al. (2012) Progression free survival of first line vascular endothelial growth factor-targeted therapy is an important prognostic parameter in patients with metastatic renal cell carcinoma. Eur J Cancer 48: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 30.Team RDC (2011) R: A language and environment for statistical computing R Foundation for Statistical Computing: Vienna, Austria.
- 31. Knox JJ (2008) Progression-free survival as endpoint in metastatic RCC? Lancet 372: 427–429. [DOI] [PubMed] [Google Scholar]