Summary
Background
No standard therapy exists for refractory or relapsed, advanced thymic epithelial tumors (TETs). We investigated the efficacy of cixutumumab, a fully human, IgG1 monoclonal antibody targeting the insulin-like growth factor-1 receptor, in TETs after failure of prior chemotherapy.
Methods
In this multicentre, open-label, phase 2 trial we enrolled patients aged 18 years or older with histologically confirmed recurrent or refractory TETs, who had progressed after at least one prior platinum-containing chemotherapy regimen, had Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease and adequate organ function. Eligible patients received cixutumumab (20 mg/kg, intravenous) every three weeks until disease progression or development of intolerable toxicity. The primary endpoint was response rate, analyzed on an intention-to-treat basis. Multiple pharmacodynamic studies were performed. This trial has completed enrollment and is registered with ClinicalTrials.gov, number NCT00965250.
Findings
Between August 25, 2009, and March 27, 2012, 49 patients were enrolled (37 thymomas; 12 thymic carcinomas) and received a median of six cycles of cixutumumab (range 1–46). At final analysis median potential follow-up was 24 months (IQR 17·3–36·9). In the thymoma cohort five (14%) of 37 patients (95% CI 5–29%) achieved a partial response, 28 had stable disease and four had progressive disease. Corresponding numbers for the thymic carcinoma cohort were zero of 12 patients (95% CI 0–26%), five and seven. The most common grade 3–4 adverse events in both cohorts combined were hyperglycemia (5 [10%] of 49 patients), lipase elevation (3 [6%]), weight loss, tumor pain, and hyperuricemia (2 each [4%]). Nine (24%) of 37 patients with thymoma developed autoimmune conditions (five new-onset) during treatment, the most common of which was pure red cell aplasia. Two (4%) of 49 treated patients died while on study. One case was attributed to disease progression and the other to disease–related complications (respiratory failure, myositis, and an acute coronary event), which could have been precipitated by treatment with cixutumumab.
Interpretation
Cixutumumab monotherapy is well tolerated and active in relapsed thymoma. Development of autoimmunity during treatment needs further investigation.
Funding
Division of Cancer Treatment and Diagnosis, National Cancer Institute/National Institutes of Health and ImClone Systems.
Introduction
Thymic epithelial tumors (TETs) are rare mediastinal tumors that are associated with relatively slow growth and a reasonably good prognosis.1 The association between a myriad of autoimmune diseases and thymoma is well described. Alterations in cellular and humoral immunity provide an explanation for the development of autoimmune disorders in these patients.2,3 Thymic carcinomas, the most aggressive form of TETs, are usually not associated with autoimmune diseases.2
TETs are relatively sensitive to chemotherapy.4 However, few effective options exist for the treatment of relapsed or refractory disease. Initial studies of targeted therapy have yielded disappointing results.4
The insulin-like growth factor (IGF) receptor family of tyrosine kinases is expressed in normal and neoplastic tissues.5 Activation of the IGF-1 receptor (IGF-1R) is ligand-dependent and promotes cell proliferation and inhibits apoptosis. Gene amplification and activating mutations of the IGF-1R gene are rare.5 In the thymus, IGF-1 has been shown to increase the thymic epithelial cell population and influence the development of thymocytes and chemokine expression.6 TETs express IGF-1R, especially in patients with recurrent or advanced disease and aggressive histologic subtypes and IGF-1R expression in primary tumors was associated with worse progression-free survival.7
The clinical benefit of IGF-1R inhibition in TETs was first observed in phase 1 studies of monoclonal antibodies targeting the receptor. One patient with metastatic thymoma treated with figitumumab (CP-751,871) at a dose of 20 mg/kg administered once every three weeks had prolonged stable disease lasting for more than one year.8 Another patient with thymoma had disease stabilization lasting greater than 12 weeks in a phase 1 study of cixutumumab (IMC-A12; NSC 742460), which is a fully human, IgG1 monoclonal antibody that binds to IGF-1R with high affinity and induces internalization and degradation of the receptor. In this trial cixutumumab was administered once every two weeks at doses of 6 mg/kg to 15 mg/kg.9 Based on these preclinical and clinical results, we designed this multicentre, open-label, phase 2 study to assess the efficacy of cixutumumab at a dose of 20 mg/kg administered intravenously once every three weeks in patients with recurrent TETs.
Methods
Patients
Patients aged 18 years or older with histologically confirmed recurrent TETs who had progressed after at least one platinum-containing chemotherapy regimen with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST; version 1·1), and adequate organ and bone marrow function were eligible. Further details of eligibility criteria are provided in the supplementary appendix. All patients provided written informed consent prior to enrollment. The study was approved by the National Cancer Institute and Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Boards.
Procedures
Cixutumumab at 20 mg/kg was administered intravenously on day 1 and repeated every three weeks until disease progression or development of intolerable toxicity. Two dose reductions were permitted (to 16 mg/kg and 13 mg/kg) if patients developed the following cixutumumab-related adverse events (AEs): symptomatic grade 3 hyperglycemia, fasting blood glucose ≥ 16.65 mmol/L with or without symptoms, grade 4 hyperglycemia, other grade 3 non-hematological toxicity (except controlled nausea and/or vomiting), and grade 3 or 4 hematological toxicity. Toxicity was assessed using Common Terminology Criteria for Adverse Events version 3.0 (version 4·0 was utilized starting January 1, 2011). Stopping rules for treatment included grade 3 or 4 allergic reactions or acute infusional reactions, a delay of treatment for three weeks or more for any reason including treatment-related AEs and incomplete resolution of AEs despite two dose reductions. Computed tomography (CT) scans were performed after every other cycle to assess response based on RECIST criteria. Patients with scans that demonstrated neither sufficient shrinkage to qualify as an objective response nor sufficient increase to qualify as disease progression, taking as reference the smallest cumulative longest dimension since start of treatment were deemed to have stable disease. Since the first restaging CT scan was performed 6 weeks after initiation of treatment, the disease had to be stable for at least this duration to qualify as stable disease. Radiologic assessment was conducted by the investigators and an independent radiological review was not conducted for assessment of response or progression. Patient reported outcomes such as disease-specific or general health-related quality of life measures were not evaluated in this study.
To determine the IGF-1 concentration in serum and IGF-1R and phospho-Akt levels in peripheral blood mononuclear cells (PBMCs), blood samples were collected before cixutumumab infusion, 24 hours later, on days 3, 7 and 14 of cycle 1, day 1 of cycle 2 and at the end of the study. IGF-1 and IGF-1R analyses were performed using the electrochemiluminescence (ECL) technology platform from Meso-Scale Discovery (Rockville, MD) as previously described.10 The test reagents (antibodies pairs) for both IGF-1 and IGF-1R (DY291 and DYC305-2 respectively) were obtained from R&D Systems. The assay for phospho-AKT analysis was acquired from Meso-Scale Discovery (Rockville, MD) and performed per manufacturer’s protocol. Epidermal growth factor receptor (EGFR) and IGF-1R expression in tumor tissue was not examined in this study.
For multiparameter flow cytometric analysis, whole blood samples were collected in cell preparation (CPT) tubes with sodium citrate (BD Vacutainer CPT Tubes; BD Biosciences, San Jose, CA) at baseline, in the second week of cycle 1 and before cycle 2. Details of sample handling and analysis are provided in the supplementary appendix.
Peripheral blood was also collected in one 10 ml serum separator tube at baseline, at each treatment cycle, and at the end of the study for anticytokine autoantibodies against interferon (IFN)α, IFNγ, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-12p70, IL-17A and IL-22 as described previously.11
Statistical analysis
The primary endpoint was assessment of the objective response rate (ORR) in two patient cohorts: thymoma and thymic carcinoma. Secondary endpoints included assessment of duration of response, progression-free survival (presented as time to progression; the event is progression) and overall survival. For each cohort, Simon’s optimal two-stage design was used with alpha = 0·10 and beta = 0·10.12 The study was designed to rule out an ORR of 5% (p0=0·05) and target an ORR of 20% (p1=0·20). Twelve patients were to be enrolled in each cohort initially. If one or more responses were seen in the first 12 patients, then accrual was to continue until 37 patients were enrolled and treated in that cohort. Further, if four or more responses were observed among 37 patients enrolled in each cohort then the result was to be interpreted as sufficiently interesting to warrant further studies.
Exploratory analyses were performed to determine the association between a variety of parameters, response, survival and autoimmune syndromes. In particular, 44 exploratory survival analyses were performed, with results obtained to be interpreted in that context. Comparisons between dichotomous parameters and response were determined by Fisher’s exact test, while responses were compared according to reported race/ethnicity using Mehta’s modification to Fisher’s exact test.13 A Cochran-Armitage test for trend was used to determine the significance of the association between ECOG status and response. Comparisons between response categories for continuous parameters were made using an exact form of a Wilcoxon rank sum test. SAS 9.3 (SAS Institute, Inc., Cary NC) was used for statistical analyses. Phospho-AKT analysis presented in the supplemental section was performed using GraphPad Prism v6.0. Additional details pertaining to statistical analysis are provided in the supplementary appendix.
Role of the funding source
This trial was sponsored and approved by the Division of Cancer Treatment and Diagnosis, National Cancer Institute/ National Institutes of Health (NCI/NIH) under a Collaborative Research and Development Agreement with ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company. This work was supported by the Intramural Research Program of the NCI/NIH. All authors were involved in data collection, data interpretation and writing of this report. The sponsor, AR, AB, SMS and GG had access to the raw data. The corresponding author (GG) had full access to all the data and had final responsibility for the decision to submit the manuscript for publication.
Results
Forty-nine patients were enrolled from two centers between August 25, 2009 and March 27, 2012 (41 patients from NCI and 8 patients from MSKCC). The thymic carcinoma cohort was closed after enrollment of 12 patients due to lack of activity. Patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics
| Parameter | Thymoma | Thymic carcinoma | Total |
|---|---|---|---|
| Total number | 37 | 12 | 49 |
| Median age (range), years | 52 (31 – 86) | 55 (26 – 71) | 52 (26 – 86) |
| Gender: | |||
| Male | 19 | 7 | 26 |
| Female | 18 | 5 | 23 |
| ECOG Performance Status: | |||
| 0 | 3 | 1 | 4 |
| 1 | 29 | 8 | 37 |
| 2 | 5 | 3 | 8 |
| Race: | |||
| Caucasian | 27 | 6 | 33 |
| Non-Hispanic | 25 | 5 | 30 |
| Hispanic | 2 | 1 | 3 |
| Black | 6 | 1 | 7 |
| Asian | 4 | 5 | 9 |
| Metastatic sites: | |||
| Median (range) | 1 (1 – 4) | 3 (1 – 5) | 2 (1 – 5) |
| Intrathoracic sites only | 18 | 0 | 18 |
| Extrathoracic sites (with or without intrathoracic sites) | 11 | 12 | 23 |
| Histology | |||
| Thymoma | 37 | 37 | |
| AB | 2 | 2 | |
| B1 | 4 | 4 | |
| B2 | 15 | 15 | |
| B2/B3 | 3 | 3 | |
| B3 | 9 | 9 | |
| Not otherwise specified | 4 | 4 | |
| Thymic carcinoma | 12 | 12 | |
| Paraneoplastic syndromes: | 16 | 0 | 16 |
| Myasthenia gravis | 9 | 9 | |
| Shulman syndrome | 1 | 1 | |
| Pure red cell aplasia | 3 | 3 | |
| Crohn’s disease | 1 | 1 | |
| Ulcerative colitis | 1 | 1 | |
| Mucocutaneous candidiasis | 1 | 1 | |
| Prior systemic therapy: | 37 | 12 | 49 |
| Median number of regimens (range) | 3 (1 – 11) | 2 (1 – 11) | 2 (1 – 11) |
| 6 or more regimens | 10 | 2 | 12 |
| Prior Chemotherapy | 37 | 12 | 49 |
| 1 prior line | 12 | 7 | 19 |
| 2 prior lines | 8 | 2 | 10 |
| 3 or more prior lines | 17 | 3 | 20 |
| Prior Platinum | 37 | 12 | 49 |
| Prior Anthracycline | 27 | 5 | 32 |
| Investigational Agents* | 17 | 4 | 21 |
| Prior radiotherapy | 23 | 4 | 27 |
| Prior surgery: | |||
| Radical | 28 | 2 | 30 |
| Debulking | 1 | 1 | 2 |
| Biopsy | 8 | 9 | 17 |
Investigational agents were counted as prior lines of systemic therapy but not counted as prior lines of chemotherapy.
Efficacy
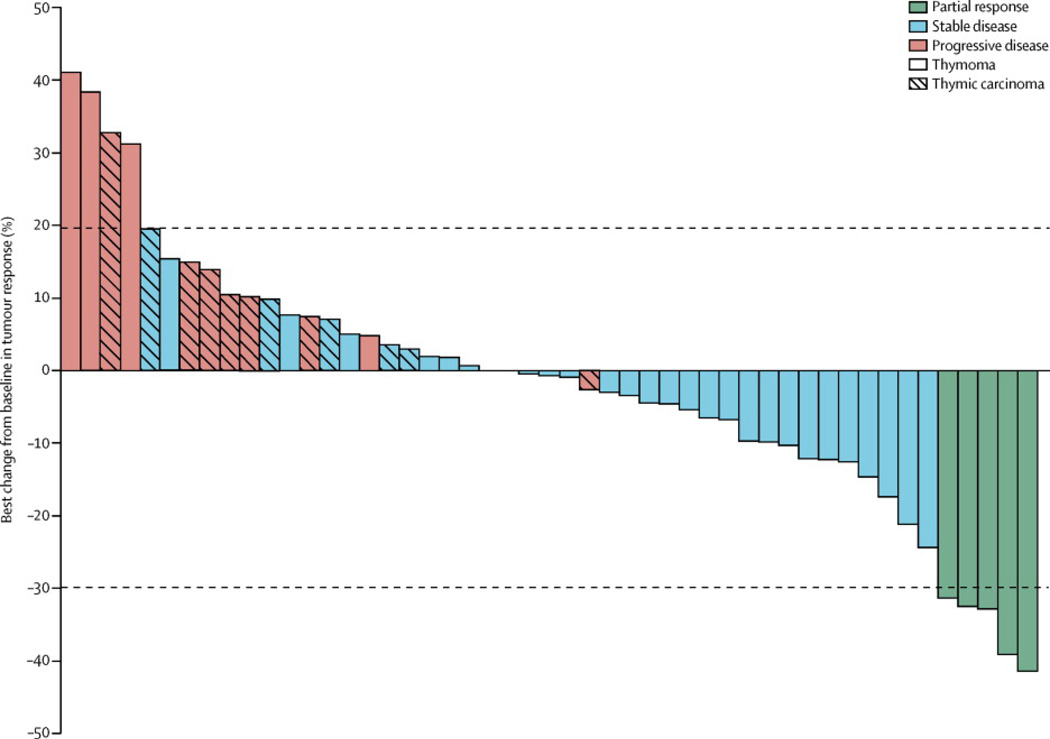
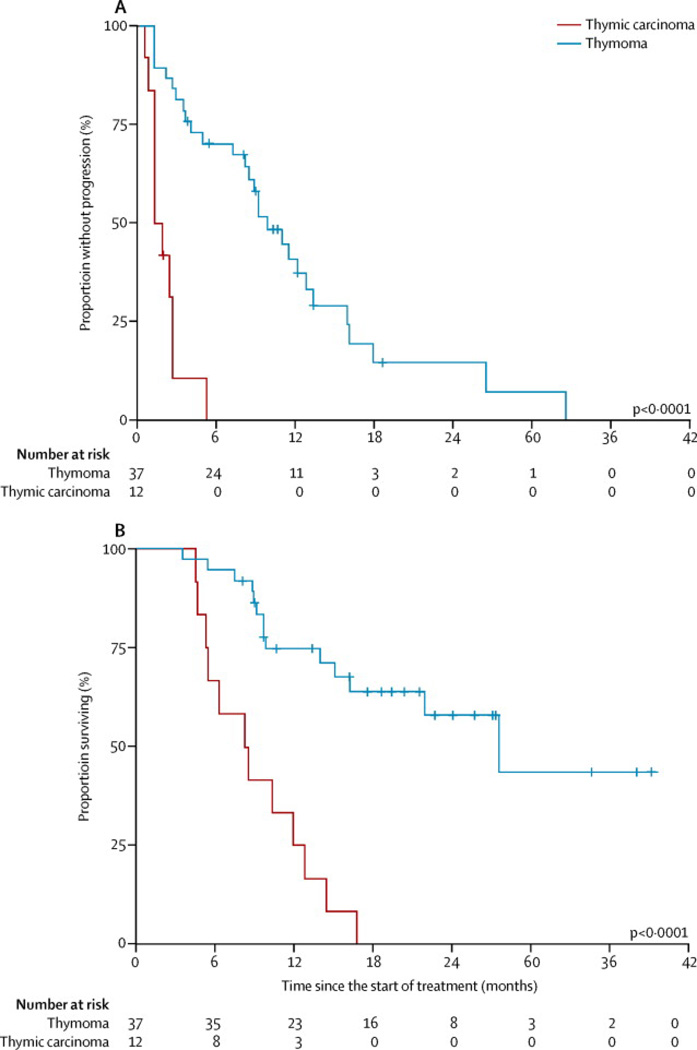
All 49 patients were evaluable for response (Table 2). The median potential follow-up for this study was 24 months (IQR17.3–36.9). Among patients with thymic carcinoma, there were no objective responses. Five (42%) of 12 patients had stable disease (SD) and seven (58%) patients had progressive disease (PD). The disease control rate (DCR; ORR plus SD) was 42% (95% CI, 15–72%), median time to progression (TTP) was 1·7 months (95% CI, 0·9 – 2·7) and median survival was 8·4 months (95% CI, 4·7 – 12·8) (Figure 1). Among patients with thymoma five (ORR 14%) of 37 patients achieved a partial response (PR) (95% CI, 5–29%). Twenty-eight (76%) patients had SD and four (11%) patients had PD. The DCR for the thymoma cohort was 89% (95% CI, 75–97%), median TTP was 9·9 months (95% CI, 7·3 – 12·8) and median survival was 27·5 months (95% CI, 15·0 – undefined) (Figure 1).
Table 2.
Response to Treatment
| Parameter | Thymoma (N=37) |
Thymic carcinoma (N=12) |
Total (N=49) |
|---|---|---|---|
| Best Response | |||
| Partial response | 5 | 0 | 5 |
| Stable disease | 28 | 5 | 33 |
| Progression | 4 | 7 | 11 |
| Response rate | 14% | 0% | 10% |
| (95% CI) | 5–29% | 0–26% | 3–22% |
| Disease Control | 89% | 42% | 78% |
| (95% CI) | 75–97% | 15–72% | 63–88% |
| Median time to progression (95%CI), months | 9·9 (7·3 – 12·8) | 1·7 (0·9 – 2·7) | 8·2 (3·0 – 11·0) |
| Median survival (95% CI), months | 27·5 (15·0 – undefined) | 8·4 (4·7 – 12·8) | 16·2 (10·4 – undefined) |
| Median number of cycles (range) | 13 (2– 46) | 2.5 (1 – 8) | 8 (1 – 46) |
Figure 1.
Kaplan-Meier curves for time to progression and overall survival. (A) Time to progression and (B) overall survival for all patients according to histology (thymoma vs. thymic carcinoma)
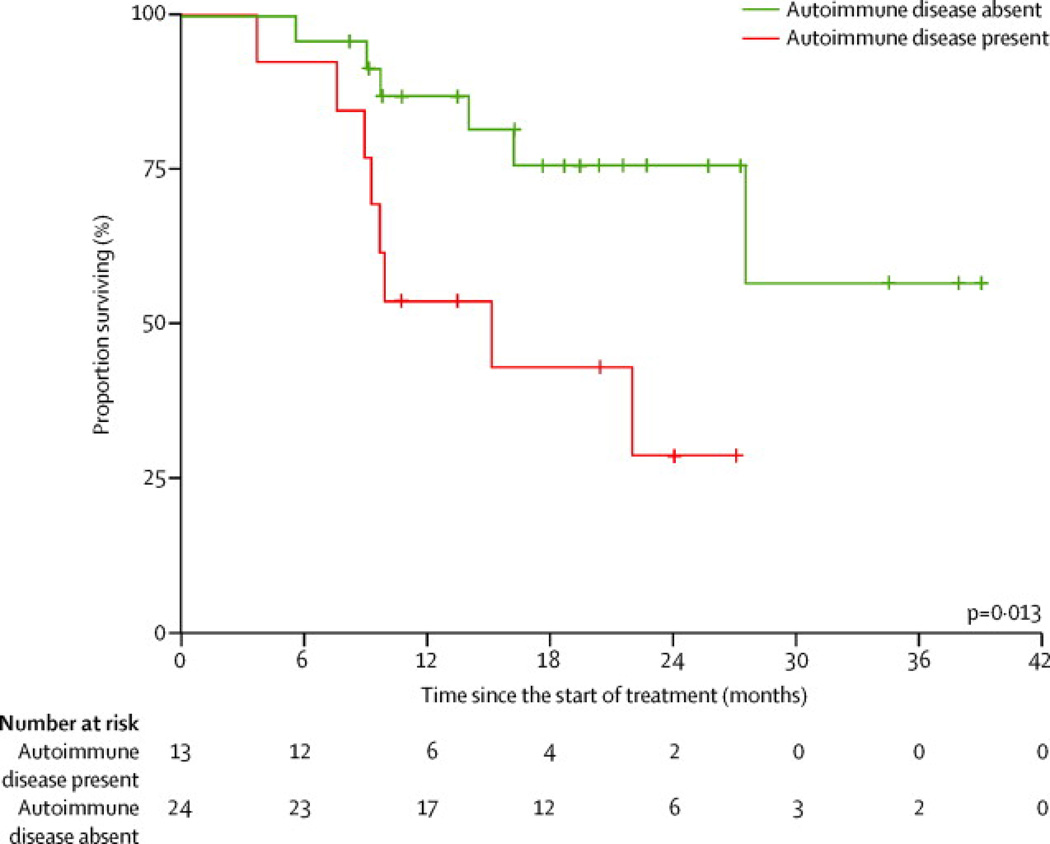
Absence of autoimmune disease at registration or during treatment in 24 (65%) of 37 patients with thymoma was associated with a significant survival benefit (p=0·013; Hazard Ratio (HR) 0·27; 95% CI for HR, 0·09–0·82) (Figure 2). On univariate analyses a variety of other clinical and biological parameters including baseline performance status in all patients and multiple parameters in patients with thymoma including age, gender, race, presence of extrathoracic metastases, prior treatments, duration of cixutumumab therapy, development of new autoimmune disease, pre-treatment anticytokine antibody titers and T-cell subsets at baseline and after treatment were all found to have no significant effect on survival (p>0·05).
Figure 2.
Kaplan-Meier curves for overall survival in patients with thymoma according to presence or absence of autoimmune disease
As of October 2013 two (5%) of 37 patients with thymoma are still receiving treatment. Accurate information on disease progression following prior treatments was available for a total of 33 (67%) of 49 patients. Of 11 patients with thymic carcinoma in this group, median TTP was 3·1 months; of 22 patients with thymoma, the median TTP was 5·9 months.
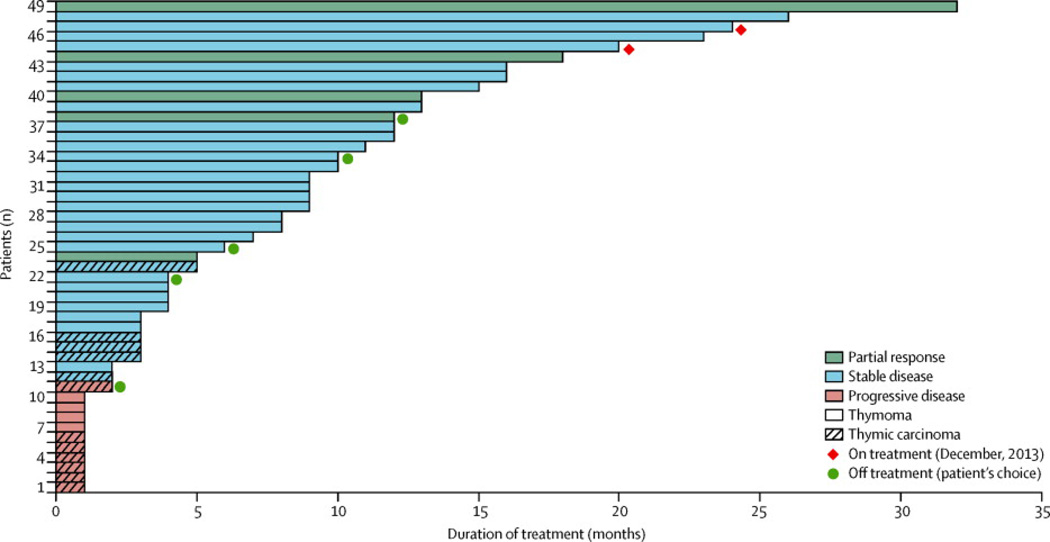
Figure 3 depicts the waterfall plot for best RECIST response according to histology. Reasons for coming off treatment included PD in 39 (80%) of 49 patients (including clinical progression in seven patients), complications of treatment in one (2%) patient, death due to complications of disease in two patients (4%) (respiratory failure in one case and myositis, respiratory failure and an acute coronary event in another case), and patient choice in five (10%) cases. Of the latter, three (60%) of five patients came off-treatment due to persistent AEs that were not severe enough for discontinuation of treatment (fatigue and mild hemoptysis in one case, diarrhea and worsening myasthenia in the second case and fatigue and anemia in the third case), one (20%) patient came offtreatment for logistical reasons (travel) and one (20%) patient opted to discontinue treatment in the absence of clear benefit but lack of RECIST-progression. Duration of treatment for all patients is presented in Figure 4.
Figure 3.
Waterfall plot of best change from baseline according to histology
Figure 4.
Duration of treatment for all patients on study
The five PRs observed in patients with thymoma included three males and two females and these patients achieved a partial response approximately 3, 4·5, 7·5, 8·5 and 19·5 months after initiation of treatment (Supplemental Table 1). No subgroup analysis was performed to study any potential correlation between the histologic subtype of thymoma and response to treatment or survival since the number of patients was considered to be too small. Three out of the five responders developed new or worsening autoimmune disorders while on treatment. Four of these patients came off study due to PD and one patient decided to discontinue treatment after 8 cycles. Responders received a median of 18 cycles of treatment (range, 6–46). The four patients who progressed on cixutumumab did so after 32, 14, 5 and 18 months after start of treatment. Four patients are alive 15, 32, 17 and 20 months after going off treatment and one patient died due to PD 17 months after stopping cixutumumab. Details of prior systemic therapy for recurrent thymoma in patients achieving a PR to cixutumumab are provided in Supplemental Table 2.
Toxicity
A total of 551 cycles of treatment were administered as of June 2013. The median number of cycles administered per patient was eight (range, 1–46). Twelve (24%) of 49patients received treatment for more than 12 months (all thymomas). AEs, at least possibly attributable to cixutumumab are reported in Table 3. Grade 3–4 AEs with an incidence greater than 5% included hyperglycemia in 5 (10%) of 49 patients and increased serum lipase observed in 3 (6%) of 49 patients. Six (12%) of 49 patients required dose reductions: three (6%) for lipase elevation after 4, 6, and 13 cycles, two (4%) for anemia after 4 and 7 cycles, and one (2%) for intolerable fatigue after 14 cycles. Nine (24%) of 37 patients with thymoma developed new onset autoimmune syndromes or worsening of a preexisting condition (Table 4).
Table 3.
Summary of toxicity experienced by at least 10% of patients (any grade) or 2% or more of patients (for grade 3 or higher). All adverse events are at least possibly attributed to cixutumumab
| Toxicity | All grades (n=49) |
Grade 1–2 (n=49) |
Grade 3 (n=49) |
Grade 4 (n=49) |
Grade 5 (n=49) |
|---|---|---|---|---|---|
| Alanine aminotransferase increased | 3 (6%) | 2 (4%) | 1 (2%) | 0 | 0 |
| Alopecia | 5 (10%) | 5 (10%) | 0 | 0 | 0 |
| Anemia | 5 (10%) | 5 (10%) | 0 | 0 | 0 |
| Anorexia | 17 (35%) | 17 (35%) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 4 (8%) | 3 (6%) | 1 (2%) | 0 | 0 |
| Creatine kinase increased | 1 (2%) | 0 | 0 | 1 (2%) | 0 |
| Creatinine increased | 12 (25%) | 12 (25%) | 0 | 0 | 0 |
| Dyspnea | 1 (2%) | 0 | 0 | 1 (2%) | 0 |
| Fatigue | 14 (29%) | 13 (27%) | 1 (2%) | 0 | 0 |
| Hyperglycemia | 26 (53%) | 21 (43%) | 5 (10%) | 0 | 0 |
| Hyperuricemia | 19 (39%) | 17 (35%) | 0 | 2 (4%) | 0 |
| Lipase increased | 4 (8%) | 1 (2%) | 3 (6%) | 0 | 0 |
| Muscle cramps | 14 (29%) | 14 (29%) | 0 | 0 | 0 |
| Myositis | 1 (2%) | 0 | 1 (2%) | 0 | 0 |
| Nail changes (nail loss, nail splitting, brittle nails, nail discoloration) | 7 (14%) | 7 (14%) | 0 | 0 | 0 |
| Nausea | 8 (16%) | 8 (16%) | 0 | 0 | 0 |
| Oral mucositis/ulceration | 7 (14%) | 7 (14%) | 0 | 0 | 0 |
| Respiratory failure | 1 (2%) | 0 | 0 | 0 | 1 (2%) |
| Thrombocytopenia | 7 (14%) | 7 (14%) | 0 | 0 | 0 |
| Tumor pain | 8 (16%) | 6 (12%) | 2 (4%) | 0 | 0 |
| Visual changes (blurred vision, floaters, flashing lights) | 7 (14%) | 7 (14%) | 0 | 0 | 0 |
| Weight loss | 5 (10%) | 3 (6%) | 2 (4%) | 0 | 0 |
Data are number of patients (%). Events are maximum grade per patient, over all cycles.
Table 4.
Autoimmune syndromes
| Patient No. |
Diagnosis | Autoimmune syndrome | Present diagnosis |
Response |
|---|---|---|---|---|
| 1 | Thymoma | Myasthenia | Yes* | Partial response |
| PRCA | No | |||
| Immune | No | |||
| thrombocytopenia | No | |||
| Myositis | No | |||
| Myocarditis | ||||
| 2 | Thymoma | PRCA | No | Stable disease |
| Food allergy (peanut) | No | |||
| 3 | Thymoma | PRCA | Yes | Stable disease |
| 4 | Thymoma | Cutaneous + GI Crohn’s | Yes* | Partial response |
| 5 | Thymoma | PRCA | Yes | Stable disease |
| 6 | Thymoma | Myasthenia | Yes* | Partial response |
| AI colitis | No | |||
| 7 | Thymoma | Myasthenia | Yes* | Stable disease |
| 8 | Thymoma | Food allergy (olives) | No | Stable disease |
| 9 | Thymoma | PRCA | No | Stable disease |
PRCA: pure red cell aplasia;
These pre-existing AI syndromes worsened during treatment
Two (4%) of 49 patients died while on study. A 45 year old female with a WHO B2 thymoma died due to respiratory failure related to PD 15 weeks after starting treatment and an 86 year old female with a WHO AB thymoma died due to disease-related complications (respiratory failure, myositis, acute coronary event) after receiving 13 cycles of treatment. In the latter case, precipitation of complications by treatment with cixutumumab could not be ruled out.
Pharmacodynamic studies
Highly significant induction of free IGF-1 was seen after administration of a single-dose of cixutumumab with a 4·7–7·2 times increase post-treatment at all three post-drug time points (each p<0·0001) (Supplemental Figure 1a, and Supplemental Table 3). As the vast majority of IGF-1 in plasma is bound by IGF-binding protein, which may influence the levels of free IGF-1, we explored the mechanism of increased IGF-1 following cixutumumab treatment by measuring total plasma IGF-1. Plasma samples were treated to release all IGF-1 which was then determined with the IGF-1 ECL assay. A highly significant, 0·8 to 1·5 times increase of total IGF-1 was observed at each time point compared to pre-treatment levels (each p≤0·0001) (Supplemental Figure 1b, and Supplemental Table 3). For example, there was a mean increase of 6·8 nmol/L (95% CI, −1·91 to 16·3 nmol/L by C1D3 compared to baseline. No statistically significant associations were observed between IGF-1 levels and response in patients with thymoma. However, there was a non-significant tendency observed towards some association between total (p=0·14) and free (p=0·07) IGF-1 levels and longer overall survival in patients with thymoma. (Supplemental Figure 2). No statistically significant association was noted between IGF-1 levels and pre-existing or new or worsening autoimmune disease in patients with thymoma (Supplemental Figure 3).
Since the most established activity of an IGF-1R therapeutic antibody is the downregulation of its receptor, IGF-1R in various preclinical models, we sought to determine the effect of cixutumumab in down-regulating IGF-1R from patients’ PBMCs. We detected significant and sustained post-treatment reduction of IGF-1R in PBMCs that was observed as early as the day after drug administration (Supplemental Table 3). However, no detectable post-treatment changes were observed in phospho-AKT expression (Supplemental Table 4).
A significant increase in IFN-γ-expressing CD4+ T cells was seen 21 days after the first dose of cixutumumab in patients as results improved from PD to SD to PR (p=0·0017). Patients with thymoma who achieved a response also showed a significant decrease in circulating endothelial progenitor cells (CEPs) after completion of one cycle of treatment (p=0·049). No significant post-treatment changes were observed in CEPs in patients with thymic carcinoma or in the number of circulating endothelial cells (CECs) in either histology. No statistically significant associations were noted in the number of other immune cell subsets including naïve, memory, and regulatory T cells between responders and non-responders.
Anti-cytokine autoantibody titers were measured in 46 paired samples pre- and post-treatment. The most frequently detected pre-treatment antibody was anti-IFNα (54%) followed by anti-IL12, anti-IL17A, and anti-IL22 in 39%, 4%, and 2% samples respectively. Pretreatment titers of anti-IFN-α and anti-IL22 antibodies were significantly lower among responders compared to non-responders (p=0·021 and p=0·0048, respectively). Conversely, pretreatment titers of anti-GMCSF antibodies were significantly higher among responders (p=0·014). Except for one patient who developed anti-IFN–α autoantibodies during the course of treatment, there was no appreciable change in titer of anticytokine antibodies during or after treatment in all patients, including patients with autoimmune syndromes.
Among the nine (24%) of 37 patients with thymoma who developed new or worsening autoimmune syndromes after treatment, a statistically higher titer of anti-IFN-γ- antibodies was observed at baseline (p=0·0091) and a significant decrease in IL17-expressing CD4+ T cells after one cycle of treatment (p=0·0058). Also seen amongst these patients were significantly lower pretreatment IFN-γ-, IL17-, and IL4-expressing CD4+ T cells (p=0·011, p=0·033 and p=0·021) and lower levels of IFN-γ- and IL4-expressing CD4+ T cells after one cycle of treatment (p=0·016 and p=0·043 respectively). Results of selected immune correlative studies are presented in Supplemental Table 5.
Discussion
Our study shows that cixutumumab monotherapy is well tolerated and active in patients with relapsed thymoma. However no activity was seen in patients with thymic carcinoma. Treatment is associated with a significant increase in serum IGF-1 levels in all patients and an increase in select T-cell subsets and a reduction in CEPs among patients with thymoma who achieved an objective response.
Five (14%) of 37 patients with thymoma achieved PR. Hence, the study met its primary endpoint for these patients. In addition, 33 (89%) of 37 patients with thymoma had SD (including 13 patients who received treatment for more than one year). Although disease stabilization could be a reflection of the indolent nature of thymoma, it should be noted that these patients had received a median of two prior lines of systemic therapy (range, 1–9) and their tumors were progressing when they entered the study. Additionally, since all patients participating in this study had advanced, unresectable disease on enrollment we do not expect the proportion of patients who had undergone prior radical surgery to have influenced the efficacy of cixutumumab in comparison to other systemic therapies for recurrent disease. (Table 5)
Table 5.
Systemic therapy in patients with relapsed Thymic Epithelial Tumors
| Intervention | Number of Patients | Response Rate |
PFS/TTP | OS |
|---|---|---|---|---|
| Pemetrexed25 | Thymoma = 16 | NR | 45·4 weeks | NR |
| Thymic carcinoma = 11 | NR | 5·1 weeks | ||
| Capecitabine + Gemcitabine26 | Thymoma = 12 | 5 | 11 months | Not reached |
| Thymic carcinoma = 3 | 1 | 6 months | ||
| Octreotide Prednisone27 |
Thymoma = 32 | 12 | 8·8 months | Not reached |
| Thymic Carcinoma = 6 | 0 | 4·5 months | 23·4 months | |
| Erlotinib + Bevacizumab28 | Thymoma = 11 | 0 | NR | Not reached |
| Thymic carcinoma = 7 | 0 | NR | ||
| Belinostat29 | Thymoma = 25 | 2 | 11·4 months | Not reached |
| Thymic carcinoma = 16 | 0 | 2·7 months | 12·4 months | |
| Amrubicin + Carboplatin*30 | Thymoma = 18 | 3 | 7·6 months | Not reached |
| Thymic carcinoma = 33 | 10 | 7·6 months | 27·3 months | |
| Cixutumumab (current study) | Thymoma = 37 | 5 | 9·9 months | 27·5 months |
| Thymic carcinoma = 12 | 0 | 1·7 months | 8·4 months | |
Only 3 of 18 patients with thymoma and 14 of 33 thymic carcinoma had received previous chemotherapy; NR: Not reported; PFS: progression-free survival; TTP: time to progression; OS: overall survival
The lack of response and shorter TTP and survival in patients with thymic carcinoma is also consistent with results of previous studies with systemic therapy (Table 5). In the absence of a targetable mutation, it is unlikely that single-agent therapy will be significantly more effective than existing treatment options for this challenging histology.
Thymic carcinomas have been shown to express IGF-1R more commonly than thymoma.7 However, all our responses were seen in patients with thymoma, and the median survival was longer in these patients compared to patients with thymic carcinoma. Tumor expression of IGF-1R might not serve as a good biomarker for response to anti-IGF therapy. Other biomarkers with the potential to predict sensitivity to anti-IGF 1R therapy are being explored including insulin-like growth factor-binding protein 5 (IGFBP-5)/IGFBP-4 expression ratio, total IGF-2 and IGFBP-2 and coexpression of IGF-1R and insulin receptor substrate-1 (IRS-1) on tumor cells.14–16
We observed a significant and sustained increase in free and total serum IGF-l after a single dose of cixutumumab. Similar observations have been made in earlier studies with cixutumumab and other IGF-1R antibodies.17–19 Since free IGF-1 accounts for only 1–2% of total plasma IGF-1, the increased total IGF-1 alone can account for the several fold changes of free IGF-1 following treatment. We did not observe an association between an increase in serum IGF-1 and response, which is similar to previous observations.18 In contrast, in a phase 1 study of chemotherapy and figitumumab in Japanese patients with advanced lung cancer, higher baseline total serum IGF-1 levels were observed among responders.17
The effects of IGF-1 on the immune system are well described and include enhancement of lymphocyte survival through activation of T cell AKT, inhibition of IL-2-dependent lymphocyte function and suppression of antibody production.20 We observed a significant increase in IFN-γ-expressing CD4+ T cells after treatment among responders. If validated in future studies, the change in this T-cell subset could potentially serve as a biomarker of response to cixutumumab in patients with thymoma.
In our study anti-IFNα and anti-IL12 autoantibodies were identified most frequently and this is consistent with previous reports.3 Interestingly, although the titer of anticytokine autoantibodies did not change during treatment, significantly higher pretreatment titers of anti-GMCSF antibodies and lower titers of anti-IFN-α and anti-IL22 antibodies were noted among responders. The relevance of these findings to the biology of thymoma needs further evaluation.
One of the most intriguing findings of this study was the clinical observation of the development of autoimmunity or worsening of preexisting autoimmune conditions in some patients including three out of five responders. We detected differences in certain immune cell subsets amongst these patients including lower levels of IFN-γ-, IL17-, and IL4-expressing CD4+ T cells and a higher titer of anti-IFN-γ-antibodies. Severe autoimmunity has been observed after treatment with anti-CTLA-4 antibodies, and responses have been associated with autoimmunity induced by treatment.21 This suggests that cixutumumab may be modulating the immune system in the milieu of a disease like thymoma that is already prone to development of autoimmunity.
Our study also uncovered a significant reduction in CEPs after treatment with cixutumumab amongst responders. Vascular endothelial growth factor (VEGF) inhibitors have been shown to inhibit bone marrow derived CEPs.22 Anti-IGF-1R therapy can have antiangiogenic effects as demonstrated by significant reductions in tumor-associated VEGF after treatment with figitumumab and a reduction in tumor microvessel density after treatment with robatumumab.23, 24 Taken together these results might help explain the decrease in CEPs observed in our study and point to an antiangiogenic action of cixutumumab.
In conclusion, our study demonstrates activity of cixutumumab in patients with relapsed thymoma that warrants further evaluation. Strategies to optimize outcome could include patient selection by utilizing newly developed predictive biomarkers that can help in the identification of patients who might benefit from anti-IGF therapy and combination of cixutumumab with chemotherapy or other targeted therapies. The immunomodulatory effects of anti-IGF therapy and development of autoimmunity in some patients observed in this study is intriguing and requires further investigation.
Panel: Research in context
Systematic review
We searched PubMed and ClinicalTrials.gov with the terms “thymoma”, “thymic carcinoma” and “IGF-1R” and did not identify any studies of anti-IGF-1R antibody therapy for TETs. To the best of our knowledge this is the first trial investigating anti-IGF-1R therapy in TETs and also the largest study of biologic therapy in TETs to date. We used preliminary data on IGF-1R expression in resected TETs from our laboratory (which has since been published; reference 7) as well as reports from phase I studies of clinical benefit of targeting IGF-1R in patients with TETs as the rationale for formulating this trial.7,8
Interpretation
Treatment with cixutumumab in patients with recurrent thymoma achieved a 14% response rate and TTP of 9·9 months which merits further investigation. These results are comparable to results of other prospective trials in this patient population. To our knowledge, this is the first time that significantly increased titers of anti-GMCSF antibodies and lower titers of anti-IFN-α and anti-IL22 antibodies before treatment and a post-treatment increase in IFN-γ-expressing CD4+ T cells have been observed among patients responding to cixutumumab. These observations need further validation and could serve as potential biomarkers of response to anti-IGF-1R treatment in TETs if confirmed in future studies. These results provide a rationale for future development of IGF-1R inhibitors in patients with thymoma.
Supplementary Material
Acknowledgments
This work was sponsored by DCTD, NCI/NIH under a Collaborative Research and Development Agreement with ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company. This trial received support from the Intramural Research Program of the NCI/NIH. We thank all patients who participated in this study and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Partially presented at the American Society of Clinical Oncology Annual Meeting in Chicago, 2012
Contributions
AR and GG designed and supervised the study. GG and AR (from 1st January, 2013) were principal investigators for this trial at the NCI and GJR was the principal investigator at MSKCC. AR, CAC, AB, RJK, AT, SK, ALC, BS, ES, GJR, and GG were responsible for patient inclusion and follow-up. AB and IB gathered the data. LC, MJL, JBT, SKB, LBR, and YY performed pharmacodynamic studies. AR, LC, MJL, JBT, SKB, SMS, and GG analyzed and interpreted the data. AR prepared the initial version of the manuscript and all authors reviewed the article for intellectual content, provided comments, and gave final approval.
Conflicts of interest
GJR has served as a consultant or advisor to Chugai, Tragara, Ariad, Daiichi and Abbott. All other authors declare that they have no conflicts of interest.
References
- 1.Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. 2004;77:1860–1869. doi: 10.1016/j.athoracsur.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Evoli A, Minicuci GM, Vitaliani R, et al. Paraneoplastic diseases associated with thymoma. J Neurol. 2007;254:756–762. doi: 10.1007/s00415-006-0429-z. [DOI] [PubMed] [Google Scholar]
- 3.Meager A, Wadhwa M, Dilger P, et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132:128–136. doi: 10.1046/j.1365-2249.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Curr Treat Options Oncol. 2008;9:277–287. doi: 10.1007/s11864-009-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 6.Chu YW, Schmitz S, Choudhury B, et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008;112:2836–2846. doi: 10.1182/blood-2008-04-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zucali PA, Petrini I, Lorenzi E, et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer. 2010;116:4686–4695. doi: 10.1002/cncr.25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 9.McKian KP, Haluska P. Cixutumumab. Expert Opin Investig Drugs. 2009;18:1025–1033. doi: 10.1517/13543780903055049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol. 2012;32:238–245. doi: 10.1007/s10875-011-9621-8. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Mehta CR, Patel NR. A Network Algorithm for Performing Fisher's Exact Test in r × c Contingency Tables. J Am Statist Assoc. 1983;78:427–434. [Google Scholar]
- 14.McCaffery I, Tudor Y, Deng H, et al. Putative Predictive Biomarkers of Survival in Patients with Metastatic Pancreatic Adenocarcinoma Treated with Gemcitabine and Ganitumab, an IGF1R Inhibitor. Clin Cancer Res. 2013;19:4282–4289. doi: 10.1158/1078-0432.CCR-12-1840. [DOI] [PubMed] [Google Scholar]
- 15.Mukohara T, Shimada H, Ogasawara N, et al. Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVPAEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 2009;282:14–24. doi: 10.1016/j.canlet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Becker MA, Hou X, Harrington SC, et al. IGFBP ratio confers resistance to IGF targeting and correlates with increased invasion and poor outcome in breast tumors. Clin Cancer Res. 2012;18:1808–1817. doi: 10.1158/1078-0432.CCR-11-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto Y, Sekine I, Tanioka M, et al. Figitumumab combined with carboplatin and paclitaxel in treatment-naive Japanese patients with advanced non-small cell lung cancer. Invest New Drugs. 2012;30:1548–1556. doi: 10.1007/s10637-011-9715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tap WD, Demetri G, Barnette P, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 2012;30:1849–1856. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 19.Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic Tlymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capillo M, Mancuso P, Gobbi A, et al. Continuous infusion of endostatin inhibits differentiation, mobilization, and clonogenic potential of endothelial cell progenitors. Clin Cancer Res. 2003;9:377–382. [PubMed] [Google Scholar]
- 23.Wang Y, Lipari P, Wang X, et al. A fully human insulin-like growth factor-I receptor antibody SCH 717454 (Robatumumab) has antitumor activity as a single agent and in combination with cytotoxics in pediatric tumor xenografts. Mol Cancer Ther. 2010;9:410–418. doi: 10.1158/1535-7163.MCT-09-0555. [DOI] [PubMed] [Google Scholar]
- 24.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loehrer PJ, Sr, Yiannoutsos CT, Dropcho S, et al. A phase II trial of pemetrexed in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol (Meeting Abstracts) 2006;24:7079. [Google Scholar]
- 26.Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs) Ann Oncol. 2010;21:1168–1172. doi: 10.1093/annonc/mdp483. [DOI] [PubMed] [Google Scholar]
- 27.Loehrer PJ, Sr, Wang W, Johnson DH, Aisner SC, Ettinger DS. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol. 2004;22:293–299. doi: 10.1200/JCO.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Bedano PM, Perkins S, Burns M, et al. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol (Meeting Abstracts) 2008;26:19087. [Google Scholar]
- 29.Giaccone G, Rajan A, Berman A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29:2052–2059. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawashima Y, Inoue A, Sugawara S, et al. Phase II study of amrubicin (AMR) and carboplatin (CBDCA) for invasive thymoma (IT) and thymic carcinoma (TC): NJLCG0803. J Clin Oncol (Meeting Abstracts) 2013;31:7530. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.