Abstract
Introduction
Exposure to low-dose radiation is widespread and attributable to natural sources. However, occupational, medical, accidental, and terrorist-related exposures remain a significant threat. Information on radiation injury to the feto-placental unit is scant and largely observational. We hypothesized that radiation causes trophoblast injury, and alters the expression of injury-related transcripts in vitro or in vivo, thus affecting fetal growth.
Methods
Primary human trophoblasts (PHTs), BeWo or NCCIT cells were irradiated in vitro, and cell number and viability were determined. Pregnant C57Bl/6HNsd mice were externally irradiated on E13.5, and placentas examined on E17.5. RNA expression was analyzed using microarrays and RT-qPCR. The experiments were repeated in the presence of the gramicidin S (GS)-derived nitroxide JP4-039, used to mitigate radiation-induced cell injury.
Results
We found that survival of in vitro–irradiated PHT cell was better than that of irradiated BeWo trophoblast cell line or the radiosensitive NCCIT mixed germ cell tumor line. Radiation altered the expression of several trophoblast genes, with a most dramatic effect on CDKN1A (p21, CIP1). Mice exposed to radiation at E13.5 exhibited a 25% reduction in mean weight by E17.5, and a 9% reduction in placental weight, which was associated with relatively small changes in placental gene expression. JP4-039 had a minimal effect on feto-placental growth or on gene expression in irradiated PHT cells or mouse placenta.
Discussion and conclusion
While radiation affects placental trophoblasts, the established placenta is fairly resistant to radiation, and changes in this tissue may not fully account for fetal growth restriction induced by ionizing radiation.
Keywords: Placenta, Trophoblast, Ionizing radiation, Microarray, JP4-039
1. Introduction
Exposure to ionizing radiation remains a reality in today's world. Worldwide, the average annual exposure to natural radiation is about 2.4 milli Sievert (mSv) [1]. Occupational exposures are most relevant to people working with nuclear fuel and medical devices, in defense-related functions, and in occupations associated with enhanced exposure to natural sources of radiation. For example, aircrew members are exposed to 5–8 mSv per hour while flying [1]. Medical sources of radiation include diagnostic procedures that expose individuals to low doses (commonly 0.1–10 mSv) and therapeutic exposures, typically 20–60 Gray (Gy), to a targeted tissue [1]. Accidental exposures in nuclear fuel processing plants typically expose workers to 1–20 Gy [1]. These risks may be greatly amplified if “dirty bombs” are deployed by terrorists against civilians [2].
Research into diagnosis, treatment, and prevention of radiation injury in pregnancy is limited by appropriate ethical concerns and by the scarcity of information on mechanisms underlying the effect of ionizing radiation on the developing feto-placental unit. Anecdotal reports or observational studies have generated some information pertaining to gestational age and radiation dose. During the pre-implantation period, as little as 0.3 Gy is lethal to the mouse embryo [3]. In the post-implantation period, the main risks from radiation include embryonic death, congenital anomalies, growth restriction, and neurologic maldevelopment [4]. Exposing mice at E14 to 0.3–1.5 Gy of whole body irradiation caused decreased neonatal body length and body weight [5]. Minimal effects on litter size or fetal growth were observed when mice at E7-16 were exposed to low dose radiation, 10–13 mSv per day over 10 days [6]. In humans, data from children exposed to in utero radiation after catastrophic events in Hiroshima, Nagasaki, and Chernobyl revealed lower height and weight in adolescence [4,7].
Ionizing radiation damages tissues through diverse mechanisms [8]. A major consequence of radiation is direct and indirect DNA damage. Direct effects include the transfer of kinetic energy from radioactive particles to the DNA backbone, which breaks phosphodiester bonds. Indirect effects include the generation of reactive oxygen species, which cause DNA double-strand breaks and cell-cycle arrest. Other types of injury include p53-dependent and -independent apoptosis [9], mitochondrial damage, loss of regenerative capacity, and premature senescence [8]. NFκB mediates several radiation-stimulated signal transduction pathways, which may explain the degree of radiation-sensitivity of differing cells types [10]. These pathways implicate CDKN1A (also known as p21, CIP1), epidermal growth factor receptors, and the apoptosis-related proteins BAX and BCL2 in radiation injury [11]. Whereas radiation-induced pathways have been interrogated in non-placental cell types, there are no studies of radiation injury to cultured primary human trophoblast (PHT) cells; there has been a single study that included the choriocarcinoma line JEG3 and showed no effect on gene expression of gap junction protein alpha 1 [12].
Methods to scavenge reactive oxygen species have been proposed to mitigate radiation damage. This effect has been attributed, at least in part, to the action of manganese superoxide dismutase (MnSOD, [13]). The nitroxides, which have superoxide dismutase–mimetic activity and inhibit lipid peroxidation [14], constitute one such class of radioprotectors. JP4-039 is a nitroxide linked to a short alkene isostere analog of hemigramicidin S, which allows concentration at the mitochondrial membrane, the site of radiation-induced lipid peroxidation [15]. It has been shown to protect against radiation damage in vivo [16,17]. In this study, we tested the hypothesis that ionizing radiation causes injury to PHT cells in vitro and to the mouse placenta in vivo. We also assessed whether the nitroxide JP4-039 mitigates that damage.
2. Materials and methods
2.1. Cell culture and irradiation in vitro
All studies involving human placental cells were approved by the Institutional Review Board at the University of Pittsburgh. For control, we used the immortalized choriocarcinoma line BeWo (ATCC, Manassas, VA), which captures aspects of trophoblast biology but maintains its undifferentiated state and proliferative capacity [18], and NCCIT cells (ATCC, Manassas, VA), a mixed germ cell tumor line that is particularly radiosensitive [19]. Term PHT cells were isolated and cultured using a modified version of the trypsin–deoxyribonuclease–dispase/Percoll method described by Kliman et al. [20,21]. After 4 h in culture, non-adherent cells and syncytial fragments were removed by washing in phosphate-buffered saline (PBS). All human placental cells were maintained, for 72 h after plating, in Dulbecco's modified Eagle's medium (DMEM, Fisher Scientific, Hampton, NH) containing 10% fetal bovine serum (Fisher Scientific), 20 mmol/l HEPES (pH 7.4, Sigma–Aldrich, St. Louis, MO), and antibiotics at 37 °C in a 21% oxygen/5% carbon dioxide atmosphere. The quality of PHT cells was routinely monitored every 24 h by cell morphology and by ELISA assay of medium human beta chorionic gonadotropin (β-hCG, DRG International, Mountainside, NJ), showing a characteristic increase in medium β-hCG as cytotrophoblasts differentiate into syncytiotrophoblasts [21,22].
The cells were irradiated 24 h after initial plating, defined as time zero. Cells were irradiated at the dosage noted in Results vs. sham, defined as 0 Gy [23], using a Clinac 600C (Varian Medical Systems, Palo Alto, CA) with a 6 MV photon beam and a dose rate of 250 cGy/min. The flasks containing the cells were placed on 1.5 cm of bolus (a tissue equivalent material) since the maximum irradiation depth was 1.5 cm, which corresponded to the plated cell layer. In some of the experiments, irradiated cultured cells were exposed to either JP4-039 (10 μM) in DMSO [24], added to the medium 1 h before radiation, or to DMSO alone. Cells were collected for microarray analysis at 4, 8, and 24 h after irradiation, and for all other analyses at 24 h after exposure to radiation.
Cell numbers (BeWo and NCCIT) were assessed by rinsing the monolayer with PBS, followed by trypsinization, resuspension in DMEM, and counting using a hemocytometer. Total cellular protein concentration was measured with the Pierce BCA Protein Assay Reagent Kit (Thermo Scientific, Rockford, IL). For DNA extraction, PHT cells were lysed in a buffer containing NaCl 0.4 M, Tris 10 mM, EDTA 2 nM, SDS 25% with proteinase K (200 μg/ml). The DNA was precipitated in saturated NaCl and isopropanol, washed, resuspended in Tris, and quantified using a NanoDrop 1000 spectrophotometer (NanoDrop, Wilmington, DE).
2.2. Irradiation of pregnant mice
All experiments using mice were approved by the Institutional Animal Care and Use Committee protocols at the University of Pittsburgh. Female C57Bl/6HNsd adult mice were fed standard laboratory chow. Mice were mated and separated the next morning (termed E0.5). Pregnancy was confirmed by weight change on E12.5. On E13.5, mice were irradiated at 0 Gy (sham) or 4 Gy using a Gamma Cell cesium irradiator (JL Shepherd, San Fernando, CA), with a dose rate of 70 cGy/min. This radiation dose was selected based on our experience with mouse irradiation [25], and because a higher dose increased the risk of fetal or early neonatal death, or severe malformation (data not shown). All mice were unanesthetized at the time of irradiation.
Ten minutes after radiation or sham, half the mice from each group received JP4-039, prepared as previously described [26,27]. JP4-039 was dissolved at a concentration of 3 mg/ml in 10% ethanol, 10% Cremophor EL (BASF SE, Limburgerhof, Germany), and 80% water and administered intravenously at a dose of 10 mg/kg, with control mice receiving solvent alone. The mice were monitored for 4 days, and on E17.5, they were re-weighed and then sacrificed. Fetuses and placentas were procured and immediately weighed. One portion of the placenta was placed in RNAlater (Qiagen, Valencia, CA) overnight at 4 °C, then frozen at –80 °C until RNA extraction. Another portion was snap-frozen in liquid nitrogen. Tissue was also fixed in 4% paraformaldehyde, paraffin-embedded, sectioned, stained with hematoxylin and eosin, and microscopically examined using a Nikon Eclipse 90i microscope equipped with Nikon Elements software (both, Nikon Corporation, Tokyo). The image was then imported into ImageJ, version 1.44k (Rasband, W.S., ImageJ, U. S. NIH, Bethesda, MD), for stereologic analysis. Using the methods outlined by Howard [28], we processed vertical uniform random sections of mouse placenta with the Grid plugin. Systematic random vertical lines, 500,000/μm2 with random offset, were used to measure the height of the labyrinth and junctional zones.
2.3. RNA extraction and processing for RT-qPCR and microarrays
RNA was extracted from PHT cells using both TRI Reagent (Molecular Research Center, Cincinnati, OH) and the RNeasy Mini Kit (Qiagen) and following the respective manufacturers’ instructions. For extraction of mouse placental RNA, each placental specimen was homogenized and RNA extracted using TRI Reagent (Molecular Research Center) as above. RNA samples were incubated with DNase I, using a TURBO DNase Kit (Life Technologies, Grand Island, NY), and RNA quality and quantity were determined using a NanoDrop 1000 spectrophotometer (NanoDrop) as well as visualization on a denaturing agarose gel.
Reverse transcription of 1 μg of total RNA to cDNA was performed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies), with amplification in a Veriti thermal cycler (Applied Biosystems/Life Technologies Corp). Synthesized cDNA was then diluted 1:5 using nuclease-free water. Quantitative PCR was performed in duplicate. For in vivo analysis, ribosomal protein L32 (Rpl32) was the internal control, while for in vitro analysis, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ) was the internal control. Quantitative PCR was performed using a 384-well plate with a total reaction volume of 10 μl that included 3 μl of cDNA, 1 μl of forward primer, 1 μl of reverse primer, and 5 μl of SYBR Green PCR Master Mix (Life Technologies). Quantitative PCR was performed using a 7900HT Fast Real-Time PCR System (Applied Biosystems). Because each placental preparation yielded cultured trophoblasts that were subject to radiation or control, gene expression in vitro was performed using the delta delta CT method [29], whereas the delta CT method was used for samples derived from in vivo experiments.
We used high-throughput microarray analysis to screen for radiation-induced transcriptional changes in cultured PHT cells or mouse placentas. All samples were first examined with an Agilent High-Resolution C Scanner (Agilent Technologies, Santa Clara, CA) to ensure RNA integrity and quality. For cultured PHTs, we analyzed the RNA using the Agilent SurePrint G3 Human GE 8 × 60K arrays (Agilent Technologies). Mouse placental RNA was analyzed using the MouseWG-6 Expression BeadChip arrays (Illumina, San Diego, CA). Microarray data were analyzed using a moderated t-statistic [30]. We then ranked the log2 expression ratio (radiation:sham) for each significantly changed transcript. For the PHT RNA data, which encompassed 3 time points (4 h, 8 h, and 24 h), we ranked transcripts by the maximum log2 expression ratio over the entire 24 h time course. We then selected a merged subset of the top 1% and bottom 1% of differentially expressed RNA from PHTs and from mouse placentas.
2.4. Western immunoblotting
PHT proteins were extracted with cell lysis buffer (Tris–HCl 50 mM pH 7.4, NaCl 150 mM, Triton-X100 1%) containing protease inhibitors. Protein concentrations were measured with the Pierce BCA Protein Assay Reagent Kit. Each protein lysate (75 μg) was loaded into each lane of 12% polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride (PVDF) membrane. Mouse anti-CDKN1A antibody (1 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti poly (ADP-ribose) polymerase (PARP, 0.1 μg/ml, EMD Millipore, Billerica, MA), mouse monoclonal anti-cytokeratin 18 (0.1 μg/ml, Roche Diagnostics, Indianapolis, IN), or mouse anti-MnSOD (0.1 μg/ml, R&D, Minneapolis, MN) were used to detect protein expression at 4 °C overnight, while mouse anti-actin antibody [0.08 μg/ml] (EMD Millipore) was used to measure β-actin, which served as a loading control.
2.5. Statistical analysis
We used the Q–Q plot graphical method to determine whether the data were normally distributed. Missing data were removed after informal tests to ensure they were missing at random. When there were no correlated data, we analyzed the data with linear regression. When there were correlated data, we used linear mixed-effects modeling. The clustered variables were the mouse placenta in vivo or PHT cultures in vitro. For mouse placentas, radiation and JP4-039 were used as categorical predictor variables. The likelihood ratio test was used to determine whether a fixed or random effect was a significant contributor to the model. Alpha values < 0.05 were considered significant when building multivariable models. Parameters were estimated using the restricted maximum likelihood approach. All linear mixed-effects models were examined to ensure homoscedasticity of the response variable. We used R, version 2.15.1, for all our statistical analyses [31]. For linear mixed-effects model analyses, we used the lme4 package [32,33].
3. Results
3.1. The effect of cell irradiation on PHT cells in vitro
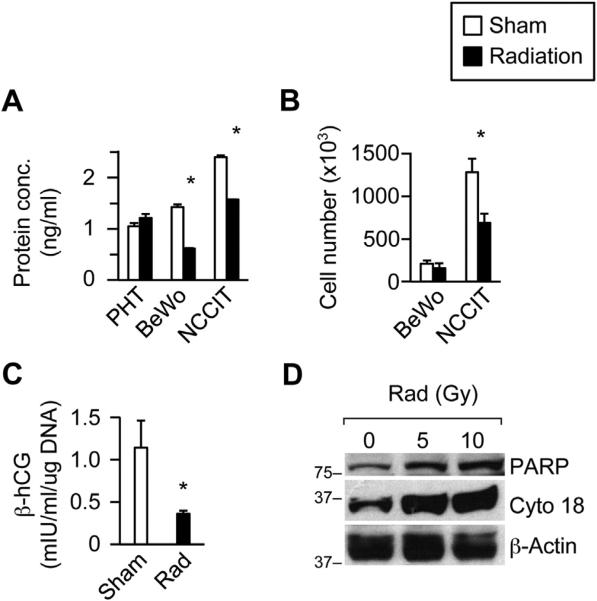
We first examined the effect of in vitro irradiation on PHT cell number. We measured total protein as a surrogate for cell number, because PHT cells do not divide in vitro, but differentiate into syncytia. As shown in Fig. 1A, radiation had no effect on protein content in PHT cells, determined 24 h after exposure. In contrast, radiation markedly reduced protein content in the BeWo placental line, as well as in the radiosensitive NCCIT cells, both capable of proliferation in vitro. We obtained similar results by measuring cellular DNA content (not shown). Exposure to radiation also reduced cell number in the radiosensitive NCCIT line, as expected, but not in BeWo cells (Fig. 1B). As shown in Fig. 1C, hCG production was diminished in irradiated PHT cells, compared to control, and was accompanied by a 73% reduction in hCG mRNA (p < 0.0001) This was accompanied by enhanced apoptosis, determined by the increased expression of PARP and cytokeratin 18 (Fig 1D).
Fig. 1.
The effect of ionizing radiation in vitro on cultured human trophoblasts. Radiation was delivered as described in Methods. (A) Protein concentration, (B) Cell number, (C) Medium β-hCG levels normalized to total cellular DNA, (D) Expression of PARP and cytokeratin 18. Note that cell number was not recorded for PHT cells, which do not proliferate in vitro. Radiation dose was 10 Gy in PHT cells (5 and 10 GY in Panel D), but only 5 Gy in the placental line BeWo or in the radiosensitive mixed germ cell tumor cells NCCIT. Sham was an identical procedure without radiation. The data are for a representative experiment, which was repeated at least three times using cells from three different placentas. *Denotes p < 0.05.
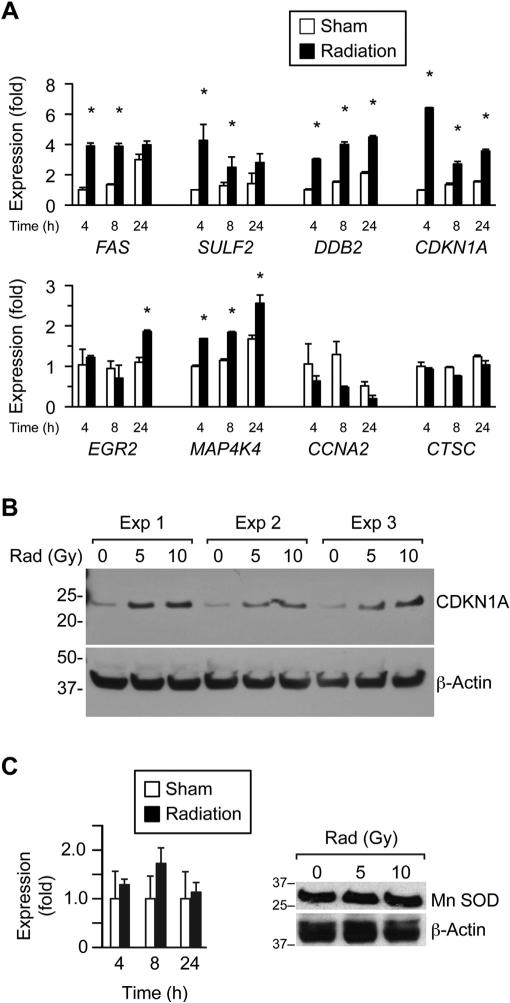
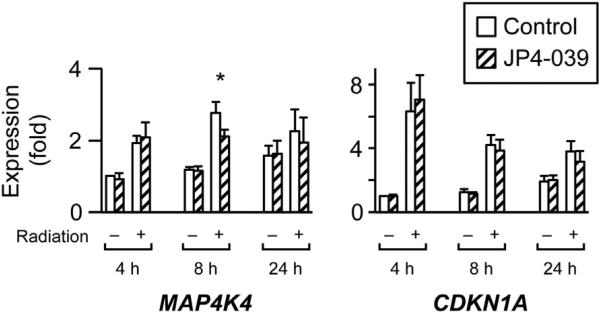
To comprehensively interrogate the impact of cell irradiation on gene expression in PHT cells, we used high-throughput microarray screening to measure gene expression at 4, 8, and 24 h after exposure to radiation, at a dose of 10 Gy (NCBI-GEO reference number GSE49924). Using a mixed-effects model with adjustment for false discovery, our microarray screen identified 1200 genes that were upregulated in irradiated PHT cells and 1050 genes that were downregulated by irradiation (q < 0.05). We ranked the log2 expression ratio of these significantly affected genes and chose a subset of the top 1% and bottom 1% for RT-qPCR validation, including the upregulated genes CDKN1A, DDB2, EGR2, FAS, SULF2, and MAP4K4 and the downregulated genes CCNA2 and CTSC. As seen in Fig. 2A, most expression changes observed in the selected genes by microarrays were verified using RT-qPCR, with the greatest effect commonly in the first 4 h after irradiation. We further confirmed our results by determining protein expression for the most upregulated mRNA, cyclin-dependent kinase inhibitor 1A (CDKN1A). As expected, the expression of the CDKN1A protein was upregulated by PHT cell irradiation (Fig. 2B). In contrast, the expression of the MnSOD, which is known to be radioprotective [34], was unchanged (Fig. 2C). To assess whether the nitroxide JP4-039 mitigates irradiation injury in PHT cells, we added the drug to the cell culture 1 h before radiation or sham exposure. We found that a single dose of 10 μM JP4-039 had no effect on medium hCG levels (not shown) or on the genes presented in Fig. 2 (including the largest effect, on CDKN1A), except for a weak attenuation of MAP4K4 expression change at 8 h (Fig. 3).
Fig. 2.
The effect of ionizing radiation in vitro on gene expression in cultured human trophoblasts. (A) Transcripts were selected for RT-qPCR assay (n = 6) based on mRNA array assays, performed as described in Methods. Radiation dose was 10 Gy, and sham was an identical procedure without radiation. *Denotes p < 0.05. (B) A western analysis of the expression change in CDKN1A at several radiation doses ranging from 0 to 10 Gy and repeated three times using three PHT cell cultures, each prepared from a different placenta. The data were normalized to actin and scanned, confirming (p < 0.05) a dose-dependent increase in CDKN1A expression. (C) The expression of MnSOD by RT-qPCR (left panel) and western analysis (right panel). None of the differences were significant.
Fig. 3.
The effect of JP4-039 on the expression of selected genes in PHTs. All transcripts shown in Fig. 2 were tested using RT-qPCR. Only MAP4K4 at the 8 h time point was affected by JP4-039. CDKN1A was the most affected transcript in Fig. 2 and is shown for comparison. DMSO was used as control for JP4-039. The data are from a representative experiment which was repeated three times. *Denotes p < 0.05.
3.2. The effect of whole body irradiation on the mouse placenta in vivo
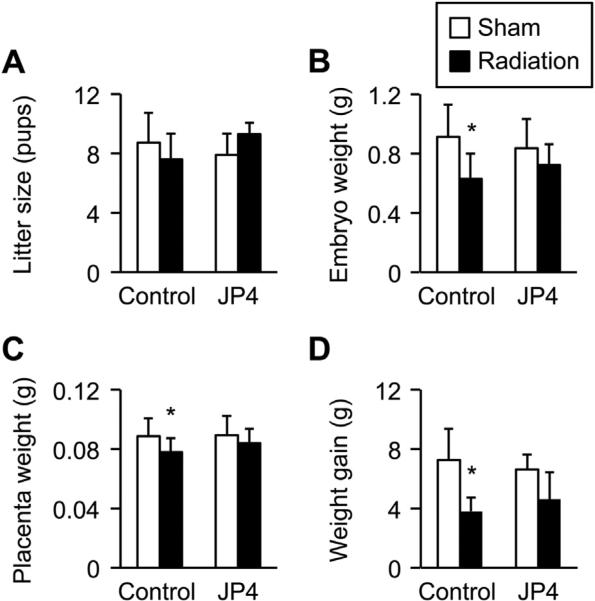
To determine the impact of radiation injury on placental function and gene expression, we exposed pregnant mice at E13.5 to whole body irradiation as describe in Methods and analyzed physiologically relevant outcomes at E17.5. As shown in Fig. 4, radiation reduced fetal weight, with an insignificant effect on litter size and a small, yet significant, effect on placental weight. No gross fetal or placental anomalies were noted. Interestingly, maternal weight gain was also reduced after exposure to ionizing radiation. None of these parameters was significantly affected by treatment with a single dose of 10 mg/kg of JP4-039, 10 min post irradiation (Fig. 4).
Fig. 4.
The effect of radiation and JP4-039 on murine pregnancy at E17.5. Whole body irradiation at 0 Gy (sham) or 4 Gy (radiation) was performed on E13.5 as detailed in Methods. JP4-039 (10 mg/kg) was administered intravenously 10 min after radiation or sham. (A) Litter size, (B) fetal weight, (C) placenta weight, (D) maternal weight gain. Data were based on non-irradiated dams (n = 30, of which 8 received JP4-039), and irradiated dams (n = 17, of which 7 received JP4-039. *Denotes p < 0.05).
To gain further insight into the effect of total body irradiation on the placenta, we used stereology, as described in Methods, to analyze irradiated placental morphology. Focusing on the labyrinthine and junctional zones, we found no significant differences between irradiated or sham-exposed placenta. Specifically, using vertical uniform random sections, the mean height of the junctional zone was 365 ± 24 μm for the sham-exposed dams, which decreased by 56 ± 43 μm in irradiated placentas (p = 0.2). The mean height of the labyrinth was 935 ± 28 μm for the sham-exposed dams, which was unchanged in the radiation-exposed dams (934 ± 45 μm, p = 0.99).
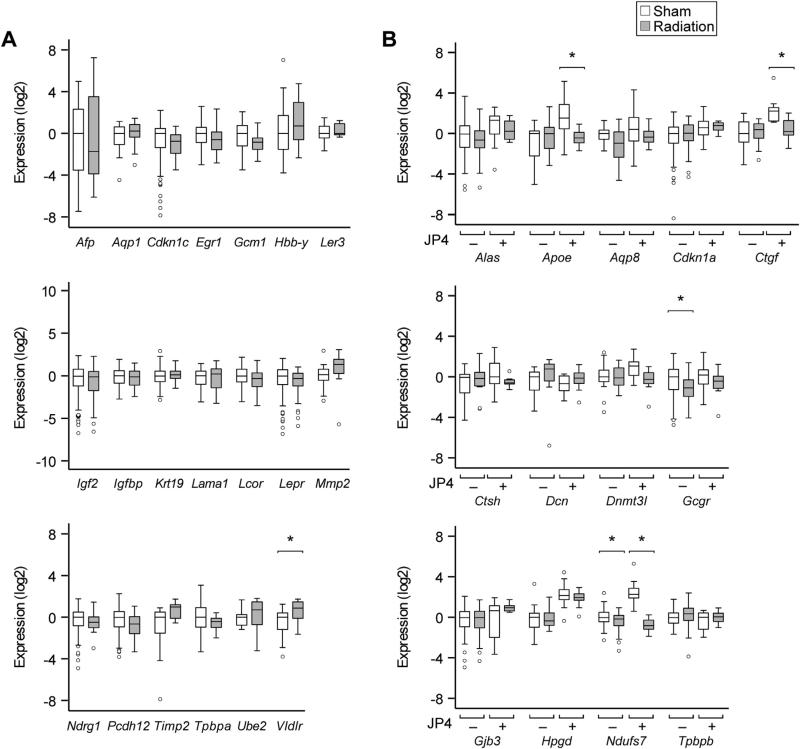
We employed high-throughput microarray screening to examine the impact of whole body irradiation on gene expression in the mouse placenta (n = 6 for sham vs. radiation, NCBI-GEO reference number GSE49924). We found that the log2 expression ratio ranged between –2.01 and 1.96, and none of the approximately 30,000 tested mRNAs was significantly different after adjustment for false discovery. We used RT-qPCR analysis of gene products exhibiting the greatest change from the 1% most up- or downregulated genes, and included genes that are relevant to mouse placental biology or to radiation injury. The expression of a subset of these mRNA was also tested in mice exposed to JP4-039. As seen in Fig. 5A–B, radiation weakly affected the expression of Vldlr (1.6-fold increase), Gcgr (1.6-fold decrease), and Ndufs7 (1.5-fold decrease). We also found that JP4-039 modified the effect of radiation on three genes: Apoe, Ctgf, and Ndufs7. Most gene products, however, were resistant to the effect of radiation or JP4-039 treatment.
Fig. 5.
The effect of radiation, with or without JP4-039, on mouse placental gene expression. Whole body irradiation at 0 Gy (sham) or 4 Gy (radiation) was performed on E13.5 as detailed in Methods. JP4-039 (10 mg/kg) was administered intravenously 10 min after radiation or sham. (A) Gene products tested for the effect of radiation and (B) gene products tested for the effect of radiation and the interaction with JP4-039. *Denotes p < 0.05.
4. Discussion
Our data indicate that, while ionizing radiation affects human trophoblasts in vitro and mouse fetal growth in vivo, the impact of radiation on placental gene expression is relatively modest and might not account for the full effect of radiation on fetal growth.
In vitro, irradiation of PHT cells led to reduced medium β-hCG levels and gene expression, and enhanced apoptosis. The extent of global gene expression was overall small. Among gene products that exhibited a significant change in vitro, the greatest effect was an increase in CDKN1A mRNA and protein expression. Indeed, CDKN1A, commonly regulated by p53, is known to be upregulated by radiation injury, resulting in G1-phase cell cycle arrest and probable protection against apoptosis [35–37].
Other gene products that we found to be regulated in vitro or in vivo might reflect radiation injury, or its consequences. FAS plays a key role in trophoblast apoptosis [38]. FAS and MAP4K4 both activate MAPK8, and are required for TNFα-induced apoptosis [35]. SULF2, a member of the heparan sulfate 6-O-endosulfatases family, regulates signaling cascades through release of growth factors from storage sites and is possibly involved in tissue regeneration [39]. Interestingly, SULF2 is a target of p53 during doxorubicin-induced DNA damage in HepG2 cells [40]. DDB2, a damage-related protein and part of a heterodimeric complex that participates in nucleotide excision repair, is increased in irradiated human fibroblasts [41]. EGR2 is a member of the early growth response family of transcription factors and is known to be upregulated after wound injury in the mouse [42]. The VLDL receptor (VLDLR) probably plays a role in placental cholesterol metabolism, which may be disrupted as a consequence of cell injury [43]. The glucagon receptor (GCGR) is implicated in maintaining adequate carbohydrate supply to the fetus. Gcgr−/− fetuses exhibit placental vascular damage, vacuolization of syncytiotrophoblasts, and diffuse interstitial trophoblast hyperplasia [44]. NDUFS7 is a complex 1 mitochondrial respiratory chain protein. In addition to a role in Leigh Syndrome, with severe lactic acidosis in infants or muscle weakness in adults, reduced levels of NDUFS7 have been associated with oxidative damage [45].
Although the changes in gene expression identified in our study may not account for the 25% reduction in mean fetal weight, we emphasize that we exposed mice to radiation injury at E13.5, a time when placental growth has nearly plateaued, yet fetal growth remains rapid [46]. This may also explain the relatively small effect of radiation on placental phenotype. Because we used whole body irradiation, the observed fetal growth restriction may reflect a direct radiation effect on the fetus, affecting neurons, soft tissue and epithelial surfaces, and thus impacting growth. Such a direct injury was not probed in the present study. It is likely that radiation at an earlier point in pregnancy might have exerted a greater impact on the developing placenta. Another confounding factor that we could not control was for the significant reduction in maternal weight gain, reflecting reduced fetal weight and a small reduction in litter size. In addition, it likely reflects reduced food intake by irradiated dams, with resultant reduction in fetal growth. It is intriguing that, despite irradiation and reduced maternal food intake, placental histology and gene expression patterns were relatively intact, further supporting the notion that the established placenta, in the last third of pregnancy, is fairly resistant to these injuries.
The dose of radiation used in our study was intended to affect fetal growth, with minimal effect on survival. Indeed, the use of 5 Gy in preliminary experiments led to a high rate of fetal death, that was prohibitive for our studies on fetal growth. A previous study showed a 5% reduction in fetal growth when Swiss albino mice were irradiated at a similar gestational age, but at ≤1.5 Gy [5]. We used C57BL/6HNsd. It is known that different mouse strains exhibit a range of susceptibility to total body irradiation [47].
Our attempt to mitigate radiation injury with the GS-nitroxide JP4-039 led to a weak and sporadic effect on several gene products. JP4-039 did not alter in vitro differentiation of control or irradiated cells (not shown). A prior study showed that JP4-039 given 1 h before radiation improved survival of 32Dcl3 cells [24], probably reflecting cell sensitivity to radiation injury. Importantly, JP4-039 did not affect fetal weight, placenta weight, or mouse weight gain. The impact of JP4-039 or other targeted reactive oxygen species scavengers [48] at different gestational ages, any beneficial or deleterious effects, the timing of administration relative to the exposure, trans-placental transport, and the optimal dose remain to be established.
Although exposure of pregnant women to less than 0.05 Gy has not been associated with an increased rate of fetal anomalies or spontaneous abortion [49], a safe radiation dose for trophoblasts has not been defined. Our data suggest that even though radiation affects placental trophoblasts, the established placenta is fairly resistant to radiation. Our study underscores the need to interrogate the impact of radiation and radiation-mitigating drugs on the feto-placental unit. Additional doses, used at early and late gestational ages, might be instrumental in guiding safety parameters in humans.
Acknowledgments
This work was supported in part by an NIH grant, U19 AI068021, to JSG; NIH grants R01HD045675 and R01ES011597 to YS; and Pennsylvania Department of Health Research Formula Funds to YS. The authors thank Judy Ziegler and Elena Sadovsky for technical assistance and Lori Rideout and Bruce Campbell for assistance in manuscript preparation.
References
- 1.Nations United . Sources and effects of ionizing radiation. United nations scientific committee on the effects of atomic radiation (UNSCEAR) 2000 report to the general assembly. United Nations; New York: 2000. [Google Scholar]
- 2.Mettler FA, Jr, Voelz GL. Major radiation exposureewhat to expect and how to respond. N Engl J Med. 2002;346:1554–61. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, Kai M, Kusama T. The embryonic and fetal effects in ICR mice irradiated in the various stages of the preimplantation period. Radiat Res. 1997;147:735–40. [PubMed] [Google Scholar]
- 4.De Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF, Caruso A. Radiation effects on development. Birth Defects Res C Embryo Today. 2007;81:177–82. doi: 10.1002/bdrc.20099. [DOI] [PubMed] [Google Scholar]
- 5.Devi PU, Hossain M. Effect of early fetal irradiation on the postnatal development of mouse. Teratology. 2001;64:45–50. doi: 10.1002/tera.1046. [DOI] [PubMed] [Google Scholar]
- 6.Howell EK, Gaschak SP, Griffith KD, Rodgers BE. Radioadaptive response following in utero low-dose irradiation. Radiat Res. 2013;179:29–37. doi: 10.1667/RR3029.1. [DOI] [PubMed] [Google Scholar]
- 7.Petrova A, Gnedko T, Maistrova I, Zafranskaya M, Dainiak N. Morbidity in a large cohort study of children born to mothers exposed to radiation from chernobyl. Stem Cells. 1997;15(Suppl. 2):141–50. doi: 10.1002/stem.5530150721. [DOI] [PubMed] [Google Scholar]
- 8.Jeggo P, Lavin MF. Cellular radiosensitivity: how much better do we understand it? Int J Radiat Biol. 2009;85:1061–81. doi: 10.3109/09553000903261263. [DOI] [PubMed] [Google Scholar]
- 9.Rodemann HP, Blaese MA. Responses of Normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17:81–8. doi: 10.1016/j.semradonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Snyder AR, Morgan WF. Differential induction and activation of NF-kappaB transcription complexes in radiation-induced chromosomally unstable cell lines. Environ Mol Mutagen. 2005;45:177–87. doi: 10.1002/em.20092. [DOI] [PubMed] [Google Scholar]
- 11.Kis E, Szatmari T, Keszei M, Farkas R, Esik O, Lumniczky K, et al. Microarray analysis of radiation response genes in primary human fibroblasts. Int J Radiat Oncol Biol Phys. 2006;66:1506–14. doi: 10.1016/j.ijrobp.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Banaz-Yasar F, Tischka R, Iliakis G, Winterhager E, Gellhaus A. Cell line specific modulation of connexin43 expression after exposure to ionizing radiation. Cell Commun Adhes. 2005;12:249–59. doi: 10.1080/15419060500514101. [DOI] [PubMed] [Google Scholar]
- 13.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–36. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 14.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–50. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci. 2012;15:1407–13. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Bernard ME, Epperly MW, Shen H, Amoscato A, Dixon TM, et al. Amelioration of radiation esophagitis by orally administered p53/Mdm2/Mdm4 inhibitor (BEB55) or GS-nitroxide. In Vivo. 2011;25:841–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberger JS, Clump D, Kagan V, Bayir H, Lazo JS, Wipf P, et al. Strategies for discovery of small molecule radiation protectors and radiation mitigators. Front Oncol. 2011;1:59. doi: 10.3389/fonc.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huppertz B, Borges M. In: Placenta trophoblast fusion. Chen EH, editor. Humana Press; Totowa, NJ: 2008. pp. 135–47. [DOI] [PubMed] [Google Scholar]
- 19.Damjanov I, Horvat B, Gibas Z. Retinoic acid-induced differentiation of the developmentally pluripotent human germ cell tumor-derived cell line, NCCIT. Lab Invest. 1993;68:220–32. [PubMed] [Google Scholar]
- 20.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y. Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol. 1999;180:896–902. doi: 10.1016/s0002-9378(99)70661-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–72. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- 23.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, et al. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603–12. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, et al. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Epperly MW, Smith T, Zhang X, Goff JP, Franicola D, Greenberger B, et al. Modulation of in utero total body irradiation induced newborn mouse growth retardation by maternal manganese superoxide dismutase-plasmid liposome (MnSOD-PL) gene therapy. Gene Ther. 2011;18:579–83. doi: 10.1038/gt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frantz MC, Pierce JG, Pierce JM, Kangying L, Qingwei W, Johnson M, et al. Large-scale asymmetric synthesis of the bioprotective agent JP4-039 and analogs. Org Lett. 2011;13:2318–21. doi: 10.1021/ol200567p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frantz MC, Skoda EM, Sacher JR, Epperly MW, Goff JP, Greenberger JS, et al. Synthesis of analogs of the radiation mitigator JP4-039 and visualization of BODIPY derivatives in mitochondria. Org Biomol Chem. 2013;11:4147–53. doi: 10.1039/c3ob40489g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. Garland science/BIOS Scientific Publishers (US distribution by Taylor & Francis); Abingdon, Oxon, UK: 2005. [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004:3. doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team . R: a language and environment for statistical computing. R foundation for statistical computing (Vienna); 2011. http://www.R-project.org/ [Google Scholar]
- 32.Bates D, Maechler M, Bolker B. lme4: linear mixed-effects models using eigen and S4. R Package Version 0999902344-0. 2012 [Google Scholar]
- 33.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, et al. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–78. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 35.Kuribayashi K, Finnberg N, Jeffers JR, Zambetti GP, El-Deiry WS. The relative contribution of pro-apoptotic p53-target genes in the triggering of apoptosis following DNA damage in vitro and in vivo. Cell Cycle. 2011;10:2380–9. doi: 10.4161/cc.10.14.16588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–8. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 37.Daino K, Ichimura S, Nenoi M. Early induction of CDKN1A (p21) and GADD45 mRNA by a low dose of ionizing radiation is due to their dose-dependent post-transcriptional regulation. Radiat Res. 2002;157:478–82. doi: 10.1667/0033-7587(2002)157[0478:eiocpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol. 1998;110:495–508. doi: 10.1007/s004180050311. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura I, Fernandez-Barrena MG, Ortiz-Ruiz MC, Almada LL, Hu C, Elsawa SF, et al. Activation of the transcription factor GLI1 by WNT signaling underlies the role of SULFATASE 2 as a regulator of tissue regeneration. J Biol Chem. 2013;288:21389–98. doi: 10.1074/jbc.M112.443440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chau BN, Diaz RL, Saunders MA, Cheng C, Chang AN, Warrener P, et al. Identification of SULF2 as a novel transcriptional target of p53 by use of integrated genomic analyses. Cancer Res. 2009;69:1368–74. doi: 10.1158/0008-5472.CAN-08-2742. [DOI] [PubMed] [Google Scholar]
- 41.Ray A, Milum K, Battu A, Wani G, Wani AA. NER initiation factors, DDB2 and XPC, regulate UV radiation response by recruiting ATR and ATM kinases to DNA damage sites. DNA Repair (Amst) 2013;12:273–83. doi: 10.1016/j.dnarep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grose R, Harris BS, Cooper L, Topilko P, Martin P. Immediate early genes krox-24 and krox-20 are rapidly up-regulated after wounding in the embryonic and adult mouse. Dev Dyn. 2002;223:371–8. doi: 10.1002/dvdy.10064. [DOI] [PubMed] [Google Scholar]
- 43.Wittmaack FM, Gafvels ME, Bronner M, Matsuo H, McCrae KR, Tomaszewski JE, et al. Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology. 1995;136:340–8. doi: 10.1210/endo.136.1.7828550. [DOI] [PubMed] [Google Scholar]
- 44.Ouhilal S, Vuguin P, Cui L, Du XQ, Gelling RW, Reznik SE, et al. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: a role for glucagon action in pregnancy maintenance. Am J Physiol Endocrinol Metab. 2012;302:E522–31. doi: 10.1152/ajpendo.00420.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatr. 2010;67:360–8. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki K, Kobayashi M, Kobayashi K, Shiraishi Y, Goto S, Hoshino T. Structural and functional change of blood vessel labyrinth in maturing placenta of mice. Placenta. 1997;(Suppl. 18):155–64. [Google Scholar]
- 47.Wallace M, Coates PJ, Wright EG, Ball KL. Differential post-translational modification of the tumour suppressor proteins Rb and p53 modulate the rates of radiation-induced apoptosis in vivo. Oncogene. 2001;20:3597–608. doi: 10.1038/sj.onc.1204496. [DOI] [PubMed] [Google Scholar]
- 48.Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51:462–75. doi: 10.1002/em.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ACOG Committee Opinion Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647–51. doi: 10.1097/00006250-200409000-00053. [DOI] [PubMed] [Google Scholar]