Abstract
Background & Aims
Reliable community-based colorectal adenoma prevalence estimates are needed to inform colonoscopy quality standards and to estimate patient colorectal cancer risks; however, minimal data exist from populations with large numbers of diverse patients and examiners.
Methods
We evaluated the prevalence of adenomas detected by sex, age, race/ethnicity, and colon location among 20,792 Kaiser Permanente Northern California members ≥50 years of age who received a screening colonoscopy exam (102 gastroenterologists, years 2006-2008).
Results
Prevalence of detected adenomas increased more rapidly with age in the proximal colon (adjusted odds ratio [OR] = 2.39; 95% confidence interval [CI]: 2.05-2.80; 70-74 vs. 50-54 years) than in the distal colon (OR=1.89; 95% CI: 1.63-2.19). Prevalence was higher among men vs. women at all ages (OR=1.77; 95% CI: 1.66-1.89), increasing in men from 25% to 39% at ≥70 years and in women from 15% at 50-54 years to 26 % (P<0.001). Proximal adenoma prevalence was higher among blacks than whites (OR=1.26; CI: 1.04-1.54); although total prevalence was similar, including for persons <60 years old (OR=1.17; 95% CI: 0.91-1.50).
Conclusions
Prevalence of detected adenomas increases substantially with age and is much higher in men; proximal adenomas are more common among blacks than whites, although the total prevalence and the prevalence for ages <60 years were similar by race. These demographic differences are such that current adenoma detection guidelines may not be valid, without adjustment, for comparing providers serving different populations. The variation in prevalence and location may also have implications for the effectiveness of screening methods in different demographic groups.
Keywords: adenoma/diagnosis, colonic neoplasms/diagnosis, polyps/diagnosis, colonoscopy/methods, colonoscopy/standards
Introduction
Colorectal cancer is the second leading cause of cancer death in the United States.1-2 Colonoscopy is the primary screening test or the follow-up method for all screening strategies and is one of the most commonly performed medical procedures in the United States.3-4 Its use can reduce colorectal cancer mortality through the detection of early-stage adenocarcinomas and the detection and removal of precursor adenomatous polyps;3-4 however, there are minimal detailed demographic data on adenoma prevalence at colonoscopy from large, community-based sources.
Accurate community-based estimates of the prevalence of colorectal neoplasms (adenomatous polyps and cancer; hereafter collectively referred to as adenomas) detected at colonoscopy by age, sex, and race/ethnicity are needed for two reasons: to serve as reference “normal ranges” for developing colonoscopy exam quality standards and to provide estimates of individual-level cancer risk for different demographic groups. The clinical and medicolegal ramifications of cancers developing despite screening have prompted the need for quality standards for exam performance, particularly for colonoscopy, given its frequency of use.5-6 The adenoma detection rate of examiners – the percentage of a physician's patients undergoing colonoscopy in whom a colorectal adenoma or cancer is found7 – has emerged as the main potential measure of colonoscopy performance quality; such rates may predict subsequent colorectal cancer risk following a screening colonoscopy.8 Current guidelines suggest that adenomas should be detected in ≥15% of women and in ≥25% of men who undergo screening colonoscopy;6 however, these estimates were originally obtained from small referral populations, and there are minimal data on the prevalence of adenomas detected in diverse populations from large, community-based sources.3 Community-based estimates of the prevalence of detected adenomas by age, sex and race/ethnicity would better inform standards and allow accurate comparisons of colonoscopists who practice within different patient populations.
Adenoma prevalence has been used to estimate cancer risk and to identify which demographic populations may experience the highest potential benefit from adenoma removal. Individualized screening strategies based on patient risk and benefit have been advocated by the National Cancer Institute and by experts in the field as a potential means of increasing screening effectiveness while simultaneously decreasing cost.9-10 The goal is to create screening and surveillance methods that are patient centered, effective, efficient, timely (occurring at appropriate time points and intervals, balancing risk and benefit), equitable (equal access to care), and safe.9
Although adenoma prevalence data are needed, to our knowledge, only one estimate exists from a large community-based population in the United States. That estimate, from the Clinical Outcomes Research Initiative (CORI), primarily used polyp size without pathology for many analyses, because there was not comprehensive access to pathology data.11 Calculating an accurate prevalence for detected adenomas requires linkages between clinical and pathology databases that are difficult to achieve. Thus, almost all prior reports originated from small populations, referral centers, had relatively few colonoscopists (limiting generalizability), lacked data by age or race/ethnicity, or lacked comprehensive pathology data on all patients.8, 11-17
The present study was undertaken to provide community-based estimates for the prevalence of adenomas detected during screening colonoscopy within a large population of patients, across multiple centers, and with a large number of examiners. The primary aims were to contrast prevalence and adjusted risks by age, sex, race/ethnicity, and location within the colon.
Methods
Setting
This is a retrospective cohort study among members of Kaiser Permanente Northern California (KPNC), an integrated health services delivery organization which serves approximately 3.3 million people across 15 medical centers in urban, suburban, and semi-rural regions within a large geographic area. Its membership demographics closely approximate the diverse underlying population of Northern California, as compared with census demographics, including members with Medicare, Medicaid (low-income), and commercial insurance; thus, studies within this setting provide results that can be generalized to a large region.18-19 The study was approved by the KPNC institutional review board. Patients included had undergone a screening colonoscopy between January 1, 2006 and December 31, 2008 and were ≥50 years of age at the time of the examination.
Colonoscopy Exposure Ascertainment
Electronic records of colonoscopy exams were retrieved from electronic databases using Current Procedural Terminology (CPT) codes.20 We included only exams performed by board certified gastroenterologists who had performed at least 300 total and 75 screening exams during the study period and excluded a small number of exams performed by physicians at facilities outside their regular service area.
Colonoscopy procedure indications (screening vs. non-screening) were assigned using information from electronic gastroenterology referral forms along with electronic laboratory, pathology, radiologic, and diagnostic databases. Similar to other previous large screening studies,12, 15 we included examinations performed on patients with a family history of colorectal cancer. Examinations were considered non-screening and excluded from analysis if either the gastroenterology referral or the databases indicated any of the following: symptoms in the preceding 6 months (e.g., abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, occult or overt blood in stools, unexplained weight loss, abnormal abdominal imaging, or diverticulitis); a prior colorectal adenoma; a prior history of colorectal cancer; an inflammatory bowel disease diagnosis within the previous 10 years; a colonoscopy within the previous 10 years; a sigmoidoscopy within the previous 5 years; or a positive test for stool hemoglobin within the previous 1 year.
Outcome Assignment
Adenoma prevalence (synonymous with adenoma detection rate) was defined as the proportion of patients undergoing a screening examination in which one or more histologically confirmed colorectal adenomas or adenocarcinomas were detected. We refer to the combined prevalence of detected adenomas and adenocarcinomas, pooled across many providers, collectively throughout this report as adenoma prevalence, consistent with prior reports of adenoma detection rates for colonoscopy quality studies.21 Few patients had cancers and almost all patients with cancers had co-existing adenomas.
Adenoma presence and location were determined with Systematized Nomenclature of Medicine (SNOMED) codes in an electronic pathology database, using the colorectal “T” location code for colon segment and “M” pathology codes for histology (e.g., tubular adenoma, tubulovillous adenoma, and adenocarcinoma). Cancers were identified using a Kaiser Permanente cancer registry that reports to the Surveillance, Epidemiology and End Results (SEER) registry. Since more than one polyp from any given location may have been placed in a single specimen container, it was not possible to determine the exact number of adenomas detected per location. Since polyp size is only included as free text in procedure reports, and is not used as a quality measure in current guidelines, size is not reported in the current analysis. Proximal adenomas were defined as those occurring in the cecum, ascending colon, hepatic flexure, and transverse colon; distal adenomas were those occurring in the splenic flexure, descending colon, sigmoid colon, and rectum.
Other Variables
Sex, age, and race/ethnicity were obtained from electronic medical records; family history of colorectal cancer was recorded from the gastroenterology referral indication field and from the family history field within the electronic medical record.
Validation Studies
We performed several validation studies to contrast the accuracy of the electronic data capture methods described with the results from manual chart abstractions of progress, pathology and procedure notes (details not shown). These evaluations confirmed a very high level of agreement and/or sensitivity for: capture of colonoscopy exam performance compared with manual procedure log books (99%); assignment of a screening vs. non-screening exam indication (98%); assignment of adenoma status (yes/no) (100%); assignment of polyp histology for each container in patients with multiple containers (e.g. any adenoma vs. all non-adenoma); and assignment of adenoma location compared with the location described in the colonoscopy reports.
For approximately 7.5% of all patients (31% of patients with adenomas), the SNOMED location code indicated only “colon” or did not specify a colon location. The proportions of such examinations were approximately evenly distributed by sex, age, and racial/ethnic groups. We manually evaluated detailed endoscopy and pathology reports from a random sample of 125 such patients; 65% were found to be in the distal colon and the endoscopist had assigned a measurement (e.g. “polyp at 25 centimeters”) rather than a colon location. We explored sensitivity analyses using four different strategies: 1) exclusion of these patients; 2) inclusion with imputation to proximal, distal, or both locations using the exact proportions found in the validation study; 3) assignment of all such adenomas to a distal location; and 4) assignment of all to a proximal location. The risk estimates and conclusions by sex, age, and race/ethnicity were similar for all four approaches; thus, the location estimates presented utilize the imputed values with the exact proportions obtained from the validation study.
Statistical Analyses
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina) and STATA version 10.1 (StataCorp, College Station, Texas).
Comparisons of categorical data were performed using Pearson's chi-square test of independence. Evaluation of trends for prevalence across age categories used the Cochrane-Armitage test for trend.
Unadjusted prevalence estimates and 95% confidence intervals (CI) were determined for each category of age (50-54, 55-59, 60-64, 65-69, 70-74, and ≥75 years), including stratifications by race/ethnicity and sex. These statistics were also determined separately for proximal and distal locations.
We evaluated the associations between the prevalence of detected adenomas and patient sex, age category, and race/ethnicity using multilevel logistic regression models. We estimated risk using odds ratios (OR) and 95% confidence intervals (CI), with clustering on physician and medical service area; this method accounts for within-physician and within-service area correlation of patient outcomes. Although no interaction by sex was detected with age or race/ethnicity, we present most results stratified by sex for ease of comparison to other studies. Stratified risk estimates were adjusted for age, race/ethnicity, and family history of colorectal cancer.
Results
Between January 2006 and December 2008, we identified 20,792 screening colonoscopies among 125,462 total colonoscopy exams performed for any indication, for members ≥50 years of age. Exams were excluded for the following reasons: diagnostic or surveillance exam indications (including prior history of an adenoma) (n=73,922); colonoscopy within the previous 10 years, sigmoidoscopy within the previous 5 years, or a positive stool-blood test within the previous 1 year (n=28,890); procedures performed by an examiner with fewer than 300 total exams and 75 screening exams during the 3-year study period (n=1,855); and exams performed by physicians at facilities outside their regular service area (n=3). This resulted in 20,792 screening exams performed by 102 physicians. As expected, and consistent with reports from other institutions, there was substantial variation in adenoma detection rates between individual examiners; the lowest average for an examiner was 6.6 patients with at least one adenoma detected per 100 patients undergoing colonoscopy and the highest average for an examiner was 48.1. The mean was 24. 1 (men and women combined).
Of the patients undergoing screening exams (Table 1), 12,006 (58%) were female, 64% were white, 48% were <60 years of age at the time of their procedure, and 16% had a family history of colorectal cancer. At least one adenomatous polyp was found in 24.6% of all patients, and a colorectal cancer (including intramucosal cancer) was found in 1.5%. Almost all patients with a cancer were also diagnosed with an adenoma.
Table 1.
Patient demographics.
| Total n (%) |
Women n (%) |
Men n (%) |
|
|---|---|---|---|
| Total | 20,792 (100) | 12,006 (58) | 8,786 (42) |
|
| |||
| Age, years | |||
|
| |||
| 50-54 | 5,593 (27) | 3,195 (27) | 2,398 (27) |
| 55-59 | 4,493 (22) | 2,599 (22) | 1,894 (22) |
| 60-64 | 4,591 (22) | 2,718 (23) | 1,873 (21) |
| 65-69 | 2,903 (14) | 1,665 (14) | 1,238 (14) |
| 70-74 | 1,807 (9) | 1,052 (9) | 755 (9) |
| ≥75 | 1,405 (7) | 777 (6) | 628 (7) |
|
| |||
| Race/Ethnicity | |||
|
| |||
| White | 13,266 (64) | 7,877 (66) | 5,389 (61) |
| Black | 892 (4) | 543 (5) | 349 (4) |
| Asian | 2,152 (10) | 1,290 (11) | 862 (10) |
| Native American | 64 (0) | 31 (0) | 33 (0) |
| Multiracial | 290 (1) | 185 (2) | 105 (1) |
| Hispanic | 1,361 (7) | 817 (7) | 544 (6) |
| Unknown | 2,767 (13) | 1,263 (11) | 1,504 (17) |
|
| |||
| Family History of CRC | 3,240 (16) | 1,977 (16) | 1,263 (14) |
CRC, colorectal cancer.
Prevalence and Associations by Sex and Age
Adenoma prevalence was lower in women (20.2% had any adenoma, 1.2% had cancer [20.2% total]) than in men (30.6% had any adenoma with 1.8% cancer [30.6% total]). For all ages combined, men were more likely to have had colorectal adenomas than women (OR = 1.77; 95% CI: 1.66-1.89).
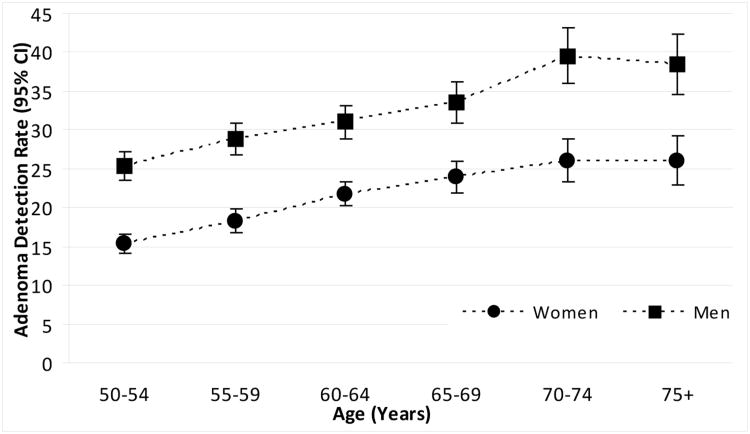
The estimated risk of an adenoma doubled from age 50-54 to age 70-74 (OR 2.00, 95% CI 1.76-2.26); the increase with age was similar for both men and women (test for interaction by sex, P=0.83). Adenoma prevalence rose across all 5-year age categories, reaching a peak at 70-74 years of age (test for trend: P<0.001 respectively) (Figure 1, Tables 2 and 3). Among women, the crude prevalence increased from 15% in patients 50-54 years of age to a high of 26% by ≥75 years of age (test for trend: P<0.001). Among men, the crude prevalence increased from 25% at 50-54 years of age to a high of 39% by 70-74 years of age (P<0.001).
Figure 1. Colorectal adenoma prevalence at screening colonoscopy for women and men, by age.
Table 2. Colorectal adenoma prevalence at screening colonoscopy.
| Total % (95% CI) |
Women % (95% CI) |
Men % (95% CI) |
|
|---|---|---|---|
|
| |||
| Age, years | |||
|
| |||
| 50-54 | 20 (19, 21) | 15 (14, 17) | 25 (24, 27) |
| 55-59 | 23 (22, 24) | 18 (17, 20) | 29 (27, 31) |
| 60-64 | 26 (24, 27) | 22 (20, 23) | 31 (29, 33) |
| 65-69 | 28 (26, 30) | 24 (22, 26) | 34 (31, 36) |
| 70-74 | 32 (30, 34) | 26 (23, 29) | 39 (36, 43) |
| ≥75 | 32 (29, 34) | 26 (23, 29) | 38 (35, 42) |
| Total | 25 (24, 25) | 20 (20, 21) | 31 (30, 32) |
| Trend: | P<0.001 | P<0.001 | P<0.001 |
|
| |||
| Race/Ethnicity | |||
|
| |||
| White | 24 (24, 25) | 20 (19, 21) | 30 (29, 32) |
| Black | 27 (24, 30) | 22 (19, 26) | 34 (29, 39) |
| Asian | 26 (24, 28) | 22 (19, 24) | 32 (29, 35) |
| Native American | 31 (20, 43) | 23 (7, 38) | 39 (22, 57) |
| Multiracial | 25 (20, 30) | 19 (14, 25) | 34 (25, 44) |
| Hispanic | 25 (22, 27) | 18 (16, 21) | 34 (30, 38) |
| Unknown | 25 (23, 26) | 20 (18, 22) | 29 (26, 31) |
| Total | 25 (24, 25) | 20 (20, 21) | 31 (30, 32) |
CI, confidence interval.
Table 3. Adjusted odds ratios for colorectal adenoma at screening colonoscopy.
| Total OR* (95% CI) |
Women OR (95% CI) |
Men OR (95% CI) |
|
|---|---|---|---|
|
| |||
| Sex | |||
|
| |||
| Women | 1.00 | ||
| Men | 1.77 (1.66, 1.89) | ||
|
| |||
| Age, years | |||
|
| |||
| 50-54 | 1.00 | 1.00 | 1.00 |
| 55-59 | 1.26 (1.14, 1.39) | 1.25 (1.08, 1.44) | 1.27 (1.10, 1.46) |
| 60-64 | 1.47 (1.34, 1.62) | 1.52 (1.32, 1.74) | 1.42 (1.23, 1.63) |
| 65-69 | 1.64 (1.47, 1.83) | 1.71 (1.47, 1.99) | 1.57 (1.35, 1.84) |
| 70-74 | 2.00 (1.76, 2.26) | 1.97 (1.66, 2.35) | 2.04 (1.70, 2.45) |
| ≥75 | 1.96 (1.71, 2.24) | 1.94 (1.60, 2.36) | 2.00 (1.65, 2.43) |
| Trend: | P<0.001 | P<0.001 | P <0.001 |
|
| |||
| Race/Ethnicity | |||
|
| |||
| White | 1.00 | 1.00 | 1.00 |
| Black | 1.15 (0.98, 1.35) | 1.16 (0.93, 1.45) | 1.14 (0.90, 1.45) |
| Asian | 0.96 (0.86, 1.08) | 0.98 (0.84, 1.14) | 0.96 (0.82, 1.13) |
| Native American | 1.48 (0.85, 2.56) | 1.21 (0.51, 2.88) | 1.78 (0.86, 3.70) |
| Multiracial | 0.92 (0.70, 1.22) | 0.86 (0.59, 1.26) | 1.04 (0.68, 1.59) |
| Hispanic | 0.98 (0.85, 1.12) | 0.84 (0.69, 1.02) | 1.17 (0.96, 1.42) |
| Unknown | 1.10 (1.00, 1.22) | 1.15 (0.98, 1.35) | 1.07 (0.93, 1.22) |
|
| |||
| Family History of CRC | 1.07 (0.98, 1.18) | 1.03 (0.91, 1.17) | 1.13 (0.99, 1.30) |
Odds ratios adjusted for age, race/ethnicity, and family history of colorectal cancer with clustering on physician and service area.
OR, odds ratio; CI, confidence interval; CRC, colorectal cancer.
Prevalence and Associations by Race/Ethnicity
Adenoma prevalence was consistently higher for men than women among all racial/ethnic groups overall (Table 2) and at each age interval (Figure 2), but there were no significant differences in adenoma prevalence between men or women across racial/ethnic groups (all ages combined) (Table 2) or within each age category (Figures 1 and 2). Prevalence trends for women were lowest among Hispanics at 18% and highest for Native Americans at 23%. For men, prevalence was lowest among whites at 30% and highest among Native Americans at 39%; however, due to small numbers, the estimates for Native Americans had wide confidence intervals (Table 2). In comparison to whites, adenoma risks did not differ significantly for other racial/ethnic groups (Table 3). In addition, in men and women combined, adenoma risk in blacks compared to whites did not differ significantly within age categories, or for persons <60 years (OR=1.17; 95% CI 0.91-1.50) and ≥60 years of age (OR=1.16; 95% CI 0.94-1.45); this was also the case for each sex separately (data not shown).
Figure 2. Colorectal adenoma at screening by age and race/ethnicity (women, panel A; men, panel B).
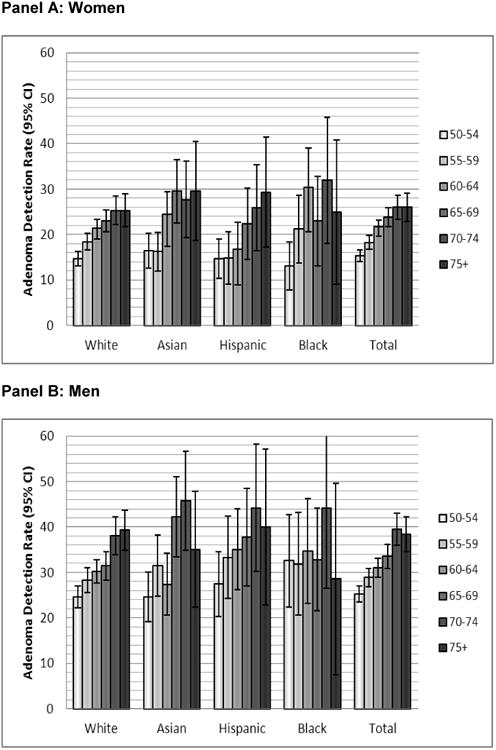
Panel A: Women
Panel B: Men
Note: On Panel B, the upper CI for 70-74 year old Black men is truncated from 61.7%.
Prevalence and Associations by Colon Location
The proportion of patients (men and women combined) with adenomas found only in the proximal colon approximately doubled with increasing age, from 7.0% at age 50-54 to 13.8% at age ≥75 (test for trend: P<0.001) (Table 4). A similar doubling was found for patients with adenomas in both the proximal and distal colon (increasing from 2.9% to 6.8%, P<0.001). Trends were similar for both men and women in stratified analyses, although adenoma prevalence was higher in men. In contrast, the prevalence of patients with only distal adenomas was relatively stable with age, increasing about one-fourth, from 9.8% to a high of 12.2% (P<0.001) (Table 4). For all ages combined, among women, adenomas were found only in the distal colon in 8.9% of examinations, only in the proximal colon in 8.3%, and in both locations in 3.1%. Among men, adenomas were detected only in the distal colon in 12.6% of examinations, only in the proximal colon in 11.9%, and in both locations in 6.1% (Table 4).
Table 4. Colorectal adenoma prevalence by colon location.
| Distal Only | Proximal Only | Distal and Proximal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | [95% CI] | n | (%) | [95% CI] | n | (%) | [95% CI] | |
| Sex | |||||||||
|
| |||||||||
| Women | 1,068 | (8.9) | [ 8.4, 9.4] | 993 | (8.3) | [ 7.8, 8.8] | 367 | (3.1) | [ 2.7, 3.4] |
| Men | 1,107 | (12.6) | [ 11.9, 13.3] | 1,047 | (11.9) | [ 11.2, 12.6] | 535 | (6.1) | [ 5.6, 6.6] |
|
| |||||||||
| Age, years | |||||||||
|
| |||||||||
| 50-54 | 547 | (9.8) | [ 9.0, 10.6] | 389 | (7.0) | [ 6.3, 7.6] | 161 | (2.9) | [ 2.4, 3.3] |
| 55-59 | 459 | (10.2) | [ 9.3, 11.1] | 401 | (8.9) | [ 8.1, 9.8] | 162 | (3.6) | [ 3.1, 4.2] |
| 60-64 | 458 | (10.0) | [ 9.1, 10.8] | 497 | (10.8) | [ 9.9, 11.7] | 216 | (4.7) | [ 4.1, 5.3] |
| 65-69 | 333 | (11.5) | [ 10.3, 12.6] | 331 | (11.4) | [ 10.2, 12.6] | 148 | (5.1) | [ 4.3, 5.9] |
| 70-74 | 221 | (12.2) | [ 10.7, 13.7] | 228 | (12.6) | [ 11.1, 14.2] | 123 | (6.8) | [ 5.6, 8.0] |
| ≥75 | 157 | (11.2) | [ 9.5, 12.8] | 194 | (13.8) | [ 12.0, 15.6] | 92 | (6.5) | [ 5.3, 7.8] |
| Trend: | P<0.001. | P<0.001 | P<0.001 | ||||||
|
| |||||||||
| Race/Ethnicity | |||||||||
|
| |||||||||
| White | 1,344 | (10.1) | [ 9.6, 10.6] | 1,304 | (9.8) | [ 9.3, 10.3] | 567 | (4.3) | [ 3.9, 4.6] |
| Black | 86 | (9.6) | [ 7.7, 11.6] | 102 | (11.4) | [ 9.3, 13.5] | 50 | (5.6) | [ 4.1, 7.1] |
| Asian | 239 | (11.1) | [ 9.8, 12.4] | 216 | (10.0) | [ 8.8, 11.3] | 98 | (4.6) | [ 3.7, 5.4] |
| Native American | 8 | (12.5) | [ 4.2, 20.8] | 8 | (12.5) | [ 4.2, 20.8] | 4 | (6.3) | [ 0.2, 12.3] |
| Multiracial | 31 | (10.7) | [ 7.1, 14.3] | 31 | (10.7) | [ 7.1, 14.3] | 10 | (3.4) | [ 1.3, 5.6] |
| Hispanic | 133 | (9.8) | [ 8.2, 11.4] | 131 | (9.6) | [ 8.1, 11.2] | 72 | (5.3) | [ 4.1, 6.5] |
| Unknown | 334 | (12.1) | [ 10.9, 13.3] | 248 | (9.0) | [ 7.9, 10.0] | 101 | (3.7) | [ 3.0, 4.3] |
|
| |||||||||
| Total | |||||||||
|
| |||||||||
| Total | 2,175 | (10.5) | [ 10.0, 10.9] | 2,040 | (9.8) | [ 9.4, 10.2] | 902 | (4.3) | [ 4.1, 4.6] |
Distal refers to the splenic flexure, descending colon, sigmoid colon, and rectum.
Proximal refers to the cecum, ascending colon, hepatic flexure, and transverse colon.
CI, confidence interval.
The adjusted odds ratios for any proximal adenoma and any distal adenoma are presented in Table 5. In men and women combined, the prevalence of any proximal adenoma (including patients with proximal location only and both proximal and distal locations) increased more rapidly with age than did that of any distal adenoma (ages 70-74 vs. 50-54; proximal adenomas OR=2.39; 95% CI: 2.05-2.80; distal adenomas OR 1.89, 95% CI: 1.63-2.19) (Table 5).
Table 5. Adjusted odds ratios for colorectal adenoma by colon location.
| Total OR* (95% CI) |
Women OR (95% CI) |
Men OR (95% CI) |
||
|---|---|---|---|---|
| Any Proximal Adenoma | ||||
|
| ||||
| Sex | ||||
|
| ||||
| Women | 1.00 | |||
| Men | 1.88 (1.73, 2.04) | |||
|
| ||||
| Age, years | ||||
|
| ||||
| 50-54 | 1.00 | 1.00 | 1.00 | |
| 55-59 | 1.39 (1.22, 1.58) | 1.30 (1.08, 1.57) | 1.48 (1.24, 1.77) | |
| 60-64 | 1.80 (1.59, 2.03) | 1.86 (1.56, 2.23) | 1.71 (1.43, 2.04) | |
| 65-69 | 1.90 (1.65, 2.18) | 1.91 (1.57, 2.33) | 1.89 (1.56, 2.30) | |
| 70-74 | 2.39 (2.05, 2.80) | 2.39 (1.91, 2.98) | 2.42 (1.94, 3.02) | |
| ≥75 | 2.43 (2.06, 2.88) | 2.21 (1.72, 2.83) | 2.71 (2.15, 3.41) | |
| Trend: | p<0.001 | p<0.001 | p<0.001 | |
|
| ||||
| Race/Ethnicity | ||||
|
| ||||
| White | 1.00 | 1.00 | 1.00 | |
| Black | 1.26 (1.04, 1.54) | 1.26 (0.96, 1.66) | 1.25 (0.95, 1.65) | |
| Asian | 0.94 (0.82, 1.08) | 0.93 (0.76, 1.13) | 0.94 (0.77, 1.14) | |
| Native American | 1.55 (0.79, 3.02) | 1.17 (0.39, 3.49) | 2.00 (0.84, 4.75) | |
| Multiracial | 0.88 (0.62, 1.25) | 0.81 (0.49, 1.33) | 1.02 (0.62, 1.70) | |
| Hispanic | 1.03 (0.87, 1.22) | 0.85 (0.66, 1.08) | 1.24 (0.99, 1.56) | |
| White | 1.03 (0.90, 1.17) | 1.12 (0.91, 1.38) | 0.98 (0.82, 1.16) | |
|
| ||||
| Family History of CRC | 1.08 (0.96, 1.22) | 1.13 (0.96, 1.33) | 1.06 (0.89, 1.26) | |
|
| ||||
| Any Distal Adenoma | ||||
|
| ||||
| Sex | ||||
|
| ||||
| Women | 1.00 | |||
| Men | 1.81 (1.67, 1.96) | |||
|
| ||||
| Age, years | ||||
|
| ||||
| 50-54 | 1.00 | 1.00 | 1.00 | |
| 55-59 | 1.18 (1.05, 1.33) | 1.18 (0.99, 1.41) | 1.17 (0.99, 1.38) | |
| 60-64 | 1.31 (1.16, 1.47) | 1.35 (1.14, 1.59) | 1.25 (1.06, 1.48) | |
| 65-69 | 1.52 (1.33, 1.73) | 1.60 (1.33, 1.93) | 1.43 (1.19, 1.72) | |
| 70-74 | 1.89 (1.63, 2.19) | 1.80 (1.45, 2.23) | 2.00 (1.62, 2.47) | |
| ≥75 | 1.74 (1.47, 2.06) | 1.77 (1.39, 2.25) | 1.72 (1.36, 2.18) | |
| Trend: | p<0.001 | p<0.001 | p<0.001 | |
|
| ||||
| Race/Ethnicity | ||||
|
| ||||
| White | 1.00 | 1.00 | 1.00 | |
| Black | 1.09 (0.90, 1.33) | 1.11 (0.84, 1.46) | 1.07 (0.80, 1.43) | |
| Asian | 0.99 (0.87, 1.13) | 1.02 (0.85, 1.23) | 0.96 (0.79, 1.17) | |
| Native American | 1.47 (0.76, 2.85) | 1.21 (0.41, 3.57) | 1.68 (0.72, 3.94) | |
| Multiracial | 0.89 (0.63, 1.25) | 0.82 (0.51, 1.32) | 1.00 (0.60, 1.67) | |
| Hispanic | 1.01 (0.86, 1.19) | 0.86 (0.68, 1.09) | 1.20 (0.95, 1.50) | |
| Unknown | 1.14 (1.01, 1.28) | 1.20 (0.99, 1.45) | 1.10 (0.94, 1.29) | |
|
| ||||
| Family History of CRC | 1.06 (0.95, 1.19) | 0.95 (0.81, 1.12) | 1.19 (1.01, 1.39) | |
Proximal refers to the cecum, ascending colon, hepatic flexure, and transverse colon. “Any proximal adenoma” included patients with only proximal adenomas plus those who had both proximal and distal adenomas.
Distal refers to the splenic flexure, descending colon, sigmoid colon, and rectum.
“Any distal adenoma” included patients with only distal adenomas plus patients who had both proximal and distal adenomas.
Odds ratios are the odds of an adenoma in a given location compared to odds of no adenoma in any location and were adjusted for age, race/ethnicity, and family history of colorectal cancer with clustering on physician and service area.
OR, odds ratio; CI, confidence interval; CRC, colorectal cancer.
The risk of any proximal adenoma was higher among blacks than whites (OR=1.26; 95% CI 1.04-1.54; men and women combined) (Table 5) and there was no evidence of interaction by sex (OR women: 1.26; 95% CI: 0.96-1.66 vs. OR men: 1.25; 95% CI: 0.95-1.65). In contrast, the risk of any distal adenoma was similar between blacks and whites (OR: 1.09; 95% CI: 0.90-1.33; men and women combined) (Table 5).
Discussion
The success of colorectal cancer screening programs largely depends upon the identification and removal of precancerous adenomas and early-stage cancers amenable to treatment. Prior publications have evaluated differences in colorectal cancer incidence by sex, age, and race/ethnicity; however, this is the first publication, to our knowledge, to report the detailed prevalence of detected colorectal adenomas at screening colonoscopy by patient demographics within a large multi-medical center, community-based population with comprehensive access to procedure, pathology, and medical data. This study found that adenomas were more common among men than women, and that the prevalence of detected adenomas – particularly proximal adenomas – increased markedly with age in both sexes. These findings complement prior reports of sex differences in detected colorectal adenoma and cancer prevalences from Veterans Affairs medical centers;12 populations in Israel,13 South Korea;22-23 Poland;8 and from the Clinical Outcomes Research Initiative (CORI), a consortium of gastroenterology practices in the United States.14-17 The CORI studies, to our knowledge the only prior large studies in the United States, primarily reported polyps (without pathology) for most analyses or evaluated a subset of practices that reported pathology, the availability of which varied for certain racial/ethnicity subgroups.11, 14-15 The current results have several implications for both the development of individualized screening programs, based on the likelihood of finding identifiable neoplasms at colonoscopy, as well as the ability to provide more precise normative values for the development of quality metrics for colonoscopy, one of the most commonly performed procedures in the United States.6, 8, 12, 24-25
First, our findings suggest that the current proposed standards for adenoma detection rates, if not adjusted for patient demographics, may substantially underestimate mean adenoma prevalence, particularly in older populations, which could bias comparisons of providers and programs that serve different populations. Current gastroenterology society guidelines, originally based on small populations, suggest that, among healthy asymptomatic patients greater than 50 years of age undergoing screening colonoscopy, adenomas and cancers should be detected in ≥15% of women and ≥25% of men; however, the low end of the suggested prevalence standard was found only in the youngest age group in the current study.6 Increased detection of adenomas with age was also found in a subset of the CORI population.14 Thus, a physician who primarily performed screening examinations in 50-55 year old men, for example, would be expected to have a much lower mean adenoma detection rate (approximately 25%) than an equally skilled provider who primarily performed examinations on men in their early seventies (approximately 39% prevalence). These findings underscore the importance of considering demographic case mix when developing quality standards for physician and program adenoma detection rates.14
Second, the prevalence of detected adenomas and colorectal cancer incidence data appear discordant between men and women. In contrast to the current study's finding of marked sex differences in adenoma prevalence between men and women across all age groups (41%-67% higher in men compared to women), a recent analysis of lifetime colorectal cancer risk (all locations) found comparable cancer risk estimates for men and women and for blacks and whites (lifetime risks 5.3% white males, 4.9% black males, 4.9% white females, and 5.2% black females).26 SEER cancer incidence reports, which include biases not present in the lifetime risk estimates, found higher cancer rates among men vs. women (52/100,000 among men vs. 39/100,000 among women; 2008),27 but this 34% difference is comparatively smaller than the differences in adenoma prevalence between men and women. This apparent disparity by sex in adenoma prevalence vs. cancer incidence raises two possibilities: 1) the finding of an adenoma may differentially estimate future cancer risk in men vs. women and; 2) the effectiveness of colorectal cancer screening methods that aim primarily to identify and remove adenomas may differ for men vs. women. Of note, a trend toward a lower protective value for colonoscopy among women was suggested by a recent publication of the National Polyp Study.4 Further research on these possibilities is needed to better elucidate the discordance by sex between colorectal adenoma prevalence and cancer risk.
Third, the current study also found proximal adenomas were more likely to be detected among blacks compared to whites (Table 5), although no significant differences by race were found for the risk of a distal adenoma. A relatively higher risk of proximal (but not consistently distal) colorectal cancer has also been reported among blacks;26, 28-30 thus, the current study's findings provide support for an increased risk of proximal cancer. These results expand on results from earlier single-center reports with small sample sizes (98-141 patients),31-33 and on results from CORI which found an increased risk in blacks vs. whites of any large polyp (>9 mm, pathology not available for all, but presumed adenoma; adjusted OR:1.16; 95% CI: 1.01-1.34)15 and of any proximal polyp.34 Of interest, the racial differences found in the CORI database varied between practice settings, raising the possibility that factors contributing to disparities in race might include practice setting or health care access;11 no differences or even nonsignificant trends in large polyp size by race were seen in the subset of CORI patients, for example, treated within the Veterans Affairs system.15 The current finding of no difference in the detection of distal adenomas by race is also supported by the recent results of a sigmoidoscopy trial, the Prostate, Lung, Colorectal, and Ovarian (PLCO) study, which found comparable rates of distal adenomas among whites and blacks.35
Fourth, the demonstrated differences in adenoma distribution within the colon suggest the potential for variability in effectiveness at preventing colorectal cancer for screening tests in different demographic groups. For example, sigmoidoscopy, when used alone, may be relatively less effective in demographic groups with a higher proportion of proximal adenomas, such as blacks and persons over the age of 60 years. The current study did not find marked sex differences in the prevalence of any adenoma by colon location; this contrasts with a prior colonoscopy study among 1463 women in a veterans population, which looked only at advanced neoplasia (adenomas at least 1 cm in diameter, villous adenomas, adenomas with high-grade dysplasia, or cancer), and suggested a higher risk of advanced proximal neoplasia among women.36
Community-based demographic data, such as those presented herein, can meaningfully inform risk stratification. The evaluation of more individualized screening strategies, based on patient risk and benefit, have been advocated by the National Cancer Institute to potentially increase screening effectiveness while simultaneously decreasing cost and risk.9-10 An example is a widely used clinical decision-making tool in cardiovascular disease that uses demographic data, personal history, and laboratory test results to recommend preventive interventions.37 Another recently-developed tool for breast cancer combined demographic data, a physical measurement (waist-hip ratio) and a blood-based genetic test to stratify the top 20% of women into a group with a 354% higher cancer risk (vs. the lowest 20% of women) while improving case findings.38-39 Future research in colorectal cancer might combine demographic factors that increase adenoma/cancer risk with other risk factors to develop risk profiles to improve both the effectiveness and efficiency of screening and surveillance.9
Strengths of the current study include its use of validated approaches to capture screening examinations and pathology data in a defined, community-based population with comprehensive capture of procedures; the manual validation of electronic codes and indications to confirm accuracy; histological confirmation of adenomas; a large pool of experienced colonoscopists; a wide distribution of patients by sex, age, and racial/ethnic groups; numerous medical centers in urban, rural, and semi-rural areas with inherent local variation in practices and populations; and the ability to adjust risk estimates for several factors. The study's community-based design is less subject to selection bias of subjects and examiners. The supplementation of referral indications with comprehensive laboratory, diagnostic, pathology, and radiologic records permitted exclusion of patients with non-screening exams and prior recent colonoscopies. Although disparities exist in all systems, integrated medical systems that may provide relatively equal access to care for members, such as the one in the current study, may also provide estimates that are less biased by demographic and socioeconomic differences in receipt of care.40-41
Potential limitations include a lack of detailed information on polyp size, details of bowel preparation, and extent of exam. However, the availability of these data would not be likely to substantially bias the results given that prior studies suggest that few patients (3-8%) have incomplete exams or bowel preparations of insufficient quality to perform adequate examinations.12, 34 Also, we are not aware of evidence that these factors are likely to be substantially differentially distributed across sex, age, or racial/ethnic groups, which would be required for them to bias the results. We did not have data on other potential confounders, such as diet, smoking, exercise, obesity, or aspirin use, which may vary between different demographic groups. Colonoscopy is imperfect at detecting adenomas; thus, the reported values represent detected colorectal adenomas and cancers, not true prevalence. Adenoma detection rates for individual physicians vary and contribute to some of the variability in observed detection rates; however, the analytic methods utilized in the present analysis, with clustering on physician and medical center, yielded odds ratios and confidence intervals adjusted for variability between physicians. We did not detect an association between the presence of adenomas and an exam indication of family history of colorectal cancer. The use of “family history” as an indication field in electronic records likely does not capture the complexity of risk, and may include second degree relatives, older first degree relatives, etc. who have only a modest influence on CRC risk. It is also possible that family history of colorectal cancer modifies cancer risk to a different degree than it does adenoma prevalence. A more precise assessment of the association between family history and adenoma prevalence would require more exact details of family history than are typically available in clinical records. Finally, for the analyses by colon location, the location of the adenoma was not explicitly coded in a portion of the colonoscopy reports; however, a range of sensitivity analyses (see methods section) provided similar results and the proportion of such examinations was approximately evenly distributed across age and racial/ethnic groups, making it unlikely that this biased the risk estimates by adenoma location.
In conclusion, the prevalence of adenomas detected at screening colonoscopy varied substantially by patient sex and age and, to a lesser extent, by race/ethnicity within a large, community-based, multi-medical center population with comprehensive access to health care, procedure, and pathology data. The prevalence of adenomas detected increased with age and was more common among men; proximal adenomas were more common among blacks than whites, although overall prevalence was comparable by race. The current results, combined with data from CORI,14 provide more precise normative data than was previously available in the United States. The prevalence values found are higher than those previously suggested for colonoscopy quality standards, particularly for older populations; this suggests colonoscopy performance quality measures should incorporate patient demographic differences in the expected prevalence of adenomas. Differences in adenoma distribution by sex, age, and race/ethnicity suggest that the effectiveness of different screening strategies may vary by patient demographics. In particular, the disparity by sex in the prevalence of adenomas detected across all age groups vs. colorectal cancer incidence raises the possibility that the presence of adenomas may differentially predict risk in men vs. women and that the effectiveness of colorectal cancer screening methods that aim primarily at identifying and removing adenomas may differ for men vs. women. Future studies are needed to determine whether different thresholds of adenoma detection influence the subsequent risk of colorectal cancer, particularly between men and women, and whether more individualized risk stratification for colorectal cancer screening would impact key outcomes such as adherence, effectiveness, efficiency, and safety.
Acknowledgments
Funding Support: The project was supported by grants from the Kaiser Permanente Community Benefits program and from the National Cancer Institute (U54 CA163262)
Abbreviations
- ADR
adenoma detection rate
- CI
confidence interval
- CORI
Clinical Outcomes Research Initiative
- CPT
Current Procedural Terminology
- ICD
International Classification of Diseases
- KPNC
Kaiser Permanente Northern California
- OR
odds ratio
- SNOMED
Systematized Nomenclature of Medicine
- SEER
Surveillance Epidemiology and End Results
Footnotes
Disclosures: Authors have no potential conflicts to disclose.
Author Contributions: DAC participated in study concept and design, obtaining of funding, study supervision, acquisition and interpretation of data, statistical analysis, and critical revision of the manuscript for important intellectual content. CDJ, ARM, and WKZ participated in acquisition and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. JDB participated in acquisition of data and administrative support. TRL participated in study concept and design and critical revision of the manuscript for important intellectual content. CD participated in interpretation of the data and critical revision of the manuscript for important intellectual content. BHF and CPQ participated in study concept and design, statistical analysis, and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008 May-Jun;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012 Feb 23;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004 Dec;127(6):1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006 Apr;101(4):873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 7.Church J. Adenoma detection rate and the quality of colonoscopy: the sword has two edges. Dis Colon Rectum. 2008 May;51(5):520–523. doi: 10.1007/s10350-008-9239-y. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010 May 13;362(19):1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed Oct 26, 2011];Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR) http://appliedresearch.cancer.gov/funding/prospr/
- 10.Roy HK, Bianchi LK. Colorectal cancer risk: black, white, or shades of gray? JAMA. 2008 Sep 24;300(12):1459–1461. doi: 10.1001/jama.300.12.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayanga AJ, Waljee AK, Kaiser HE, Chang DC, Morris AM. Racial clustering and access to colorectal surgeons, gastroenterologists, and radiation oncologists by African Americans and Asian Americans in the United States: a county-level data analysis. Arch Surg. 2009 Jun;144(6):532–535. doi: 10.1001/archsurg.2009.68. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000 Jul 20;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 13.Strul H, Kariv R, Leshno M, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006 Feb;101(2):255–262. doi: 10.1111/j.1572-0241.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 14.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011 May 23; doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008 Sep 24;300(12):1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B, Holub J, Peters D, Lieberman D. Prevalence of Colon Polyps Detected by Colonoscopy Screening of Asymptomatic Hispanic Patients. Dig Dis Sci. 2011 Sep 15; doi: 10.1007/s10620-011-1898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008 Oct;135(4):1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992 May;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Oakland, CA: Kaiser Permanente Division of Research; 2006. Jun, Available from: http://www.dor.kaiser.org/dor/mhsnet/public/kpnc_community.htm. [Google Scholar]
- 20.CPT 2011 Professional Edition. American Medical Association Press; 2010. [Google Scholar]
- 21.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011 Sep;74(3):656–665. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Lee SY, Choi KS, et al. The usefulness of colonoscopy as a screening test for detecting colorectal polyps. Hepatogastroenterology. 2007 Dec;54(80):2240–2242. [PubMed] [Google Scholar]
- 23.Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010 Mar;25(3):519–525. doi: 10.1111/j.1440-1746.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 24.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 25.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009 Jul;7(7):770–775. doi: 10.1016/j.cgh.2008.12.030. quiz 711. [DOI] [PubMed] [Google Scholar]
- 26.Merrill RM, Anderson AE. Risk-adjusted colon and rectal cancer incidence rates in the United States. Dis Colon Rectum. 2011 Oct;54(10):1301–1306. doi: 10.1097/DCR.0b013e3182242bd3. [DOI] [PubMed] [Google Scholar]
- 27.SEER Cancer Statistics Review, 1975-2008. National Cancer Institute. Based on November 2010 SEER data submission, posted to the SEER website. 2011 Available at: http://seer.cancer.gov/csr/1975_2008.
- 28.Thomas CR, Jr, Jarosz R, Evans N. Racial differences in the anatomical distribution of colon cancer. Arch Surg. 1992 Oct;127(10):1241–1245. doi: 10.1001/archsurg.1992.01420100107018. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997 Jul 15;80(2):193–197. doi: 10.1002/(sici)1097-0142(19970715)80:2<193::aid-cncr4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007 Jul;99(7):733–748. [PMC free article] [PubMed] [Google Scholar]
- 31.Rex DK, Khan AM, Shah P, Newton J, Cummings OW. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000 May;51(5):524–527. doi: 10.1016/s0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 32.Ozick LA, Jacob L, Donelson SS, Agarwal SK, Freeman HP. Distribution of adenomatous polyps in African-Americans. Am J Gastroenterol. 1995 May;90(5):758–760. [PubMed] [Google Scholar]
- 33.Johnson H, Jr, Margolis I, Wise L. Site-specific distribution of large-bowel adenomatous polyps. Emphasis on ethnic differences. Dis Colon Rectum. 1988 Apr;31(4):258–260. doi: 10.1007/BF02554356. [DOI] [PubMed] [Google Scholar]
- 34.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007 Jul;99(7):723–728. [PMC free article] [PubMed] [Google Scholar]
- 35.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: healthcare utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005 May 19;352(20):2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 37.Association AH. [Accessed November 15, 2010];Heart Attack Risk Calculator. 2010 http://www.americanheart.org/gglRisk/locale/en_US/index.html?gtype=health.
- 38.Zheng W, Wen W, Gao YT, et al. Genetic and clinical predictors for breast cancer risk assessment and stratification among Chinese women. J Natl Cancer Inst. Jul 7;102(13):972–981. doi: 10.1093/jnci/djq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mealiffe ME, Stokowski RP, Rhees BK, Prentice RL, Pettinger M, Hinds DA. Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst. 2010 Nov 3;102(21):1618–1627. doi: 10.1093/jnci/djq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SL, Shekherdimian S, Chiu VY. Effect of race and socioeconomic status in the treatment of appendicitis in patients with equal health care access. Arch Surg. 2011 Feb;146(2):156–161. doi: 10.1001/archsurg.2010.328. [DOI] [PubMed] [Google Scholar]
- 41.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009 Sep;24(9):1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]