Abstract
Context
Guideline directed care for diabetes calls for control of glycemia, blood pressure and cholesterol (composite goal). Most patients treated medically do not reach this goal.
Objective
Determine the efficacy and safety of Roux-en-Y gastric bypass (RYGB) added to lifestyle modification and intensive medical management (LS/IMM) to achieve control of all 3 endpoints.
Design
Two-arm unblinded randomized clinical trial with 120 participants. The primary endpoint of the composite outcome was assessed at 12 months. The study began in April 2008 and completed one year follow-up in all participants in December 2012.
Setting
Four academic teaching hospitals in the U.S. and Taiwan, involving five operating surgeons.
Participants
Inclusion criteria for the Diabetes Surgery Study (DSS) included HbA1c ≥ 8.0%, BMI 30.0-39.9 kg/m2, diagnosis and treatment of type 2 diabetes for at least six months, and stimulated C peptide > 1.0 ng/ml.
Interventions
All patients received lifestyle intervention modeled after the Look AHEAD study. Medications for hyperglycemia, hypertension, and dyslipidemia were prescribed according to protocol. RYGB techniques were standardized.
Main Outcome Measure
Attainment of a composite goal: HbA1c < 7.0%, LDL-C < 100 mg/dl, and SBP < 130 mmHg.
Results
One hundred and twenty participants were randomized with equal probability into LS/IMM or RYGB (60 in each group). Baseline characteristics were similar between groups. Mean BMI was 34.6 kg/m2 (95% CI 29.2 to 40.8 kg/m2) with 71 (59%; 95% CI 50% to 68%) participants having BMI < 35 kg/m2, and mean HbA1c was 9.6% (95% CI 9.4% to 9.8%). At 12 months the followup rate was 95%, and 11 (19%) in the LS/IMM group and 28 (49%) in the RYGB group achieved the primary endpoint (OR = 4.8, 95% CI 1.9 to 11.6). RYGB participants required 3.1 fewer medications than LS/IMM (4.8 versus 1.7, 95% CI -3.6 to -2.3). Weight loss was 7.9% LS/IMM vs. 26.1% RYGB (difference 18.2% 95% CI 14.2% to 20.7%). Regression analyses indicate that achieving the composite endpoint was primarily attributable to weight loss. There were 22 serious adverse events in the RYGB group, including one cardiovascular event, and 15 in the LS/IMM group. There were 4 peri-operative complications and 6 late postoperative complications in the RYGB group. Nutritional deficiency of iron, vitamin B12 and albumin were observed more frequently with RYGB.
Conclusions
In mild to moderately obese patients with type 2 diabetes addition of RYGB to LS/IMM resulted in greater likelihood of achieving the composite treatment goal. RYGB participants required fewer medications but had more complications.
Introduction
The foundation of treatment for type 2 diabetes mellitus is weight loss, achieved through reduction of energy intake and increased physical activity via lifestyle modification.1 Results from the Look AHEAD trial show that sustained weight loss through lifestyle modification improves diabetic control, but this is difficult to achieve and maintain over time.2 Medications to improve glycemia and control cardiovascular risk are also important, but up to 90% of patients with type 2 diabetes do not reach treatment goals designed to reduce long term risk of complications.3
Results from the Swedish Obesity Subjects Study indicate that patients after bariatric surgery had greater mean weight loss, reduced incidence of type 2 diabetes, and less mortality than obesity-matched control patients.4,5 Randomized clinical trials evaluating bariatric surgery as treatment for type 2 diabetes have shown that laparoscopic adjustable gastric banding (LAGB),6 Roux-en-Y gastric bypass (RYGB),7,8 vertical sleeve gastrectomy (VSG) ,7 and duodenal switch/biliopancreatic diversion (DS/BPD)8 produced more weight loss and better glycemic control than typical medical therapy. Whether the surgical advantage remains when compared with optimal medical and lifestyle treatment is unknown.
The results of bariatric surgery must be balanced against adverse events. In experienced centers, operative mortality of bariatric surgery has decreased to between 0.1% - 1%, but other less severe adverse outcomes are common.9 Our rationale for conducting the present study was that a randomized trial was needed to better define the benefits and short-term risks of bariatric surgery compared with optimal medical treatment. The present study addresses important needs in the evidence base: 1) existing data from recent randomized clinical trials does not readily fit into established clinical practice guidelines for type 2 diabetes, such as those recommended by the American Diabetes Association (ADA)1; 2) current randomized clinical trials report from a single surgical center, making outcomes difficult to generalize; 3) weight loss in the medical control group of published surgical randomized clinical trials is less than that achieved by previous rigorous behavioral weight loss trials; 4) inclusion of various surgical interventions prevents adequately powered analysis of specific procedures; and 5) to date, there are published data for only 14 patients with BMI < 35 kg/m2 who had RYGB surgery as part of a controlled clinical trial.7
We report here the one year results of the Diabetes Surgery Study (DSS), a prospective randomized clinical trial comparing RYGB with intensive lifestyle modification and protocol-driven medical management in obese patients with type 2 diabetes whose glycemic control was inadequate with standard medical therapy. The primary endpoint was a composite of three principal treatment goals for people with diabetes: hemoglobin A1c (HbA1c) < 7.0%, LDL cholesterol < 100 mg/dl, and systolic blood pressure < 130 mmHg.1 Achieving a HbA1c of 7.0% or below has been shown to protect against the microvascular complications of diabetes. Decreasing LDL cholesterol and blood pressure have both been shown to reduce the risk of macrovascular events in diabetic populations.2,3,10
Methods
The study was conducted at four sites: the University of Minnesota (UMN, starting in 2008), Columbia University Medical Center (CUMC, starting in 2009), two academic clinics in Taiwan (National Taiwan University Hospital and Min Sheng General Hospital, together called Taiwan, starting in 2009), and the Mayo Clinic in Rochester, Minnesota (Mayo, starting in 2010). All sites had IRB approval for the study and obtained IRB-approved written informed consent from each patient (registered in Clinical Trials: NCT00641251). The interventions included three components: lifestyle modification and intensive medical management (LS/IMM) for all participants and the addition of RYGB for half of the participants. The intervention was provided without charge, except that participants at the New York site were required by law to make standard insurance co-payments for medications. Compensation differed across sites, ranging from $25 to $400 for the 12 month visit.
Patient Eligibility and Enrollment
Between April 2008 and December 2011, 120 participants were randomized with equal probability to LS/IMM or RYGB in addition to LS/IMM. Patient eligibility, recruitment and enrollment are described in detail elsewhere.11 Briefly, recruitment was through mass media, contact with professional groups, presentations at public events, and a practice-based database. Key inclusion criteria were the following: age 30-67, under a doctor's care for type 2 diabetes for at least 6 months prior to recruitment; a HbA1c ≥ 8.0% at the time of entry; and a serum C-peptide level > 1.0 ng/ml 90 minutes after a liquid mixed meal of Ensure ® (250 calories, 6 g fat/40 g carbohydrate/9 g protein). Participants had a BMI of 30.0-39.9 kg/m2 and were willing to accept randomization to either treatment arm and follow the full treatment protocol. Additional criteria included the absence of conditions that would contraindicate surgery, such as serious cardiovascular disease, previous gastrointestinal surgery, psychological concerns, or history of malignancy. Randomization assignment was unblinded. The randomization schedule used permutated blocks of random length within each site, so that each site would have nearly equal proportions in each arm. Investigators were blinded to aggregate outcomes until the final patient completed 12 month follow-up.
Intensive Medical Management
LS/IMM consisted of two components - lifestyle modification designed to produce maximum achievable weight loss, and medications to control glycemia and cardiovascular disease risk factors while facilitating weight loss. Only U.S. Food and Drug Administration-approved medications were used.
Lifestyle Modification
The study lifestyle intervention was modeled on recent successful clinical trials, particularly the Diabetes Prevention Program (DPP)12 and Look AHEAD.2 Participants were instructed to weigh themselves and to record eating and exercise behaviors on a daily basis. Both groups were advised to progressively increase their level of moderate-intensity physical activity (such as walking) to a total of 325 minutes per week. LS/IMM participants were given calorie intake targets of 1,200, 1,500 or 1,800 kilocalories per day, depending on body weight, with the goal of producing a weight loss of 1-2 pounds per week. Portion-controlled diets using meal replacements, structured menus and calorie counting were encouraged to help participants stay within calorie limits. Both groups met regularly with a trained interventionist to discuss strategies for facilitating weight management and increasing physical activity, including self-monitoring, stimulus control, problem solving, social support, cognitive behavior modification, recipe modification, eating away from home and relapse prevention. Counseling sessions were comprised of 24 weekly meetings over the first 6 months, bi-weekly meetings between months 7 and 9, and monthly meetings between months 10 and 12. The lifestyle intervention protocol was similar for participants in both treatment arms. RYGB participants, however, delayed initiation of the lifestyle intervention until they could tolerate solid foods (typically about 3-4 months after surgery), did not have calorie ceilings during the period of rapid weight loss, and received additional instruction regarding food volume and adequate protein intake.
Medications
Follow-up evaluations were scheduled monthly for 6 months and at least quarterly thereafter. When the lifestyle intervention did not produce adequate weight loss, orlistat could be added to the treatment program. Sibutramine was also used for weight management until it was withdrawn from the U.S. market. Medications for glycemic control were added in the following order: 1) metformin, 2) a glucagon-like peptide-1 analog (GLP-1) or dipeptidyl peptidase 4 inhibitor (DPP-4), 3) sulfonylurea or pioglitazone, and 4) insulin. LDL cholesterol control was pursued with HMG-CoA reductase inhibitors first, followed by ezetimibe if necessary. Blood pressure medications were used in the following order: 1) angiotensin converting enzyme inhibitor (ACE) or angiotensin receptor blocker (ARB), 2) diuretic, 3) beta blocker, and 4) additional agents as necessary. The first approach to reducing elevated triglycerides was control of hyperglycemia; however, if triglycerides remained > 300 mg/dl, fenofibrate or fish oil was added. Smoking cessation was strongly recommended for all participants. An ACE or ARB was provided for all participants with micro- or macro-albuminuria. Aspirin (81-100 mg daily) was added consistent with evolving recommendations from the ADA and when not otherwise contraindicated. Vitamin and mineral supplements were prescribed for all patients based on routine testing; RYGB participants were prescribed additional calcium, iron, vitamin D and vitamin B12 supplements regardless of routine tests. The same medication treatment goals and algorithms were used for all participants with some qualifications. Occasionally, a participant refused or did not tolerate recommended medications or was controlled using medications differing from the algorithm and initiated prior to study entry. Medications for control of glycemia, dyslipidemia, and blood pressure were reduced or discontinued in RYGB participants immediately after surgery because of fluid and caloric decreases early postoperatively and were restarted as necessary to accomplish treatment goals.
Laparoscopic Roux-en-Y Gastric Bypass
Participants randomized to the RYGB group were placed on a low calorie diet with meal replacements 2 weeks prior to the operation. Staff surgeons with extensive experience (> 300 cases) performed the RYGB. A single surgeon performed the surgeries at each of the four sites, except at one site, which had two surgeons. The laparoscopic RYGB technique was standardized across all sites and was performed with construction of a 20 ml lesser curvature gastric pouch, a 100 cm biliopancreatic limb, and an antecolic 150 cm Roux limb with closure of all mesenteric defects.13 All surgeons committed to following this protocol, which was reviewed at an onsite meeting. Technical skill of each surgeon was established by personal observation by the principal investigator. On postoperative day 1, RYGB participants underwent a routine upper gastrointestinal (UGI) contrast study and were started on a clear liquid diet if the study showed no leak. Participants were typically discharged on postoperative day 2. At home, participants remained on a clear liquid diet for one week and were advanced gradually to pureed and then to solid foods as tolerated.
Outcomes
The primary outcome was success or failure in achieving the composite ‘triple endpoint’: HbA1c < 7.0%, serum LDL cholesterol < 100 mg/dL, and systolic blood pressure (SBP) < 130 mmHg, measured at the 12-month visit. Secondary outcome measures included weight loss, adverse events, fasting glucose, HbA1c < 6.0%, HDL cholesterol, triglycerides, and diastolic blood pressure, waist circumference and medications.
Measurement and Data Collection
Data were collected at baseline and at monthly medical visits in months 1-6 and quarterly medical visits thereafter and included the following: height, weight, blood pressure, pulse rate, medications used, and adverse events. Laboratory measurements collected included blood levels of HbA1c, fasting lipid profile, complete blood count, electrolytes, hepatic panel, ferritin, vitamin B1, vitamin B12, vitamin D, parathyroid hormone, fasting and 90 minute post-meal glucose and C-peptide and urine microalbumin/creatinine ratio were obtained at minimum at baseline and one year following randomization.
Statistical design and analysis
The primary analysis was intention-to-treat. For participants who did not have complete triple endpoint data at 12 months, methods of multiple imputation14 based on all prior data were applied. Logistic regressions stratified by site were used to compare proportions of success in the two groups (SAS PROC MI and SAS PROC MIANALYZE, SAS 9.2, Cary, NC).
Estimates of sample size were based on the following assumptions: (1) a two-sided significance level with p ≤ 0.05 and a standard superiority trial design; (2) 95% power; and (3) an alternative hypothesis of success rates on the triple endpoint of 65% in the RYGB group versus 30% in the LS/IMM group. These estimates were derived from previous studies. Schauer et al. reported that 82% of participants with type 2 diabetes undergoing RYGB succeeded in obtaining HbA1c < 6.0% when evaluated > 12 months after RYGB.15 The estimated success rate for the RYGB group was decreased from 82% to 65% for two reasons: a) the definition of the triple endpoint also requires serum LDL< 100 mg/dL and SBP < 130 mmHg, and the effects of RYGB are likely to be weaker on these variables; and b) study participants had higher HbA1c than those in Schauer's report. The 30% success rate in the LS/IMM group is based on studies used to generate the ADA Standards of Care.3,6,10,16 This alternative hypothesis resulted in a sample size estimate of 108 participants (54 in each group). An inflation factor of 12% was included to account for possible ‘crossovers’ (see definition below) and poor compliance.
Multiple imputations were used to address the issue of missing outcome data.14 The regressions were stratified by site. The 95% confidence intervals (CI) for dichotomous variables were computed using exact methods.17 Graphs indicate means and 95% CI.
A secondary analysis was performed on an ‘as treated’ basis, restricted to participants who had 12-month data and categorizing ‘crossover’ participants based on the treatment actually received rather than their treatment assignment. ‘Crossover’ participants were either (a) randomized to RYGB, declined surgery and continued to have contact with the study or (b) randomized to LS/IMM and obtained bariatric surgery elsewhere, but continued to have contact with the study. Regression analyses, stratified by site, were carried out to examine whether or not the RYGB effect on triple endpoint success was mediated primarily by weight loss. These were exploratory analyses designed to elucidate the mechanism of the treatment effect, not intention-to-treat analyses.
Results
Participant Characteristics
The methodology of recruitment and screening11,18 is summarized in Figure 1. A total of 2,649 candidates for the study were screened to attain 120 randomized patients. Racial and ethnic information was determined by patient report, and collected related to possible influence on triple endpoint outcomes. Patient characteristics at baseline are summarized in Table 1. Participants had diabetes for an average of 9.0 years (95% CI 7.9 to 10.0 years) at enrollment. Mean BMI was 34.6 kg/m2 (SD 3.1) with 71 (59%) participants having BMI < 35 kg/m2. Mean HbA1c was 9.6% (SD 1.1). Baseline characteristics were similar across randomized groups, as expected. One participant in the RYGB group was later determined to have type 1 diabetes. The data from this subject were included in the analyses.
Figure 1.
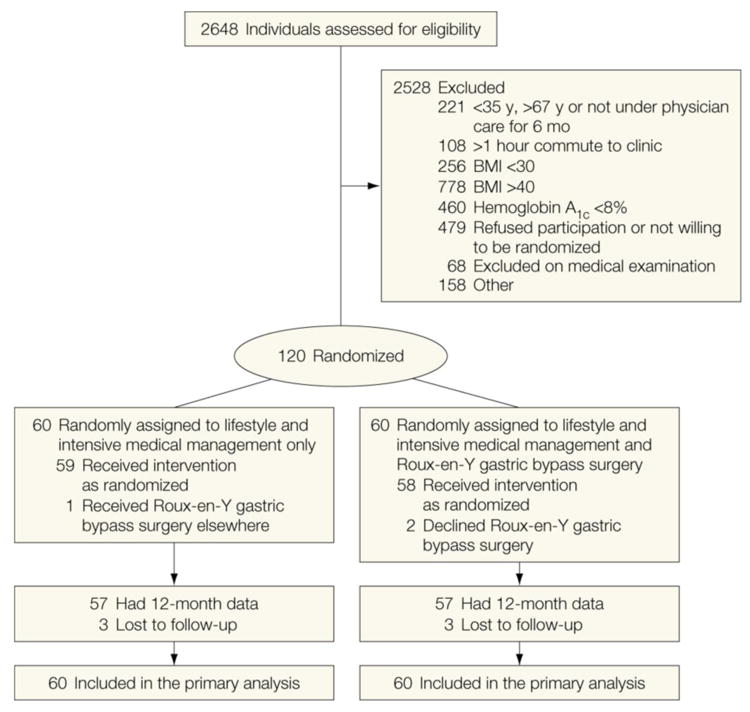
Consort Diagram.
Table 1. Baseline Data by Treatment Group.
Number (Percent)[95% CI] or Mean (95% CI)
| LS/IMM (n=60) |
RYGB (n=60) |
|
|---|---|---|
| Demographics: | ||
| Age (years) | 49 (47 to 51) | 49 (47 to 52) |
| Female | 34 (57) [43 to 69] | 38 (63) [50 to 75] |
| Race/Ethnicity: | ||
| Non-Hispanic White | 30 (50) [37 to 63] | 33 (55) [42 to 68] |
| East Asian | 17 (28) [17 to 41] | 16 (27) [16 to 40] |
| Non-Hispanic Black | 6 (10) [4 to 21] | 5 (8) [3 to 18] |
| Hispanic | 4 (7) [2 to 16] | 4 (7) [2 to 16] |
| Native American | 1 (2) [0 to 9] | 2 (3) [0 to 12] |
| Other | 2 (3) [0 to 12] | 0 (0) [0 to 6] |
| General Medical: | ||
| BMI (kg/m2) | 34.3 (33.5 to 35.1) | 34.9 (34.2 to 35.7) |
| BMI 30.0-34.9 kg/m2 | 35 (58) [45 to 71] | 36 (60) [47 to 72] |
| Height (cm) | 168 (166 to 171) | 168 (166 to 170) |
| Weight (kg) | 97.9 (93.6 to 102) | 98.8 (95.2 to 102) |
| Waist Circumference (cm) | 113 (110 to 116) | 114 (111 to 116) |
| Systolic blood pressure (mmHg) | 132 (129 to 136) | 127 (124 to 131) |
| Diastolic blood pressure (mmHg) | 79 (76 to 81) | 78 (75 to 81) |
| Years since Diabetes Diagnosis | 9.1 (7.7 to 10.5) | 8.9 (7.3 to 10.4) |
| Laboratory values (serum): | ||
| HbA1c (%) | 9.6 (9.3 to 9.9) | 9.6 (9.4 to 9.9) |
| LDL Cholesterol (mg/dL) | 105 (94 to 115) | 103 (94 to 112) |
| HDL Cholesterol (mg/dL) | 42 (40 to 44) | 41 (38 to 44) |
| Trigylcerides (mg/dL) | 249 (195 to 303) | 255 (158 to 352) |
| Total Cholesterol (mg/dL) | 189 (178 to 201) | 182 (172 to 192) |
| Creatinine (mg/dL) | 0.79 (0.74 to 0.84) | 0.81 (0.76 to 0.86) |
| Fasting C-peptide (ng/ml) | 3.0 (2.7 to 3.4) | 2.9 (2.4 to 3.3) |
| Post-meal C-peptide (ng/ml) | 4.8 (4.2 to 5.3) | 4.4 (3.8 to 5.1) |
| Fasting Glucose (mg/dL) | 207 (193 to 222) | 222 (203 to 241) |
| Fasting Glucose < 100 mg/dl | 1 (2) [0 to 9] | 1 (2) [0 to 9] |
| Alanine aminotransferase (U/L) | 37 (31to 42) | 36 (30 to 42) |
| Alkaline Phosphatase (U/L) | 84 (75 to 92) | 88 (78 to 97) |
| Serum albumin (g/dL) | 4.3 (4.3 to 4.4) | 4.3 (4.2 to 4.4) |
| Medicines: | ||
| Taking Insulin | 26 (43) [31 to 57] | 37 (62) [48 to 74] |
| Taking Other Glycemic Medicines | 57 (95) [86 to 99] | 52 (87) [75 to 94] |
| Taking Dyslipidemia Medicines | 41 (68) [55 to 80] | 39 (65) [52 to 77] |
| Taking Blood Pressure Medicines | 44 (73) [60 to 84] | 41 (68) [55 to 80] |
| Number of medications for control of glycemia, dysilipidemia and blood pressure (SD) | 4.4 (4.0 to 4.7) | 4.1 (3.6 to 4.5) |
Six (5%) of the 120 enrolled participants were lost to follow-up, three from each arm. There were also 3 crossovers: one participant randomized to LS/IMM underwent RYGB elsewhere, and two participants randomized to RYGB declined the operation. Sensitivity tests were performed on the 12-month results, using intent-to-treat with multiple imputations, intent-to-treat using all observed information, and an as treated analysis using all observed information. Because of the high level of participant compliance and follow-up, there were no material differences in p-values.
Composite and Other Endpoints
At 12 months, 11 (19%) participants in the LS/IMM group and 28 (49%) in the RYGB group achieved the primary composite endpoint (HbA1c <7.0%, LDL-C <100 mg/dl and SBP <130 mmHg) (OR 4.8, 95% CI 1.9 to 11.7) (Table 2). Among the composite endpoint components, the only significant treatment effect was for HbA1c: 18 (32%) participants met goal in the LS/IMM group versus 43 (75%) in the RYGB group (OR=6.0, 95% CI 2.6 to 13.9).
Table 2. Key Outcomes at 12 Months.
Number (Percent) [95% CI for Percent] or Mean (95% CI)
| LS/IMM | RYGB | Difference (with P-value) | |
|---|---|---|---|
| Primary Outcome | |||
| Meets Triple Endpoint | 11 (19)[10 to 32] | 28 (49)[36 to 63] | -30[-46 to -13]; <0.001 |
| HbA1c < 7.0% | 18 (32)[20 to 46] | 43 (75)[62 to 86] | -43[-60 to -27]; < 0.001 |
| LDL cholesterol < 100 mg/dL | 38 (70)[56 to 82] | 45 (79)[66 to 89] | -9[-24 to 7]; 0.27 |
| SBP < 130 mmHg | 44 (79)[66 to 88] | 48 (84)[72 to 93] | -6[-20 to 9]; 0.28 |
| Glycemia | |||
| HbA1c (%) | 7.8 (7.4 to 8.2) | 6.3 (6.1 to 6.5) | 1.5 (1.0 to 1.9); < 0.001 |
| HbA1c < 6.0% | 5 (9)[3 to 20] | 25 (44)[31 to 58] | -35[-50 to -20]; < 0.001 |
| Fasting Glucose (mg/mL) | 153 (137 to 169) | 111 103 to 120) | 42 (23 to 60); < 0.001 |
| Glucose < 100 | 7 (14)[6 to 26] | 25 (44)[31 to 58] | -30[-46 to -14]; < 0.001 |
| Serum Lipids | |||
| LDL Cholesterol (mg/dL) | 89 (80 to 97) | 83 (77 to 90) | 5 (-5 to 16); 0.27 |
| HDL Cholesterol (mg/dL) | 42 (39 to 44) | 50 (47 to 54) | -9 (-13 to -4); < 0.001 |
| Total Cholesterol (mg/dL) | 162 (151 to 172) | 153 (145 to 162) | 8 (-5 to 22); 0.12 |
| Trigylcerides (mg/dL) | 182 (142 to 222) | 104 (92 to 117) | 78 (36 to 120); < 0.001 |
| Blood Pressure | |||
| Systolic Blood Pressure (mmHg) | 124 (121 to 127) | 115 (112 to 119) | 8 (4 to 13); < 0.001 |
| Diastolic Blood Pressure (mmHg) | 74 (72 to 76) | 68 (66 to 71) | 6 (3 to 9); < 0.001 |
| Weight | |||
| Weight (kg) | 90.1 (85.7 to 94.5) | 73.0 (69.5 to 76.5) | 17.1(11.5 to 22.8); < 0.001 |
| BMI (kg/m2) | 31.6 (30.6 to 32.6) | 25.8 (24.9 to 26.7) | 5.8 (4.5 to 7.1); < 0.001 |
| Percent Weight Change (%) | -7.9 (-9.9 to -5.8) | -26 (-28 to -24) | 18.2 (15.1 to 21.2); < 0.001 |
| Waist Circumference (cm) | 105 ( 102 to 108) | 90 (87 to 93) | 15 (11 to 19) ; < 0.001 |
| Other | |||
| Medications for control of glycemia, dyslipidemia and blood pressure (n) | 4.8 ( 4.3 to 5.3) | 1.7 ( 1.3 to 2.2) | 3.1 (2.4 to 3.8); < 0.001 |
Emerging evidence about risks and benefits of blood pressure control has led the ADA to recommend less aggressive goals.19 By these new standards, 15 (26%) of LS/IMM and 31 (54%) of RYGB participants achieved the triple endpoint (OR=3.8, 95% CI 16 to 8.7).
The LS/IMM group lost 7.9% (SD 7.8%) of starting weight at one year. Most of the weight loss occurred in the first 6 months (Fig 2). Weight loss in the RYGB group was 26.1% (SD 8.7%) of starting weight at one year (difference 17.5%, 95% CI 14.2% to 20.7%). Although the rate of weight loss was greater in the first six months, weight loss continued during the last six months.
Figure 2.
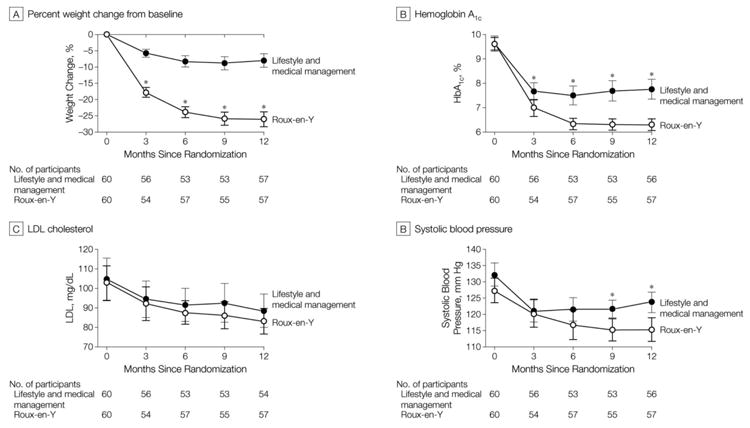
Key Quantitative Outcomes over Time
On average, the RYGB group used 3.1 fewer medications to manage glycemia, dyslipidemia and hypertension compared to LS/IMM group (95% CI 2.3 to 3.6).. The RYGB group also had significantly better results for the secondary outcomes of glycemia, HDL cholesterol, triglycerides, and diastolic blood pressure.
RYGB status was a significant predictor of triple endpoint success (OR=4.7; 95% CI=1.9 to 11.2) in as-treated models adjusted only for site. When both RYGB and weight loss are present in a logistic regression model stratified by clinic, the odds ratio for RYGB becomes non-significant (OR =0.96, 95% CI 0.22 to 4.24), while the odds ratio for triple endpoint success associated with a 10% weight loss is significant (OR=2.3, 95% CI 1.2 to 4.5). In regression analyses by treatment group, the effect of 10% weight loss was significant and similar (OR 2.6 and 2.2 in LS/IMM and RYGB respectively), indicating a consistent effect of weight loss (P=0.80 for difference). This implies that weight loss explains most of the effect of assignment to the RYGB group.
Adverse Events
Table 3 presents serious adverse events for both treatment groups, as well as the incidence of hypoglycemia and nutritional deficiencies. Overall, there were 22 serious adverse events in the RYGB group and 15 in the LS/IMM group. There were 4 perioperative complications and 6 late postoperative complications in the RYGB group. While there was no mortality, two postoperative complications merit particular attention. One RYGB participant developed a leak from the jejuno-jejunostomy with severe systemic consequences. The leak was not evident in the patient's routine post-operative upper gastrointestinal contrast study; it was detected hours later based on patient symptoms. Ultimately, this participant required extracorporeal membrane oxygenation and suffered anoxic brain injury, lower extremity amputation and long-term disability. Another RYGB participant developed a leak from the gastrojejunostomy. The intraoperative leak test was negative, but a leak was detected in the immediate post-operative period using the UGI contrast study, and the patient was reoperated laparoscopically and discharged without further sequelae. One participant in the LS/IMM group experienced pancreatitis. Two other participants were diagnosed with pancreatic cancer well into the first year of enrollment; one had received treatment with GLP-1 mimetics. Symptomatic hypoglycemia with neuroglycopenia was reported by 2 participants in the LS/IMM group and by 5 patients in the RYGB group (one of whom had declined surgery). Nutrient deficiencies in the participants (RYGB versus LS/IMM) included iron deficiency (13 versus 0), hypoalbuminemia (4 versus 0), one or more vitamin B deficiencies (11 versus 2) and low serum vitamin D (4 versus 5). The adverse events are reported based on original treatment assignment. There were no deaths reported in either group, but as discussed above, a cerebrovascular event occurred in one RYGB patient as a complication of surgery.
Table 3. Adverse Events.
| LS/IMM | RYGB | |
|---|---|---|
| Serious Adverse Events: | ||
| Postoperative Complications | ||
| Anastomotic leak | NA | 2* |
| Wound infection | NA | 1 |
| Wound hematoma | NA | 1 |
|
| ||
| Late RYGB Complications | ||
| Stricture | NA | 2 |
| Bleeding anastomotic ulcer | NA | 1 |
| Gastritis proximal pouch | NA | 1 |
| Small bowel obstruction | NA | 2 |
|
| ||
| Gastrointestinal Disorders | ||
| Diarrhea | 0 | 1 |
| Abdominal pain | 0 | 1 |
| Duodenitis | 1 | 0 |
| Acute pancreatitis | 1 | 0 |
|
| ||
| Infections | ||
| Urinary tract infection | 0 | 1 |
| Buttock abscess | 0 | 1 |
| Pyelonephritis | 1 | 0 |
| Bronchitis | 1 | 0 |
|
| ||
| Neurologic | ||
| Herniated disc | 1 | 1 |
| Foot drop | 0 | 1 |
| 3rd cranial nerve palsy | 1 | 0 |
| Bell's palsy | 1 | 0 |
|
| ||
| Psychologic – Suicide attempt | 1 | 0 |
|
| ||
| Vascular – Toe amputation | 0 | 1 |
|
| ||
| Non-operative Trauma | ||
| Motor vehicle crash | 2 | 0 |
| Ankle burn from a motorcycle accident resulting in a below the knee amputation | 0 | 1 |
|
| ||
| Other | ||
| Hypertension/headache | 0 | 1 |
| Back pain | 0 | 1 |
| Pregnancy | 0 | 1 |
| Chest pain | 1 | 0 |
| Uterine bleeding | 1 | 0 |
| Uretal stone | 1 | 1 |
| Pancreatic cancer | 2 | 0 |
|
| ||
| Total Serious Adverse Events | 15 | 22 |
|
| ||
| Selected Non-serious Adverse Events Commonly Associated with Diabetes or RYGB: | ||
|
| ||
| Cataract (severe) | 0 | 1 |
|
| ||
| Nephrolithiasis | 2 | 1 |
|
| ||
| Peripheral neuropathy | 2 | 2 |
|
| ||
| Hypoglycemia without need for assistance | 2 | 71 |
|
| ||
| Nutritional | ||
| Iron deficiency | 0 | 13 |
| Hypoalbuminemia | 0 | 4 |
| Vitamin B deficiency (B1, B6, B12) | 2 | 11 |
| Vitamin D drop to ≤ 30 ng/dl | 5 | 4 |
| Electrolyte/Fluid disorder | 0 | 1 |
| Hypertriglyceridemia | 5 | 1 |
Adverse events are reported based on randomization assignment. Two of the hypoglycemic incidents were in a patient randomized to RYGB who declined surgery.
Complications for one patient with anastomotic leak included sepsis, stroke, leg amputation, renal failure, other organ failures, coma, and extensive blood loss/clotting disorder.
Discussion
In participants with a BMI 30.0-39.9 kg/m2 and sub-optimally controlled type 2 diabetes, RYGB resulted in more participants (49%) reaching established diabetes management goals compared to LS/IMM alone (19%). In this population with type 2 diabetes, only about half of the participants achieved the composite treatment goal despite RYGB and maximum medical and lifestyle therapy. RYGB participants achieved the composite goal with 65% fewer medications than LS/IMM participants.
To our knowledge, this is the first trial comparing RYGB to intense lifestyle and medical management of type 2 diabetes using composite specified therapeutic goals. Previous randomized trials of bariatric surgery for patients with diabetes reported effects on glycemia, and sometimes blood pressure and lipids, as individual variables but not as a composite endpoint. The proportion of participants in both LS/IMM and RYGB groups who achieved the composite goal was greater than the 10.2% cross-sectional rates for the National Health and Nutrition Survey database3 and 10.1% in the baseline Look AHEAD study population.20 The Look AHEAD intensive lifestyle intervention improved achievement of the composite goal from 10.8% to 23.6% of participants at one year,10 similar to 19% (95% CI 10 to 32) in the current LS/IMM group. In our trial, the proportion of patients in both groups who achieved the composite goal was less than we projected in our power analysis. This was due to smaller than expected improvements in SBP and serum LDL in both groups. Between-group differences in the triple endpoint were consistent with our projections.
Glycemic control results are comparable to the experience of other controlled trials testing bariatric surgery treatment of diabetes. In the current study the mean HbA1c at baseline was 9.6% (SD 1.1%) in this study, substantially greater than the baseline in Look AHEAD,20 the LAGB trial of Dixon, et al6 and the bariatric surgery study of Mingrone8 et al, but similar to the trial of Schauer and colleagues.7 The generally greater mean HbA1c likely reflects our entry criteria requiring an HbA1c > 8.0%, and is relevant to evaluation of risk balancing for surgical approaches to poorly controlled diabetes. The glycemic goal of HbA1c < 7.0%, achieved by 75% of the RYGB group, cannot be directly compared to the other randomized trials of bariatric surgery for diabetes because the current study target was different. The mean HbA1c in our RYGB group was 6.3% (SD 0.9%) at one year, comparable to 6.4% for RYGB in the Schauer, et al study and 6.3% for RYGB at two years in the Mingrone et al study. The LS/IMM group improved their mean HbA1c to 7.8% (SD 1.5%), similar to 7.2% in the Look AHEAD intense treatment group, and the medical treatment arms of the other three studies of the effect of bariatric surgery on diabetes. Overall, both of the treatment groups in the current study were in accord with our prior hypotheses with regard to glycemic control.6,7,8
The RYGB did not significantly improve LDL cholesterol or blood pressure outcomes as over 70% of both groups achieved these goals. The mean one-year LDL of 89 mg/dl (SD 31 mg/dl) in the LS/IMM subjects was lower than both the Look AHEAD intense treatment group and the Mingrone, et al study medical arm. Our RYGB group LDL was 83 mg/dl (SD 25 mg/dl), similar to the Mingrone, et al RYGB group. The 84 and 79% respective rates in RYGB and LS/IMM of achieving blood pressure goal roughly approximate the Look AHEAD and other diabetes focused bariatric surgery studies.2,7,8
Compared to previous randomized surgical studies, we pursued optimal medical management including the use of weight lowering medications. In addition to lifestyle modification as employed in the Look AHEAD study, sibutramine (until removed from the market) and orlistat were used to facilitate weight loss. GLP-1 mimetics, known to produce sustained weight loss in this population, were used early in the diabetes treatment algorithm. Weight loss in the LS/IMM group averaged 7.9% (SD 7.8%) at one year, which is greater than reported in other randomized trials comparing bariatric surgery to medical therapy. Interestingly, all metabolic benefits in the LS/IMM group were realized by 6 months with subsequent decrease in the number meeting composite goal by 12 months. In contrast, treatment benefits continued to increase in the RYGB group throughout the year.
The mechanisms responsible for improvement in diabetes and cardiovascular risk factors in the study cannot be determined with certainty. The underlying assumption for the original application of bariatric surgery to treat type 2 diabetes was that greater sustained weight loss would benefit these patients. In other studies, weight loss in the Dixon et al LAGB study was correlated with type 2 diabetes improvement,6 but other bariatric surgery studies have not found a correlation with weight loss or reduction of BMI.7,8,21 Regression analyses of the present data indicate that the effect of RYGB on achieving the composite endpoint is attributable to weight loss. This finding does not preclude the possible contribution of changes in the secretion of gastrointestinal hormones to glucose control improvement,22 nor take into account between-group differences in medication use.
There was substantial difference in the frequency of serious adverse events between LS/IMM and RYGB groups. Total serious adverse and non-serious adverse events were 50% and 55% greater in the RYGB group compared to LS/IMM. The two most serious complications of RYGB were related to problems with gastrointestinal anastomotic leakage. All surgeons performing RYGB in this study were experts, thus the occurrence of serious complications must be factored into the design of larger trials of effectiveness for patients with moderate obesity. While the published incidence of anastomotic leakage after RYGB has decreased from initial reports as high as 5%23 to the current incidence of 0.8%,9 even in the hands of experienced surgeons, serious complications occur at a modest rate. Our leak rate of 3% is likely a function of random effects but it is important to emphasize the differences in our patient population compared to other studies reporting complication outcomes. The reported complication rates reflect data to one year and do not reflect internal hernias, the potential for later development of anastomotic ulcers, suicide, substance addiction, and failure of maintenance of weight loss known to occur beyond the first year after RYGB. As expected, the number of nutritional deficiencies was greater in the RYGB group despite monitoring of lab values and prescription of appropriate nutritional supplements.
Proponents have suggested that bariatric surgery for type 2 diabetes be considered earlier and for patients with lower BMIs, based on evidence of less mortality, decreased rate of malignancy, and better glycemic control durability.24,25 Others hesitate to recommend widespread use of a costly surgical procedure with inherent risks without support from large, prospective randomized clinical trials. The ADA and the NIH have been conservative about application of bariatric surgery in treatment algorithms for type 2 diabetes.17,21 Emerging data suggest recurrence of type 2 diabetes associated with weight regain after bariatric surgery.26 It is critical to demonstrate that bariatric surgery can produce a meaningful difference in established clinical endpoints compared to the very best lifestyle and medical management practices, and that it can do so over the long-term, across diverse sites and with well trained surgeons, and at reasonable cost and risk. While longer-term data will be helpful, it would be more useful in the form of a large-scale, multi-institutional study powered to examine the benefits of hard cardiovascular endpoints as well as to detect the deleterious effects of bariatric surgery.
Strengths of this study include randomized design, multiple sites and surgeons, and an intention-to-treat comparison to a group treated with best practices for lifestyle and pharmacological managements, as well as examining RYGB in combination with existing best medical practices. A high level of participant follow-up was obtained. Weaknesses include relatively small sample size, use of surrogate endpoints for cardiovascular disease and evaluation of the primary outcome at one year.
It is important to comment that recruitment for the study with four participating clinical centers proved to be considerably more difficult than anticipated: for every patient enrolled in the study an additional 22 potential participants were screened.11
Conclusion
The Diabetes Surgery Study examined RYGB as an adjunct to intensive behavioral intervention and intensive medical management in patients with BMI 30.0-39.9 kg/m2, using a composite primary endpoint of cardiovascular disease risk factors in the treatment of diabetes. This report provides data regarding treatment efficacy and safety for the first year of treatment.
The merit of RYGB treatment of moderately obese patients with type 2 diabetes depends on whether potential benefits make risks acceptable. Bariatric surgery can result in dramatic improvements in weight loss and diabetes control in moderately obese patients with type 2 diabetes who are not successful with lifestyle changes and medical management. The benefits of applying bariatric surgery must be weighed against the risk of serious adverse events.
Acknowledgments
See Funding/Support section for institutional support from Covidien for the DSS study. Dr. Ikramuddin serves on an advisory board member for Novo Nordisk, USGI and Medica, consults for Metamodix Inc and receives grant support from Covidien, EnteroMedics and ReShape Medical. Dr. Korner reports institutional and personal support from Covidien, she is on a Scientific Advisory Board for NutriSystem, consults for Federal Table Commission, and receives personal support for expert testimony. Dr. Connett reports institutional and personal grant support from Covidien, NIH grant support, and travel expenses for FDA Advisory Panel on Pulmonary Drugs. Dr. Billington reports grant support from Covidien and personal support for consultancy from EnteroMedics Inc. Avis Thomas receives salary support from Covidien for DSS, as well as supplemental salary support from Minnesota Obesity Center (CON 000000004485). Dr. Ahmed reports institutional grant support from Covidien. Dr. Vella receives consulting support from Sanofi-Aventis, Roche, and Novartis; institutional consulting support from Merck, and institutional grant support from Covidien, Daiichi-Sankyo, Merck, and GI Dynamics. Dr. Bessler reports institutional grant support from Covidien, personal consult support from Geshon Lehmar, and personal support for malpractice review. Dr. Swain receives grant support from EnteroMedics, Inc and ReShape Medical. Patricia Laqua reported institutional NIH grant support from Covidien. Dr. Jensen serves on an Advisory Board for Vivus and reports institutional grant support from Aspire Bariatrics. Dr. Bantle reports institutional support for the Look AHEAD study.
Funding/Support: The Diabetes Surgery Study (DSS) was supported by Covidien, Mansfield, MA. Covidien provided funds for these five clinical locations: University of Minnesota, Minneapolis, MN, USA; Mayo Clinic, Rochester, MN, USA; Columbia University, New York City, NY, USA; National Taiwan University Hospital, Taipei, Taiwan; Min-Sheng General Hospital, Taoyuan, Taiwan. This publication was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156 to Columbia University.
Role of the Sponsors: The sponsoring agency had no role in the collection, management, analysis, and interpretation of the study data; and had no part in the preparation of the manuscript. The sponsor was allowed to review the manuscript prior to submission.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT00641251, URL: http://clinicaltrials.gov/show/NCT00641251
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
No other authors reported disclosures.
Additional Contributions: Study Coordinators: Joyce Schone, RD and Nyra Wimmergren, RN, University of Minnesota; Heather A. Bainbridge, RD, CDN from Columbia University Medical Center; Amy E. Reynolds, RN, Mayo Clinic; Shu-Chun Chen, Linda Huang, and Meng-Jie Chen, National Taiwan University. Data Safety Monitoring Board members: David Nelson, PhD, Minnesota VA Health Care System, Minneapolis, MN; Victor J. Stevens, PhD, the Center for Health Research, Portland, OR; J. Michael Gonzalez-Campoy, MD, PhD, FACE, Medical Director and CEO of the Minnesota Center for Obesity, Metabolic and Endocrinology, PA, Eagan, MN; and Raymond Drew, MD, Abbott Northwestern Hospital, Minneapolis, MN. The investigators would like to recognize the contributions of the following individuals at the University of Minnesota Data Coordinating Center: Merrie J Harrison, Alain DuChene, Terri Schultz, and Greg Thompson. Also at the University of Minnesota, we recognize the contributions of Stanley E. Williams, PhD and acknowledge the tremendous assistance of Bridget M Slusarek, RN.
Dr. Michelle Lee is currently Associate Medical Director of Discovery Medicine and Clinical Pharmacology in CV/Metabolics at Bristol-Myers Squibb, Princeton, New Jersey, but was formerly the Assistant Professor of Clinical Medicine at Columbia University Medical Center for patient care. We extend our appreciation to Drs. Farah S. Khan, Munir Abid, and Gregory T. Mucha for patient referrals to our study. They did not receive compensation for those referrals.
Access to Data: John Connett Ph.D. and Avis Thomas M.S. of the Data Coordinating Center had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Statements P. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1)(October 2011):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong K, Glovaci D, Malik S, et al. Comparison of demographic factors and cardiovascular risk factor control among U.S. adults with type 2 diabetes by insulin treatment classification. J Diabetes Complicat. 2012;26(3):169–74. doi: 10.1016/j.jdiacomp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. New Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes. JAMA. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18212316. [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New Engl J Med. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. New Engl J Med. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 9.Hutter MH, Schirmer BD, Jones BD, et al. First Report from the American College of Surgeons Bariatric Surgery Center Network: Laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–422. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas AJ, Bainbridge HA, Schone JL, et al. Recruitment and screening for a randomized trial examining Roux-en-Y gastric bypass as an addition to intensive medical management for treatment of type 2 diabetes. Surg Obes Relat Dis. 2012 doi: 10.1016/j.soard.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Prevention (DPP) Research Group. The Diabetes Prevention Program (DPP). Description of lifesytle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikramuddin S, Kendrick ML, Kellogg TA, Sarr MG. Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg. 2007 Feb;11(2):217–28. doi: 10.1007/s11605-006-0028-4. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. [Accessed February 13, 2013];Stat Med. 1991 10(4):585–98. doi: 10.1002/sim.4780100410. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2057657. [DOI] [PubMed] [Google Scholar]
- 15.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surgery. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Classification I. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934:26404–413. [Google Scholar]
- 18.Connett JE, Ikramuddin S, Thomas AJ, et al. A randomized trial comparing Roux-en-Y gastric bypass to intensive medical management in obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. (in press). Available at: http://dx.doi.org/10.1016/j.soard.2012.08.011.
- 19.American Diabetes Association. Standards of medical care in diabetes---2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertoni AG, Clark JM, Feeney P, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complicat. 22(1):1–9. doi: 10.1016/j.jdiacomp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson LMS, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. New Engl J Med. 2012;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 22.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 23.DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235(5):640–5. doi: 10.1097/00000658-200205000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese type 2 diabetes. [Accessed February 5, 2013];Arquivos brasileiros de endocrinologia e metabologia. 2011 55(6):367–82. doi: 10.1590/s0004-27302011000600003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22011853. [DOI] [PubMed] [Google Scholar]
- 25.Ryan DH. BMI guidelines for bariatric surgery in diabetes: how low can we go? Diabetes Care. 2012;35(7):1399–400. doi: 10.2337/dc12-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2009;6(3):249–53. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]


