Abstract
Objective
Obesity is an important risk factor for osteoarthritis and is associated with changes in both the biomechanical and inflammatory environments within the joint. However, the relationship between obesity and cartilage deformation is not fully understood. The goal of this study was to determine the effects of body mass index (BMI) on the magnitude of diurnal cartilage strain in the knee.
Methods
Three-dimensional maps of knee cartilage thickness were developed from 3T magnetic resonance images of asymptomatic age- and sex-matched subjects with normal (18.5–24.9 kg/m2) or high (25–31 kg/m2) BMI. Site-specific magnitudes of diurnal cartilage strain were determined using aligned images recorded at 8:00 AM and 4:00 PM on the same day.
Results
High BMI individuals had significantly thicker cartilage on the patella and femoral groove than the normal BMI individuals. Diurnal cartilage strains were dependent on location as well as BMI. Subjects with high BMI exhibited significantly higher compressive strain in tibial cartilage than did those with normal BMI. Cartilage thickness decreased significantly on both femoral condyles from the AM to PM time point; however, there was no significant effect of BMI on diurnal cartilage strain in the femur.
Conclusions
Increased BMI is associated with increased diurnal strains in the articular cartilage of both the medial and lateral compartments of the knee. The increased cartilage strains measured in high BMI individuals may, in part, explain the elevated OA risk associated with obesity or may reflect altered cartilage mechanical properties in subjects with high BMI.
Keywords: obesity, MRI, stress, adipokine, chondrocyte, biomechanics
Introduction
Osteoarthritis (OA) is a debilitating disease that afflicts an estimated 27 million adults in the U.S. (1). One of the most common and potentially preventable risk factors for OA is obesity, which increases relative risk 2–10 times (2). Obesity is associated with OA in multiple joints, particularly the knee (2–4). However, the precise mechanisms by which obesity increases OA risk are not well understood. While a number of studies have attributed this association primarily to altered joint loading (5, 6), recent evidence suggests that both biomechanical and inflammatory factors play an important role in this relationship (2, 7, 8).
Despite focus in the literature on biomechanical factors (5, 9–11), the effects of obesity on cartilage loading are not fully understood. Some studies indicate that static coronal plane knee alignment plays a role in the progression of OA in obese subjects (5, 11). However, static malalignment may not be a risk factor for OA, but rather a marker of disease severity or progression (12, 13). Other investigators have emphasized the importance of dynamic measurements of knee loading, such as the adduction moment, using in-ground force plates and gait analysis techniques (10, 14–16). Although these static and dynamic measurements may be predictive of elevated medial compartment loads (5, 10, 11, 15, 17), they may not fully reflect alterations to the local mechanical environment of cartilage. In this regard, measurements of cartilage strain at the tissue level provide direct information on the biophysical environment of chondrocytes (18, 19). Alterations in this environment may predispose the joint to osteoarthritic degeneration (20, 21). Thus, in vivo measurements of cartilage strain may provide critical insights into the potential mechanisms by which obesity elevates the risk for OA.
The mechanical environment of chondrocytes in cartilage is characterized by a complex array of time-varying stresses, strains, fluid flow, and other biophysical factors arising from the activities of daily living. Due to the viscoelastic nature of cartilage and its repeated loading throughout the day, the resulting cartilage deformation may not completely recover, resulting in diurnal changes in cartilage thickness (22, 23). These changes have been shown to result in significant diurnal strains that vary with location in the joint (23). Increasing evidence suggests that chondrocytes have the ability to perceive and respond to biophysical phenomena that are directly associated with tissue strain, such as changes in interstitial osmolarity (24, 25). Thus, the objective of this study was to quantify the magnitude of diurnal strain induced in the articular cartilage of the knee of subjects with normal (BMI: 18.5–24.9 kg/m2) and high (BMI: 25–31 kg/m2) BMIs. Our hypothesis was that subjects with high BMI would experience higher magnitudes of diurnal strain than normal BMI subjects, and that these cartilage strains would vary with site in the joint.
Methods
A total of twenty subjects (n=10 with BMI of 18.5–24.9 kg/m2 and n=10 with BMI of 25–31 kg/m2) participated in this IRB approved study. No subjects had a history of knee injury or knee surgery. Subjects in each group were age- and sex- matched. Each subject was imaged twice in one day (at 8:00 AM and 4:00 PM) using a 3T MRI scanner (Trio Tim, Siemens Medical Solutions USA, Malvern, Pennsylvania) and an eight channel knee coil. Subjects were instructed to refrain from exercise or any strenuous activity prior to the morning scan. Subjects were scanned while lying supine, with the knee in a relaxed, slightly flexed position (26). A double-echo steady-state sequence (DESS, field of view: 15×15 cm, matrix: 512×512 pixels, slice thickness: 1 mm, flip angle: 25°, repetition time: 17 ms, echo time: 6 ms) was used to generate sagittal plane images of the knee (Figure 1) (23, 26). Total scan time was approximately 9 min. Subjects were asked to perform their normal activities throughout the day. Subjects returned to the same facility at 4:00PM and were imaged immediately upon arrival using the same protocol. In addition, each participant wore a pedometer to provide a measure of the number of steps taken between MR imaging sessions.
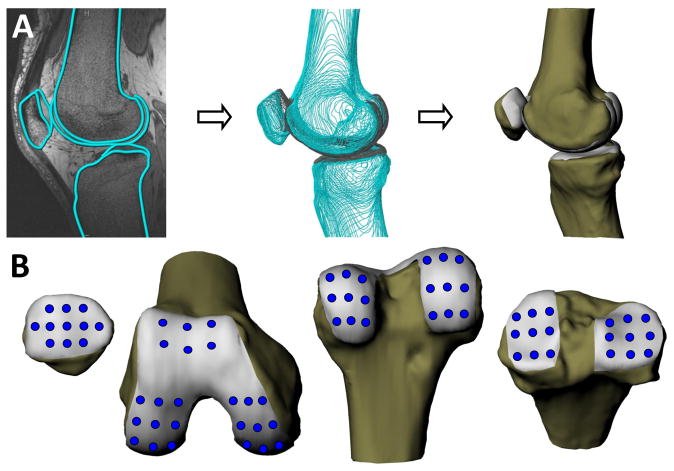
Figure 1.
A. MR images were used to create 3D models of the knee, including the femur, tibia, patella, and the corresponding articular surfaces. B. A grid sampling system was used to characterize cartilage thickness and strain in different regions of the femur, tibia, and patella.
The MR images were used to generate 3D models of each subject’s knee, including models of the femur, tibia, patella, and the corresponding articular surfaces (Figure 1) (23). In each image, the outer margins of the cortices and articular surfaces of cartilage were segmented using a solid modeling software (Rhinoceros, McNeel and Associates, Seattle, WA) (23, 27). These curves were then used to generate 3D surface mesh models of each surface (Studio, Geomagic Inc., Research Triangle Park, NC) (Figure 1). AM and PM models of the femur, tibia, and patella were aligned using an iterative closest point technique so that thickness measurements and strain calculations could be made at the same locations in each model (23). A grid sampling system was used to characterize cartilage thickness in different regions of the joint (23). Specifically, cartilage thickness on the tibia was sampled over a grid of nine evenly spaced points spanning both the medial and lateral tibial plateaus, for a total of 18 points (Figure 1). Eleven points spanned the surface of the patella and six points were sampled on the subpatellar region of the femur. A total of 36 points were measured across the surfaces of the medial and lateral femoral condyles. Thickness was defined as the minimum distance from the articular surface to bone at each mesh point on the model (Figure 2). Thickness was averaged across each mesh point within a 2.5mm radius of the sampling point. In this fashion, diurnal strain was calculated using the ratio of the change in thickness (final thickness minus initial thickness) to the initial thickness at the same location (23). This methodology has been previously validated for measuring cartilage thickness in the tibiofemoral joint (28). A more recent study from our laboratory reported a coefficient of repeatability of 0.03mm; thus, we estimate the resolution of strain measurements to be less than 1% (23).
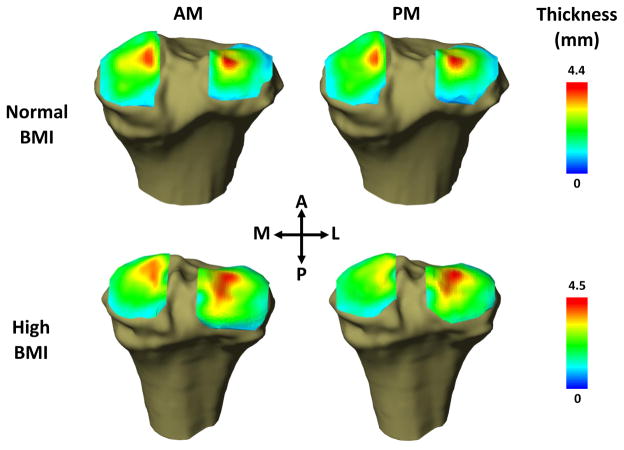
Figure 2.
Representative cartilage thickness maps of the tibia from normal and high BMI individuals in the morning (AM) and evening (PM). Significant changes in cartilage thickness were observed in the cartilage of the tibial plateau between the AM and PM time points.
Statistical Analysis
Statistical analyses were performed using Statistica (StatSoft, Inc., Tulsa, Oklahoma). Subject characteristics of normal and high BMI groups were compared with t-tests. The effects of location and BMI on undeformed cartilage thickness were compared using ANOVA. Diurnal and BMI effects on cartilage thickness were compared with ANOVA. Strain within each compartment was assessed for difference from zero and between BMI groups with t-tests. Bonferroni corrections were applied to p-values to account for multiple comparisons where appropriate.
Results
Subjects in the normal and high BMI groups did not differ significantly in age (30±2 years versus 31±2 years, mean±SEM), proportion of males to females (8 males and 2 females in both groups), height (178±1cm in both groups), and the number of steps taken on the study day (7616±821 steps versus 7933±1033 steps, respectively). The mean BMI of the control group (22.4±0.5 kg/m2) was significantly lower than that of the high BMI group (28.6±0.6 kg/m2). All subjects in the normal group fell within the normal weight BMI range (18.5–24.9 kg/m2). Most subjects within the high BMI group fell within the overweight range (25–29.9 kg/m2), with three subjects just falling into the obese range (30 and above) (highest BMI=31.4 kg/m2).
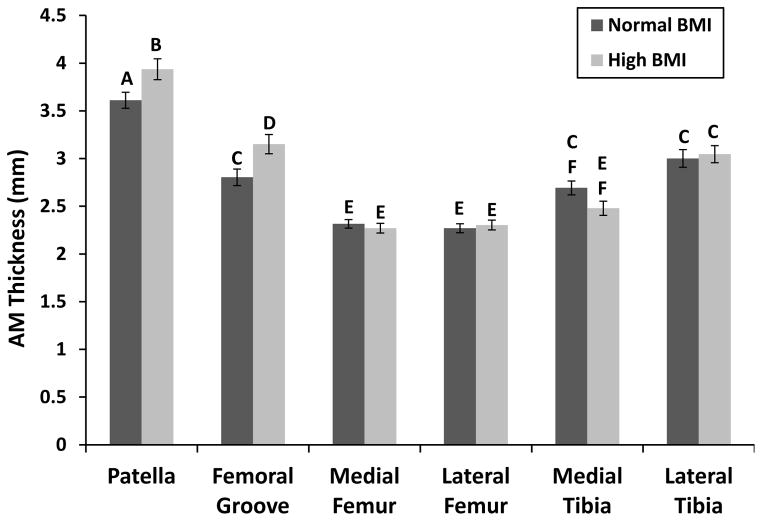
Initial (AM) cartilage thickness varied significantly with both BMI and location (2-way ANOVA, BMI x location p=0.004). The AM cartilage was significantly thicker on the patella and significantly thinner on the femoral condyles than other locations (Figure 3). High BMI individuals had significantly thicker cartilage on the patella and femoral groove than the normal BMI individuals. The high BMI group also trended towards having thinner cartilage on the medial tibia (p=0.1) than the normal BMI group.
Figure 3.
AM cartilage thickness of knee compartments varied significantly with location and BMI. Bars with different letters are significantly different from each other. All bars are mean±sem.
Patellar cartilage thickness did not change significantly between the AM and PM measurements; however, the cartilage of the high BMI group was significantly thicker than that of the normal BMI group (Figure 4a, 2-way ANOVA, diurnal p=0.2, BMI p=0.05, diurnal x BMI p=0.3). Patellar cartilage did not undergo significant diurnal strain (Figure 4b, t-test, normal p=0.1, high p=0.7). There was no effect of BMI on the magnitude of patellar strain (t-test, p=0.8).
Figure 4.
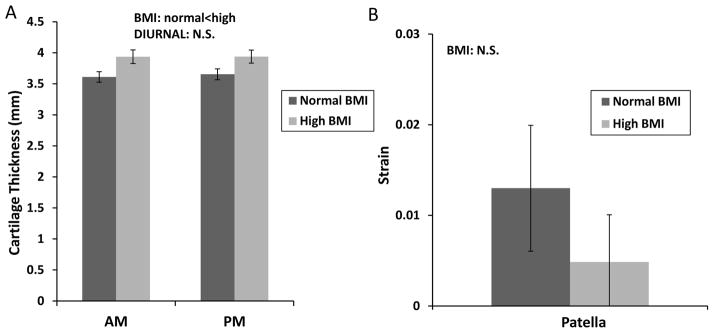
Measurements of cartilage thickness and strain in the patella. A. Cartilage thickness varied significantly with BMI, but did not vary from AM to PM. B. Cartilage strain was not significantly different from zero and did not vary with BMI. All bars are mean±sem.
In both the medial and lateral tibia cartilage, thickness decreased significantly from the AM to PM time points (Figure 5a, 2-way ANOVA, medial BMI p=0.04, diurnal p<0.00001, diurnal x BMI p=0.4; Lateral BMI p=0.9, diurnal p=0.02, diurnal x BMI p=0.08). The cartilage was also significantly thinner in the high BMI group than in the normal group for the medial tibia, but not the lateral tibia. Both medial and lateral tibial cartilage experienced significant compressive strains in the high BMI group, whereas only the medial side experienced significant strains in the normal BMI group (t-test, normal: lateral p=0.9, medial p=0.0001, high: lateral p=0.003, medial p<0.00001). BMI had a significant effect on tibial cartilage strain with high BMI individuals having significantly more compressive strain than in the normal BMI. Location also had a significant effect with compressive strain being greater on the medial than the lateral side (Figure 5b, 2-way ANOVA, location p=0.00002, BMI p=0.008, location x BMI p=0.9).
Figure 5.
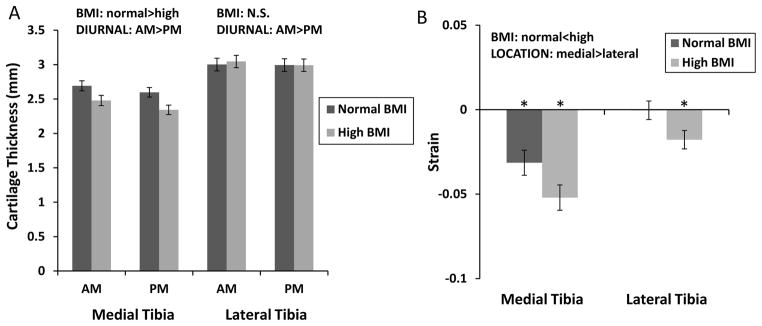
Measurements of cartilage thickness and strain in the tibia. A. Medial tibia cartilage thickness decreased significantly from AM to PM and from normal to high BMI groups. Lateral tibial cartilage thickness decreased significantly from AM to PM. B. Medial tibial cartilage undergoes significant diurnal strain and compressive strain is significantly higher in the high BMI group. Lateral tibia cartilage from the high BMI group exhibited significant diurnal strain, and compressive strain was significantly higher in the high BMI group than in the normal BMI group. All bars are mean±sem. *Strain is significantly different from zero.
Cartilage thickness on the femoral condyles did not vary significantly with BMI, whereas cartilage in the femoral groove was significantly thicker in subjects in the high BMI group than in the normal BMI group. Cartilage thickness decreased significantly on both the medial and lateral femoral condyles from the AM to PM time point (Figure 6a, 2-way ANOVA, medial diurnal p<0.00001, BMI p=0.9, diurnal x BMI p=0.9; lateral diurnal p<0.00001, BMI p=1, diurnal x BMI p=1). Cartilage thickness in the femoral groove, however, did not (2-way ANOVA, diurnal p=0.9, BMI p=0.02, diurnal x BMI p=1.0). Cartilage in the femoral groove was significantly thicker in subjects in the high BMI group than in the normal BMI group. Cartilage thickness on the femoral condyles did not vary significantly with BMI. The femoral groove did not undergo significant diurnal strain, whereas both the medial and later condyles did (Figure 6b, t-test, normal BMI: groove p=0.5, medial p=0.0001, lateral p=0.0008; high BMI: groove p=0.8, medial p=0.00004, lateral p=0.0007). There was, however, no significant effect of BMI on diurnal cartilage strain in any of the femoral locations (2-way ANOVA, location p=0.004, BMI p=0.9, location x BMI p=1). There was a significant effect of location on strain, with both condyles having greater compressive strain than the femoral groove.
Figure 6.
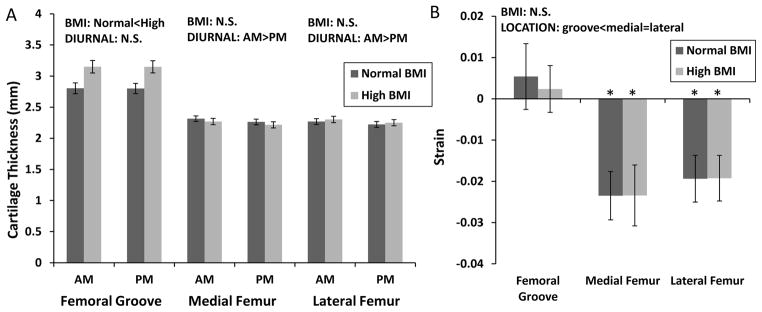
Measurements of cartilage thickness and strain on the femur. A. Cartilage thickness varied with BMI in the femoral groove and differed between AM and PM in the medial and lateral femoral condyles. B. The femoral groove did not undergo significant diurnal strain, whereas both the medial and lateral condyles did. No significant effect of BMI on diurnal cartilage strain was observed in any of the femoral locations. There was a significant effect of location on strain, with both condyles having greater compressive strain than the femoral groove. All bars are mean±sem. *Strain is significantly different from zero.
Discussion
While there is a strong association between obesity and knee OA development and progression (2, 5), the precise mechanisms by which obesity increases OA risk remains unclear. Recent studies have suggested that both mechanical and inflammatory factors play a role in this relationship (2, 29). Despite numerous studies focusing on biomechanical factors (5, 30, 31), there remains a paucity of data characterizing the effects of obesity on cartilage strains at the knee joint. Our results indicate that tibial cartilage of subjects with high BMI experienced greater diurnal compressive strains than that of subjects with normal BMI. Furthermore, high BMI subjects experienced significant strains in both the medial and lateral tibia, whereas normal BMI subjects only experienced significant diurnal strains in the medial compartment.
Although there is limited data in the literature regarding the effects of BMI on cartilage loading, our findings of regions of decreasing cartilage thickness during activities of daily living are consistent with previous studies. For example, Eckstein and colleagues measured significant changes in knee cartilage volumes in response to various in vivo activities, including deep knee bends and jump landings (32). Waterton et al. reported decreases in femoral cartilage thickness of up to 0.6mm between morning and evening MR scans (22). Furthermore, a previous study from our laboratory reported similar levels of diurnal strains in cartilage of subjects with normal BMI (23). Additionally, our findings of higher strains in the medial compartment and lower strains in the lateral compartment are consistent with previous studies measuring acute cartilage contact strains during quasi-static lunges (33) and gait (34).
Currently, it is unclear what effects obesity has on cartilage loading in the knee. A number of studies have reported that joint loads are increased with elevated body weight (9, 10, 14, 30). However, other studies have suggested that obese subjects have altered movement strategies that potentially decrease joint loading (35–37). For example, obese subjects may walk at lower self-selected speeds compared to subjects with normal BMI, which could reduce joint loading (35–37). In the current study, while taking a similar number of steps as subjects with normal BMI, subjects in the high BMI group experienced elevated strains in the tibial cartilage. These results suggest that, in response to normal activities of daily living, subjects with high BMI experience increased medial and lateral compartment cartilage strains. Because this study sought to investigate cartilage loading during the subjects’ normal routines, the timing, intensity, and types of activities performed during the day were not controlled. These factors may have an important effect on cartilage strains, since variables such as gait speed potentially alter the magnitude and frequency of joint loading (35, 36). Thus, future studies might also investigate relationships between BMI and cartilage strains during more controlled activities, such as treadmill gait at a fixed speed. This would allow for comparisons of cartilage strains between high and normal BMI subjects in response to similar loading conditions.
An important consideration in the interpretation of these data is that the increased cartilage strains observed in subjects with high BMI could reflect alterations in the mechanical properties of the cartilage that are associated with obesity or a high BMI. While there is little information available on the effects of obesity on cartilage mechanical properties, recent studies have shown that the obesity-related cytokines (i.e., “adipokines” such as leptin, adiponectin, or visfatin) can significantly increase the catabolic activities of chondrocytes (38–40). Thus, it is possible that overweight or obese subjects may exhibit changes in cartilage composition and functional properties that precede symptomatic OA, i.e., “pre-OA” (41). In this regard, quantitative MR imaging (42) could provide important insights into potential changes in mechanical properties by providing measures of cartilage composition across subjects with different BMI (43). Interestingly, however, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), which reflects cartilage glycosaminoglycan distribution and, to a certain extent, cartilage stiffness (44), shows no correlation with BMI in asymptomatic knees, but a negative correlation in OA knees (45).
Previous studies have indicated that measures of static alignment are predictive of OA progression in obese subjects. In particular, studies have focused on measurements of the orientation of the femur and tibia in the coronal plane on radiographic images obtained while standing (5, 13). These measurements potentially reflect the relative distribution of medial and lateral compartment loads, with a varus knee alignment increasing medial compartment load (11, 13). Thus, varus knee orientation is believed to be an important factor related to medial compartment OA progression in obese subjects (5). Other studies have used motion analysis techniques to predict load distributions between the medial and lateral compartments dynamically during gait (10, 14, 15). Specifically, the adduction moment at the knee, obtained from in-ground force plate measurements and inverse dynamic calculations (15), is thought to be a risk factor for the development and progression of OA (15, 31, 46). Furthermore, some studies have demonstrated that subjects with higher BMI experience increased adduction moments during gait (9, 10, 16). Although these studies provide important information regarding changes at the joint that have been correlated with OA progression, it may be difficult to infer changes in the local tissue strains from these measurements. For example, the results of the current study suggest that subjects with high BMI experienced elevated strains in both the medial and lateral compartments, as opposed to increases in just medial compartment. Thus, measurements of localized cartilage strains, as described in this study, potentially provide additional information regarding the altered loading environment within the tissue.
Increases in cartilage strain with obesity may not only affect joint biomechanics, but also the biophysical environment of the chondrocytes. Chondrocytes may respond directly to increased mechanical deformation or to the many secondary biophysical effects of cartilage tissue loading (47, 48). As cartilage is compressed, water is exuded, but the highly charged proteoglycans and associated ions remain in the tissue, increasing tissue osmolarity. Chondrocytes respond to osmotic changes both with short-term changes in signaling and also with longer term changes in gene and protein expression (19, 49, 50). Thus, increased strain in individuals with higher BMIs could affect the osmotic environment of the chondrocyte, potentially altering the normal cartilage homeostatic response to joint loading and thus contributing to the elevated OA risk associated with obesity.
In conclusion, there is limited data describing the direct effects of increased BMI on cartilage loading within the knee. Our findings show increased diurnal strains in the articular cartilage of both the medial and lateral compartments of the knee in subjects with high BMI compared to subjects with normal BMI. The increased cartilage strains measured in high BMI individuals may, in part, explain the elevated OA risk associated with obesity. Higher strains may not only result in greater wear or fatigue on the cartilage matrix, but also alter the biological response of chondrocytes to joint loading. The elevated cartilage strains observed in the high BMI group could potentially reflect alterations in the mechanical properties of the cartilage that are associated with obesity or may be the result of increased loading due to elevated body mass.
Acknowledgments
Supported in part by grants from the NIH (AR50245, AR055659, AR063325, AG15768, AR48182, AR48852), the NFL Charities, and the Arthritis Foundation.
Footnotes
The authors have no conflicts of interest to declare with regard to this research.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45(3–4):387–98. [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis and rheumatism. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT. Weight and osteoarthritis. The American journal of clinical nutrition. 1996;63(3 Suppl):430S–2S. doi: 10.1093/ajcn/63.3.430. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis and rheumatism. 2004;50(12):3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 6.Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. British journal of sports medicine. 2005;39(1):4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21(2):131–42. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 9.Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(7):822–8. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Segal NA, Yack HJ, Khole P. Weight, rather than obesity distribution, explains peak external knee adduction moment during level gait. Am J Phys Med Rehabil. 2009;88(3):180–8. doi: 10.1097/PHM.0b013e318198b51b. quiz 9–91, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf E, Bijsterbosch J, Slagboom PE, Rosendaal FR, Huizinga TW, Kloppenburg M. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(9):1117–22. doi: 10.1016/j.joca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. The Journal of bone and joint surgery. 2009;91 (Suppl 1):85–9. doi: 10.2106/JBJS.H.01409. American volume. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis and rheumatism. 2007;56(4):1212–8. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 14.Russell EM, Miller RH, Umberger BR, Hamill J. Lateral wedges alter mediolateral load distributions at the knee joint in obese individuals. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31(5):665–71. doi: 10.1002/jor.22248. [DOI] [PubMed] [Google Scholar]
- 15.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18(5):514–8. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 16.Gushue DL, Houck J, Lerner AL. Effects of childhood obesity on three-dimensional knee joint biomechanics during walking. Journal of pediatric orthopedics. 2005;25(6):763–8. doi: 10.1097/01.bpo.0000176163.17098.f4. [DOI] [PubMed] [Google Scholar]
- 17.Mundermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis and rheumatism. 2004;50(4):1172–8. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- 18.Wang CC, Guo XE, Sun D, Mow VC, Ateshian GA, Hung CT. The functional environment of chondrocytes within cartilage subjected to compressive loading: a theoretical and experimental approach. Biorheology. 2002;39(1–2):11–25. [PubMed] [Google Scholar]
- 19.Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. Journal of biomechanics. 2001;34(12):1527–35. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 20.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Annals of biomedical engineering. 2004;32(3):447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 21.Halloran JP, Sibole S, van Donkelaar CC, van Turnhout MC, Oomens CW, Weiss JA, et al. Multiscale mechanics of articular cartilage: potentials and challenges of coupling musculoskeletal, joint, and microscale computational models. Annals of biomedical engineering. 2012;40(11):2456–74. doi: 10.1007/s10439-012-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterton JC, Solloway S, Foster JE, Keen MC, Gandy S, Middleton BJ, et al. Diurnal variation in the femoral articular cartilage of the knee in young adult humans. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;43(1):126–32. doi: 10.1002/(sici)1522-2594(200001)43:1<126::aid-mrm15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, 3rd, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. Journal of biomechanics. 2013;46(3):541–7. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Browning JA, Saunders K, Urban JP, Wilkins RJ. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41(3–4):299–308. [PubMed] [Google Scholar]
- 25.Chao PG, Tang Z, Angelini E, West AC, Costa KD, Hung CT. Dynamic osmotic loading of chondrocytes using a novel microfluidic device. Journal of biomechanics. 2005;38(6):1273–81. doi: 10.1016/j.jbiomech.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Taylor KA, Terry ME, Utturkar GM, Spritzer CE, Queen RM, Irribarra LA, et al. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. Journal of biomechanics. 2011;44(3):365–71. doi: 10.1016/j.jbiomech.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischof JE, Spritzer CE, Caputo AM, Easley ME, DeOrio JK, Nunley JA, 2nd, et al. In vivo cartilage contact strains in patients with lateral ankle instability. Journal of biomechanics. 2010;43(13):2561–6. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis and rheumatism. 2009;60(12):3693–702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res Ther. 2011;13(6):R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis and rheumatism. 2005;52(7):2026–32. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 31.Russell EM, Hamill J. Lateral wedges decrease biomechanical risk factors for knee osteoarthritis in obese women. Journal of biomechanics. 2011;44(12):2286–91. doi: 10.1016/j.jbiomech.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. Journal of anatomy. 2006;208(4):491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bingham JT, Papannagari R, Van de Velde SK, Gross C, Gill TJ, Felson DT, et al. In vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology. 2008;47(11):1622–7. doi: 10.1093/rheumatology/ken345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Kozanek M, Hosseini A, Van de Velde SK, Gill TJ, Rubash HE, et al. In vivo tibiofemoral cartilage deformation during the stance phase of gait. Journal of biomechanics. 2010;43(4):658–65. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman Silvernail J, Milner CE, Thompson D, Zhang S, Zhao X. The influence of body mass index and velocity on knee biomechanics during walking. Gait & posture. 2013;37(4):575–9. doi: 10.1016/j.gaitpost.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Browning RC, Kram R. Effects of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc. 2007;39(9):1632–41. doi: 10.1249/mss.0b013e318076b54b. [DOI] [PubMed] [Google Scholar]
- 37.DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. Journal of biomechanics. 2003;36(9):1355–62. doi: 10.1016/s0021-9290(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 38.Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71(3):455–62. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 39.Iliopoulos D, Malizos KN, Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis. 2007;66(12):1616–21. doi: 10.1136/ard.2007.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNulty AL, Miller MR, O’Connor SK, Guilak F. The effects of adipokines on cartilage and meniscus catabolism. Connect Tissue Res. 2011;52(6):523–33. doi: 10.3109/03008207.2011.597902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258(2):505–14. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis. 2012;71(1):26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- 44.Baldassarri M, Goodwin JS, Farley ML, Bierbaum BE, Goldring SR, Goldring MB, et al. Relationship between cartilage stiffness and dGEMRIC index: correlation and prediction. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2007;25(7):904–12. doi: 10.1002/jor.20378. [DOI] [PubMed] [Google Scholar]
- 45.Tiderius C, Hori M, Williams A, Sharma L, Prasad PV, Finnell M, et al. dGEMRIC as a function of BMI. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(11):1091–7. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Annals of the rheumatic diseases. 2002;61(7):617–22. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. Journal of biomechanics. 1995;28(12):1529–41. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 48.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis and rheumatism. 2009;60(10):3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11(3):187–97. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- 50.Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. American journal of physiology Cell physiology. 2006;291(4):C718–25. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]