Abstract
Fibrosis in multiple organs is a prominent pathological finding and distinguishing hallmark of systemic sclerosis (SSc). Findings during the past 5 years have contributed to a more complete understanding of the complex cellular and molecular underpinning of fibrosis in SSc. Fibroblasts, the principal effector cells, are activated in the profibrotic cellular milieu by cytokines and growth factors, developmental pathways, endothelin 1 and thrombin. Innate immune signaling via Toll-like receptors, matrix-generated biomechanical stress signaling via integrins, hypoxia and oxidative stress seem to be implicated in perpetuating the process. Beyond chronic fibroblast activation, fibrosis represents a failure to terminate tissue repair, coupled with an expanded population of mesenchymal cells originating from bone marrow and transdifferentiation of epithelial cells, endothelial cells and pericytes. In addition, studies have identified intrinsic alterations in SSc fibroblasts resulting from epigenetic changes, as well as altered microRNA expression that might underlie the cell-autonomous, persistent activation phenotype of these cells. Precise characterization of the deregulated extracellular and intracellular signaling pathways, mediators and cellular differentiation programs that contribute to fibrosis in SSc will facilitate the development of selective, targeted therapeutic strategies. Effective antifibrotic therapy will ultimately involve novel compounds and repurposing of drugs that are already approved for other indications.
Introduction
Systemic sclerosis (SSc) is characterized by immune dys-regulation, obliterative microvasculopathy and fibrosis, but the relative severity and rate of progression of these processes varies from one patient to another. In the diffuse cutaneous form of SSc, fibrosis is typically the dominant feature. In contrast to organ-specific fibrosing diseases such as glomerulosclerosis, hypertrophic scars and pulmonary fibrosis, fibrosis occurs in multiple organs in SSc. Immune perturbations and vascular injury precede and contribute to the development of fibrosis, which, in turn, further exacerbates vascular and immune damage.1 To date, no therapy has been shown to reverse or arrest the progression of fibrosis, representing a major unmet medical need.
The pathogenesis of fibrosis in SSc has been the subject of several reviews published during the past 5 years.2–4 This Review highlights the most recent discoveries that are yielding a more complete—but at the same time more complex—view of fibrosis in SSc, and have opened doors for the development of targeted antifibrotic therapies.5–7 Because the skin is a prominent organ affected in SSc and is readily accessible for biopsy, much of the recently described information regarding fibrosis relates to skin cells. However, it is reasonable to presume that the pathways and mechanisms implicated in skin fibrosis are also operational in other cell types and organs. The key insights include the following: fibrosis in SSc involves largely the same effector cells and cellular transformations, signaling molecules and pathways implicated in other (organ-specific) fibrosing conditions (Figure 1); fibrosis represents deregulated wound healing, due in part to loss of intrinsic compensatory mechanisms and to aberrant recapitulation of embryological developmental programs; and, while indiscriminate immunosuppression is not effective in controlling fibrosis, this process is in fact potentially reversible.
Figure 1.
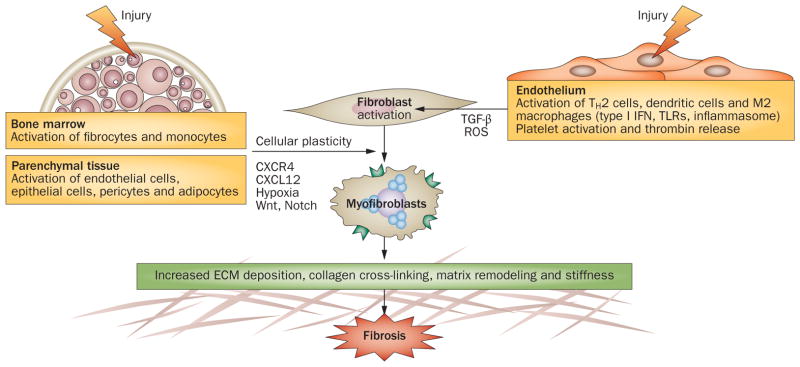
Cellular and molecular pathways underlying fibrosis in systemic sclerosis. Injury caused by viruses, autoantibodies, ischemia-reperfusion or toxins triggers vascular damage and inflammation. Activated inflammatory cells secrete cytokines and growth factors. Endothelial injury results in generation of ROS, intravascular coagulation and platelet activation with release of serotonin, vasoactive mediators, thrombin and platelet-derived growth factor, and sets in motion progressive vascular remodeling leading to luminal occlusion, reduced blood flow and tissue hypoxia. Secreted mediators, such as TGF-β and Wnt10b, cause fibroblast activation and differentiation into myofibroblasts, which produce excess amounts of collagen, contract and remodel the connective tissue, and resist elimination by apoptosis. The stiff and hypoxic ECM of the fibrotic tissue further activates myofibroblasts. Injury also directly induces transdifferentiation of pericytes, epithelial cells and endothelial cells into myofibroblasts, expanding the tissue pool of matrix-synthesizing, activated myofibroblasts. Abbreviations: CXCL12, CXC-chemokine ligand 12; CXCR4, CXC-chemokine receptor 4; ECM, extracellular matrix; IFN, interferon; ROS, reactive oxygen species; TGF-β, transforming growth factor β; TH2 cell, type 2 helper T cell; TLR, Toll-like receptor.
In SSc, the tightly regulated and self-limited response to injury that normally leads to tissue regeneration is subverted into fibrosis, with disruption of tissue architecture and loss of functional integrity. Underlying this switch is unopposed fibroblast activation due to loss of the normal constraints imposed by cytokines and receptor antagonists, intracellular nuclear receptors and microRNAs (miRNAs). Once initiated, fibrosis is escalated through multiple feed-forward amplification loops that are generated as a consequence of tissue damage, increased matrix stiffness, hypoxia, oxidative stress, and accumulation of damage-associated molecular patterns (DAMPs), which promote fibroblast activation and differentiation via innate immune signaling (Figure 2). Thus, a primary vascular or immune event causes persistent fibroblast activation and progressive injury, resulting in a vicious cycle. Intersitial and perivascular fibrosis in the lungs, heart, kidneys and other organs accounts for the late mortality of patients with SSc.
Figure 2.
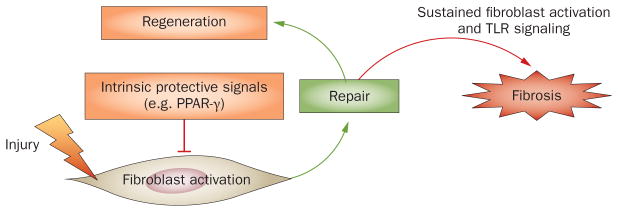
Tissue damage activates innate immune signaling, which transforms an orderly self-limited repair into a sustained, aberrant fibrogenic process. Following injury, fibroblasts undergo controlled activation, and once repair has been accomplished, tissue regeneration is complete. When tissue damage occurs from recurrent or sustained injury, damage-associated endogenous TLR ligands activate fibroblast innate immune signaling, enhancing fibrogenic responses and establishing a self-amplifying vicious cycle of fibrogenesis. Moreover, intrinsic protective signals (such as PPAR-γ) that normally act as the brakes on fibroblast activation are reduced or defective in scleroderma. Abbreviations: PPAR-γ, peroxisome proliferator-activated receptor γ; TLR, Toll-like receptor.
Fibrosis in the skin and internal organs
In fibrotic tissues, normal architecture is replaced with a largely acellular, collagen-rich, stiff connective tissue, resulting in loss of functional integrity (Figure 3). In the skin, the expanding dermis replaces the subcutaneous adipose layer and obliterates the dermal appendages. Biochemical analysis indicates excessive deposition of the fibrillar collagens (type I and type III), type V and type VII collagens and elastin fibrils, and elevated levels of enzymes that catalyze post-translational collagen modifications, such as lysyl hydroxylase and lysyl oxidase. Moreover, lesional skin shows aldehyde-derived collagen cross-links that are more typical of cartilage and bone.8 α-Smooth muscle actin (α-SMA)-positive myo-fibroblasts accumulate, whereas the scarcity of lymphatic and blood vessels gives rise to ‘capillary rarefaction’. Reduced blood supply leads to progressive tissue hypoxia, which induces local production of vascular endothelial growth factor (VEGF) and other angiogenic factors.1 Hypoxia directly and indirectly stimulates collagen synthesis and epithelial–mesenchymal transition (EMT), thereby amplifying the progression of fibrosis.9
Figure 3.
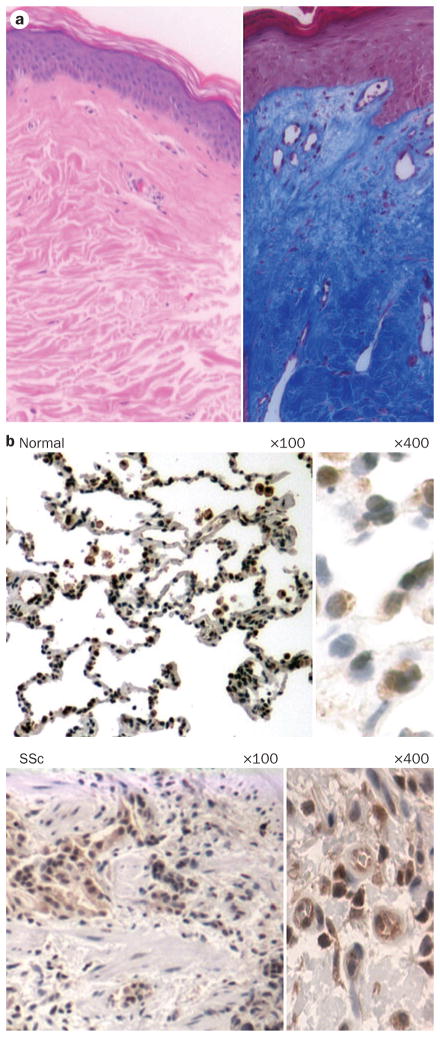
Tissue fibrosis in SSc. a | Skin biopsy from a patient with diffuse cutaneous SSc stained with hematoxylin and eosin (left) and Masson’s trichrome (right). Note the extensive dermal fibrosis, reduced cellularity and collagen deposition. b | Immunohistochemical EGR1 staining of lung tissue from a patient with SSc-associated end-stage lung fibrosis (upper panel) and a patient without lung fibrosis (lower panel). Note the dense pulmonary fibrosis, loss of normal alveolar architecture and strong upregulation of the profibrotic transcription factor EGR1 in the SSc specimen compared with normal tissue. Abbreviations: EGR1, early growth response protein 1; Sc, systemic sclerosis.
Genome-wide expression profiling
Microarray-based genome-wide expression profiling represents a robust approach to dissecting the pathways underlying fibrosis in SSc.10–13 Examination of SSc skin biopsies reveals marked alterations in gene expression, with over 2,000 genes that consistently distinguish patients with SSc from healthy controls.10,11 Reproducible and temporally stable gene expression patterns give rise to distinct “intrinsic subsets” that correspond to signaling pathways, prominent among which are transforming growth factor β (TGF-β) and Wnt pathways.10 A number of extracellular matrix genes associated with bone and cartilage, such as collagen types X and XI and cartilage oligomeric matrix protein, show increased expression in the lesional skin. Additionally, gene classes involved in innate immune signaling, lipid metabolism and hypoxia are abnormally expressed in particular patient subsets. Continuing refinements to data analysis, mapping of specific molecular signatures to individual patient subsets, longitudinal assessment of molecular signatures in serial skin biopsies, and examination of larger cohorts of clinically well-characterized patients, along with meticulous correlation of molecular fingerprints with disease phenotypes, will be needed not only to validate the distinct molecular signatures, but also to establish the significance of the subsets and, more importantly, their impact on clinical decision making (such as prediction of treatment responders). A microarray analysis of lung biopsy samples from SSc patients with end-stage lung disease demonstrated unique molecular signatures that accurately reflected the disease phenotype (pulmonary fibrosis versus pulmonary hypertension).14 SSc-affected lungs were differentially enriched with expression of genes in functional groups related to fibrosis, insulin-like growth factor signaling and caveolin endocytosis.
Fibroblasts are activated in SSc
In the 1970s, LeRoy first demonstrated that explanted SSc skin fibroblasts overproduce collagen when propagated ex vivo on plastic compared with cells from healthy controls.15,16 Subsequent studies showed that, in culture, SSc fibroblasts have a number of other characteristics: these cells secrete cytokines and chemokines, express cell-surface integrin adhesion molecules and receptors for TGF-β, platelet-derived growth factor (PDGF) and CCL2; they differentiate into myofibroblasts with prominent stress fibers; they display enhanced matrix adhesion and contractile properties; and they spontaneously generate reactive oxygen species (ROS).1,2 These phenotypic alterations persist for a number of ex vivo passages. Surprisingly, more-recent studies using genome-wide expression profiling reported that, under ex vivo culture conditions, only modest differences in gene expression are observed between SSc fibroblasts and control fibroblasts.10,11 The activated phenotype of SSc fibroblasts might reflect their persistent autocrine stimulation by TGF-β and other profibrotic stimuli,17 or cell-autonomous perturbations of intracellular signaling molecules and pathways that are inherent to SSc fibroblasts (Box 1). Alternatively, the activated phenotype could represent the durable memory of paracrine or autocrine activation and epigenetic events, such as DNA methylation mediated by DNA methyl-transferases, histone acetylation with chromatin remodeling, and downregulation of selected miRNAs.17–24 Altered patterns of histone acetylation and DNA methylation at multiple genetic loci have been demonstrated in SSc: for example, transcriptional silencing of repressive genes such as FLI1 and SMAD7 in SSc fibroblasts results in derepression of profibrotic gene expression.20,25 Histone hyperacetylation in fibrosis might result from a relative imbalance between histone acetylation and its inhibition by a family of histone deacetylase (HDAC) enzymes.18,26,27 Indeed, enhancing histone acetylation by inhibiting HDAC with agents such as trichostatin A results in abrogation of fibrotic gene expression and other TGF-β-induced responses, and might have therapeutic potential in SSc.18,28,29
Box 1. Aberrant cell-intrinsic signaling mediators in SSc.
Increased expression or activity
Decreased expression or activity
Abbreviations: CBP, CREB-binding protein; EGR, early growth response protein; ERK, extracellular signal-regulated kinase; FAK1, focal adhesion kinase; Fli-1, friend leukemia integration 1 transcription factor; miR, microRNA; PPAR-γ, peroxisome proliferator-activated receptor γ; PTEN, phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase; SMAD, mothers against decapentaplegic homolog; SSc, systemic sclerosis; TGFR1, transforming growth factor β receptor 1.
MicroRNAs
Emerging evidence assigns an increasingly important role to miRNAs in fibrosis.21 miRNAs are short (~22 nucleotides), evolutionarily conserved, single-stranded noncoding RNAs that modulate gene expression at the post-transcriptional level. The estimated 1,000 miRNAs encoded in the human genome are expressed in a tissue-specific and cell-type-specific manner. The transcription of miRNA genes can be independently regulated via their own promoters, or can be under the regulation of, and coexpressed with, the genes in which they are embedded. miRNAs selectively bind to complementary regions of mRNA transcripts and suppress gene expression via two non-mutually-exclusive mechanisms: inhibiting mRNA translation or facilitating mRNA degradation.
miRNAs are key regulators of tissue development, differentiation and repair. Microarray analysis has shown prominent changes in the expression of let-7, miR-21, miR-29 and miR-155 in lung fibrosis.22 These miRNAs are regulated by TGF-β, and have as their predicted targets genes involved in matrix repair and remodeling, such as collagens, matrix metalloproteinases and integrins, as well as Smad signaling proteins. miR-21 and miR-155 are profibrotic and show elevated expression in fibrosis, whereas let-7 and miR-29 are antifibrotic. The expression of miR-29 is reduced in cardiac fibrosis, which might cause the upregulation (derepression) of fibrotic genes.23 Markedly decreased levels of miR-29 have also been observed in lesional skin and explanted fibroblasts from patients with SSc.24 A causal role for miR-29 in fibrosis was indicated by the finding that rescue with exogenous miRNA normalized the activated SSc fibroblast phenotype in vitro.
Thus, elevated expression of profibrotic miRNAs or reduced expression of antifibrotic miRNAs are likely to be important in the development of fibrosis in SSc. Regulation of miRNA expression could be therapeutically exploited. Moreover, as miRNAs circulating as lipid-enclosed exosomes can be measured in the blood, they also represent potential biomarkers of fibrosis.
Cell fate switching in fibrosis
Although fibroproliferation is not prominent in SSc, the population of phenotypically active mesenchymal cells is expanded in lesional tissue. This might reflect transition of differentiated cells into fibroblasts or myo-fibroblasts, apoptosis resistance of myofibroblasts, or the accumulation of bone-marrow-derived mesenchymal progenitor cells. The transdifferentiation of epithelial cells, pericytes, endothelial cells and adipocytes into fibroblasts and myofibroblasts has been extensively studied in the context of organ-specific fibrosis, but has not been well documented in SSc to date. Pericytes are mesenchymal cells with smooth-muscle-cell-like properties that are normally localized to the walls of small blood vessels and capillaries. In SSc, pericytes are increased in number in the dermis and show features of myofibroblast differentiation, including expression of fibronectin extra domain A (FnEDA).30 We have shown that alveolar epithelial cells transition into myofibroblasts under hypoxic conditions, with loss of E-cadherin and acquisition of mesenchymal markers.31 A recent study, published in 2011, demonstrated that pulmonary vascular endothelial cells stimulated by TGF-β lose VE-cadherin expression and differentiate into myofibroblasts.32 This response was mediated by the tyrosine kinase c-Abl and Snail, a transcription factor with essential roles in EMT and myo-fibroblast differentiation. In light of the prominence of microangiopathy in SSc, it is tempting to consider injury-induced myofibroblast differentiation as a process linking vascular injury and fibrosis. While TGF-β seems to be the most potent stimulus for both EMT and myofibroblast differentiation, other triggers could also be important in SSc. These include Wnt ligands (Wnt3a, Wnt5 and Wnt10b), Hedgehog, Jagged–Notch signaling, hypoxia and bioactive lipids, whereas the nuclear receptor PPAR-γ (peroxisome proliferator-activated receptor γ) inhibits these transitions and promotes cellular quiescence.33,34
The Jagged–Notch pathway controls the formation of boundaries between groups of cells during embryogenesis, and mediates both EMT and myofibroblast differentiation. These responses involve proteolytic cleavage of Notch by ADAM17 and induction of Snail, which on its own is sufficient to induce scleroderma-like skin fibrosis in transgenic mice.35 Normal fibroblasts stimulated by the Notch ligand Jagged show increased collagen gene expression and myofibroblast transition,36 whereas pharmacological blockade of Notch signaling by inhibiting γ-secretase prevents TGF-β-induced EMT and ameliorates experimental skin fibrosis in mice.37 The Notch pathway has now been shown to be hyperactivated in SSc and other forms of fibrosis.36 The Hedgehog–Patched signaling pathway is also involved in organ development during embryogenesis, and aberrant Hedgehog signaling has been implicated in a variety of cancers, as well as fibrosis in the lung, liver and gastrointestinal tract.38 Hedgehog activates an osteopontin-mediated signaling pathway that induces EMT and myofibroblast differentiation. An unpublished study has demonstrated elevated Hedgehog expression and signaling in the skin of SSc patients (J. Distler, personal communication).
Persistence of the fibrotic response
A number of extracellular cues are capable of triggering fibroblast activation (Box 2). The role of these signals in fibrosis has been established in mice, where transgenic expression (gain of function) causes a spontaneous or exaggerated fibrotic phenotype, whereas knockout (loss of function) affords protection from experimentally induced fibrosis.1–3 These cues are commonly generated in response to tissue injury, and normally serve to effect rapid tissue repair and regeneration. However, their persistent paracrine or autocrine activities, coupled with loss of endogenous compensatory mechanisms and accumulation of additional stimulatory signals generated from the damaged tissue, drive unopposed fibroblast activation, resulting in progressive fibrosis.
Box 2. Extrinsic mediators of fibroblast activation implicated in SSc.
Cytokines
TGF-β: fibrogenic cytokine; expression of TGF-β receptors increased in SSc fibroblasts
IL-4, IL-10 and IL-13: expression increased in SSc fibroblasts
IL-17: increased numbers of TH17 T cells in skin and lungs of patients with SSc
IL-33: downregulated in endothelial cells in early SSc
Growth factors, peptides, bioactive lipids and ROS
Wnt proteins (e.g. Wnt3a, Wnt10b): involved in embryonic development and organogenesis; aberrantly activated in SSc and in mouse models of scleroderma
CTGF: matricellular protein; rapidly induced by TGF-β; marker of fibrosis in SSc
PDGF: PDGFRs expressed by pericytes in patients with early SSc; PDGFRα induces fibrosis in transgenic mice
IGFBP5: increased levels in idiopathic lung fibrosis tissue and explanted fibroblasts
Endothelin 1: increased levels in plasma and skin of patients with early active SSc
LPA: increased levels in SSc; LPA-knockout mice are resistant to bleomycin-induced skin and lung fibrosis
Thrombin: increased in SSc-associated interstitial lung disease and in bleomycin-induced lung fibrosis in mice
ROS: generated by oxidative stress; activates normal fibroblasts via PDGFR
Chemokines
CXCL12, CXCR4: implicated in defective vascular repair
CCL2: increased levels in SSc skin and SSc fibroblasts
Autoantibodies
Anti-topoisomerase I antibodies: specific for SSc
Anti-fibroblast antibodies: detected in SSc serum; activate normal fibroblasts
Anti-PDGFR and anti-AT1R antibodies: elevated in SSc; activate normal fibroblasts
Abbreviations: AT1R, angiotensin II type 1 receptor; CCL2, CC-chemokine ligand 2; CTGF, connective tissue growth factor; CXCL12, CXC-chemokine ligand 12; CXCR4, CXC-chemokine receptor 4; IGFBP5, insulin-like growith factor-binding protein 5; LPA, lysophosphatidic acid; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; ROS, reactive oxygen species; SSc, systemic sclerosis; TGF-β, transforming growth factor β; TH17 cell, type 17 helper T cell.
The interferon signature
Innate immune signaling is implicated not only in the immune dysregulation associated with SSc, but also in fibrosis itself. Pattern recognition receptors, such as Toll-like receptors (TLRs), lectin receptors, scavenger receptors (for example, CD36 and MARCO), complement receptors and cytosolic receptors (for example, NOD-like receptors [NLRs]), are the critical cellular sensors for exogenous danger.39 TLRs are activated not only by microbial ligands, termed pattern-associated molecular patterns (PAMPs), but also by nucleic acids and immune complexes containing autoantibodies. In plasmacytoid dendritic cells, TLR activation leads to secretion of interferon α (IFN-α), a potent immune modulator that is implicated in the pathogenesis of systemic lupus erythematosus (SLE). Increased expression of IFN-regulated genes (termed the ‘IFN signature’) correlates with disease activity and severity in SLE. The presence of an IFN signature has also been reported in SSc, albeit one of a lower magnitude than is seen in SLE.13,40–44 The IFN signature in SSc is associated with the presence of autoantibodies against topoisomerase I, and is thought to reflect endosomal TLR activation by nucleic-acid-containing immunocomplexes.40 Genome-wide association studies have revealed an association between SSc and IRF5 (interferon regulatory factor 5) and other genes involved in IFN production or signaling.45 Because IFNs generally act as negative regulators of collagen synthesis and TGF-β-mediated fibrotic responses, it remains unclear whether type I IFN signaling in SSc promotes tissue fibrosis directly via induction of other cytokines (such as IL-1 and IL-6) or indirectly via microvascular injury and apoptosis, or instead represents a protective response initiated in an attempt to limit fibroblast activation and attenuate fibrosis. This issue might be resolved experimentally with agents that block IFN signaling.
Fibroblast innate immune recognition signaling
Type I interferon production is intimately linked to innate immune signaling via TLRs. In contrast to cells of the immune system, the expression and function of TLRs in fibroblasts is poorly understood. Type I IFN stimulates TLR3 expression and TLR3-mediated responses in normal skin fibroblasts.46 The synthetic TLR3 ligand polyinosinic:polycytidylic acid (poly I:C), a mimic of viral RNA, was shown to induce the expression of several extracellular matrix genes in fibroblasts in vitro, and chronic subcutaneous administration in mice resulted in dermal fibrosis.47 In other studies, however, we found that while poly I:C caused TLR3-dependent increases in IFN-β and inflammatory cytokines and chemokines such as IL-6 and CXCL10, collagen gene expression and myofibroblast differentiation were inhibited (J. Varga, unpublished data). Moreover, in the presence of poly I:C, normal fibroblasts became unresponsive to TGF-β, suggesting that TLR3 signaling attenuates fibrosis. Indeed, mice given systemic (intraperitoneal) poly I:C were protected from bleomycin-induced fibrosis.48
In contrast to TLR3 (an endosomal receptor), TLR4 is expressed at the cell surface and seems to mediate pro-fibrotic responses. In addition to microbial ligands, fibro-blast TLR4 can be activated by DAMPs that are generated in situ as a consequence of cell damage, matrix remodeling, autoimmunity and oxidative stress.49 Endogenous ligands of TLR4 that are implicated in SSc belong to three broad categories: extracellular matrix molecules, such as hyaluronan, alternatively spliced FnEDA (which is normally expressed during embryogenesis), tenascin C and biglycan; cellular stress proteins, such as HMGB1 and HSP60; and nucleic-acid-containing immune complexes. We have demonstrated elevated TLR3 and TLR4 expression, along with striking accumulations of the endogenous TLR4 ligands hyaluronan, tenascin C and FnEDA, in lesional skin and lung biopsies from patients with SSc (S. Bhattacharyya et al., unpublished data). FnEDA is of special relevance for fibrosis, as this matrix protein has long been known to be indispensible for myofibroblast differentiation.50 Mice lacking FnEDA fail to develop lung fibrosis when challenged with bleomycin, or cardiac fibrosis following myocardial infarction.51,52
In normal mesenchymal cells, TLR4 activation by microbial ligands (such as lipopolysaccharide) or endogenous ligands induces a modest fibrotic response on its own, but is associated with synergistic stimulation of collagen, α-SMA and other markers of fibrosis in the presence of TGF-β.53 Circulating anti-fibroblast auto-antibodies, detected in the serum of some SSc patients, trigger a TLR4-mediated inflammatory response in normal dermal fibroblasts, with increased expression of inflammatory and profibrotic chemokines.54 Mechanisms underlying the profibrotic effect of TLR4 signaling include potentiation of TGF-β activity via upregulation of the TGF-β pseudoreceptor BAMBI and suppression of anti-fibrotic miRNAs such as miR-29. C3H/HeJ mice, which have a mutated TLR4, and mice with genetic ablation of TLR4, are protected from multiple types of experimental fibrosis,53,55–58 highlighting the importance of TLR4 in this process. In addition to TLRs, cellular immunity can also be triggered via the TLR-independent cytosolic receptors RIG-I, MDA5 and the NLRs NOD1 and NOD2, and activation of NALP3, a component of the inflammasome.59 Inflammasome activation and IL-1β production is implicated in numerous animal models of fibrosis, and might have a role in SSc and environment-linked scleroderma-like conditions, such as gadolinium-associated nephrogenic systemic fibrosis.60
In the skin, lungs and other affected organs in SSc, injury results in the generation of soluble or matrix-associated molecules that serve as cues to initiate or maintain the activation and differentiation of parenchymal fibroblasts and other mesenchymal cell types.
Together, these observations suggest that activated fibroblasts that are exposed to certain environmental or endogenous damage-associated TLR ligands and other danger signals display enhanced TGF-β responses, exacerbating and perpetuating the fibrotic process. In this way, fibroblast innate immune signaling triggered by endogenous ligands might be one of the key pathways for converting self-limited regenerative repair into an aberrant and intractable fibrotic process (Figure 2).
Transforming growth factor β
The pleiotropic cytokine TGF-β is recognized as a master regulator of both wound healing and pathological fibrosis. Although macrophages are an important source of TGF-β, most cells are actually capable of both secreting and responding to this cytokine. In the fibrotic cellular milieu, the bioavailability of TGF-β is regulated by its secretion from tissue-infiltrating macrophages, as well as by local activation of its matrix-bound latent form, a process mediated by cell-surface integrins (αVβ3, αVβ5 and αVβ8 on fibroblasts). Binding of latent TGF-β to these integrins causes conformational changes in the TGF-β complex that result in liberation of active TGF-β in the pericellular environment. The expression of αVβ3 and αVβ5 integrins is upregulated on SSc fibroblasts, which results in activation of latent TGF-β in the pericellular environment, causing fibroblast activation.61,62 In SSc-associated lung fibrosis, upregulation of αVβ6 integrin on airway epithelial cells is thought to be responsible for increased local TGF-β activity, and represents a promising antifibrotic drug target that is currently under intense study.63
Exposure of normal fibroblasts to TGF-β results in a robust and broad fibrotic response, with stimulation of collagen and FnEDA synthesis, production of connective tissue growth factor (CTGF; also known as CCN2) and upregulation of matrix gene expression, along with myo-fibroblast transformation and enhanced adhesion, contractility, migration and survival.64 These TGF-β responses reflect the coordinated transcriptional upregulation of hundreds of genes: microarray studies have demonstrated the presence of a strong TGF-β-activated gene signature in skin biopsies from a subset of patients with SSc.65 Individuals within this SSc subset have diffuse cutaneous disease, with more skin fibrosis and lung involvement compared with patients whose biopsies show no evidence of an activated TGF-β pathway. Elevated expression of both TGF-β receptors66–68 and TGF-β-activating integ-rins on SSc fibroblasts suggests that ongoing autocrine TGF-β stimulation accounts for the maintenance of the activated SSc fibroblast phenotype, even in the absence of exogenous stimuli. While studies provide support for the ‘autocrine TGF-β hypothesis’, inhibition of type I TGF-β receptor–SMAD signaling in SSc fibroblasts in vitro only incompletely ‘normalized’ their activated phenotype.69,70 A potential explanation could be that autocrine TGF-β stimulation contributes to the SSc fibroblast phenotype via non-canonical (SMAD2/SMAD3-independent) signaling pathways; for instance, TGF-β activates SMAD1, along with AKT and ERK1 and ERK2, each of which are constitutively expressed or phosphorylated in SSc fibroblasts.71–73 The ERK1/ERK2 pathway is involved in regulation of early growth response protein 1 (EGR1) expression and the contractile phenotype of SSc fibroblasts.71,74
Although pharmacological blockade of TGF-β is feasible as a strategy for controlling fibrosis, the pleiotropic actions of TGF-β raise concerns that interfering with this essential physiological signaling molecule could result in untoward toxicities.64 Instead, therapies that block the downstream effectors of the TGF-β response might be more selective as inhibitors of fibrosis. The pro-fibrotic responses triggered by TGF-β are mediated via both canonical SMAD and SMAD-independent pathways involving the kinases ERK1, ERK2, TAK1, AKT, JNK and p38, EGR1, FAK1 and c-Abl.74–78 In addition to constitutive SMAD2/SMAD3 phosphorylation and nuclear localization, multiple abnormalities in SMAD signaling have been identified in SSc fibroblasts.67,72,79–83 In light of the essential role of TGF-β in fibrosis, biologic agents that neutralize TGF-β or prevent its integrin-mediated activation, and small molecules that selectively block TGF-β signaling downstream of its receptor, are attractive. Indeed, several inhibitory agents are under preclinical development. It seems likely that therapies targeting TGF-β signaling will be most effective in those SSc patients who demonstrate molecular evidence of TGF-β pathway activation.
c-Abl mediates fibrotic responses
In normal fibroblasts, but not epithelial cells, both TGF-β and PDGF activate c-Abl, a non-receptor tyrosine kinase associated with chronic myelogenous leukemia (CML).84,85 Importantly, in fibroblasts, endogenous c-Abl is required for the profibrotic responses induced by TGF-β. Imatinib, a relatively selective c-Abl inhibitor that is highly effective for treating CML, blocked the stimulation of collagen synthesis and fibroblast proliferation induced by TGF-β, and suppressed collagen gene expression in SSc fibroblasts85 (S. Bhattacharyya et al., unpublished data). Moreover, imatinib also blocks PDGF receptor-mediated responses. Imatinib interfered with cutaneous wound healing in vivo,86 and in mouse models ameliorated experimental skin and lung fibrosis.84,87,88 While these observations suggest that blocking c-Abl and PDGF signaling with imatinib might control fibrosis, a multicenter randomized clinical trial showed no therapeutic efficacy of imatinib in idiopathic pulmonary fibrosis.89 Results from ongoing studies in SSc are eagerly anticipated.
Early growth response genes
EGR1 is the prototypical member of a family of zinc finger transcription factors.90 The expression of EGR1 is rapidly induced by cytokines, lipids, mechanical damage and hypoxia at sites of injury. Under these conditions, EGR1 expression and activity is short lived. We showed that, in normal fibroblasts, EGR1 is also induced by, and mediates some of the fibrotic activities of, TGF-β.74,85,91,92 The induction of EGR1 was also blocked by imatinib.85 Levels of EGR1 are elevated in skin and lung fibroblasts explanted from patients with SSc, and elevated EGR1 expression is seen in lesional skin and fibrotic lungs (Figure 3). Moreover, an EGR1-regulated gene signature can be detected in lesional skin biopsies from some patients with diffuse cutaneous SSc.93,94 As EGR1 directly induces a broad range of profibrotic genes and genes involved in wound healing, including NOX4 and TGF-β itself, sustained EGR1 expression in SSc might exacerbate fibroblast activation and contribute to the progression of fibrosis.
Connective tissue growth factor
CTGF is a 38 kDa cysteine-rich member of a family of matricellular proteins that is strongly induced by TGF-β, and is overexpressed in most fibrotic diseases. While initial studies implicated CTGF as a major downstream mediator of the fibrotic responses elicited by TGF-β in fibroblasts, CTGF is currently considered to be more of a TGF-β cofactor that enhances TGF-β binding to its receptors. CTGF seems to be required for maximal induction of some TGF-β target genes.95 It has been proposed that CTGF signals through cell-surface integrins to promote adhesive signaling in fibroblasts; however, a bona fide CTGF signaling receptor has not been identified to date, and the mechanisms responsible for CTGF-mediated fibrotic responses remain controversial. Constitutive fibroblast-specific Ctgf expression in transgenic mice was associated with the development of a scleroderma-like phenotype, with myofibroblast accumulation and dermal and perivascular fibrosis.96 Mice with targeted deletion of Ctgf die shortly after birth, whereas mice with conditional Ctgf deletion only in fibroblasts were resistant to bleomycin-induced fibrosis.97 Explanted SSc fibroblasts overexpress CTGF in vitro, and elevated CTGF levels have been reported in lesional skin and the serum of patients with SSc. A genetic study demonstrated an association between SSc and a single nucleotide polymorphism in the CTGF promoter causing elevated CTGF expression.98
Endothelin 1
The endothelins are potent vasomodulatory peptides produced by endothelial cells, but also by macrophages, fibroblasts and other cell types. Endothelin 1 (ET-1) signaling via the endothelin receptors A (ETRA) and B (ETRB) on fibroblasts induces fibroblast migration and myofibroblast differentiation in vitro.99 These cellular responses seem to be mediated via SMAD-independent pathways involving TAK1. The expression of ET-1 and its receptors is elevated in SSc skin.100 The expression of ET-1 is induced by TGF-β, and like CTGF, ET-1 is considered to be a downstream mediator of some profibrotic TGF-β responses.101 Although blocking ET-1 signaling in mice prevented the development of bleomycin-induced pulmonary fibrosis,102 controlled clinical trials using the dual ET-1 receptor antagonist bosentan failed to show significant antifibrotic efficacy of ET-1 inhibition.103
Wnt–β-catenin signaling
The Wnts are poorly soluble secreted glycoproteins with key roles in embryonic development. Intracellular Wnt signaling involves the Frizzled and LRP5/LRP6 receptors and canonical β-catenin pathway.104 The intensity and duration of Wnt–β-catenin signaling is normally tightly regulated by endogenous inhibitors, such as secreted Frizzled-related proteins (sFRP), Dkk1 and Wnt inhibitory factor (WIF). Expression of a large number of genes is regulated by Wnt–β-catenin signaling in a cell-type-specific manner.105 Stimulation of normal fibroblasts with Wnt ligands results in β-catenin-mediated expression of collagen and other matrix genes, enhanced myofibroblast differentiation and increased cell migration.106–109 Genome-wide expression profiling demonstrated elevated expression of several Wnt ligands and Wnt–β-catenin target genes in pulmonary fibrosis and SSc.10,110–112 Other studies demonstrated nuclear β-catenin localization in fibroblast-like cells present in affected tissues, indicative of activated Wnt–β-catenin signaling.113–115 Chronic β-catenin signaling in fibrotic tissues represents aberrant recapitulation of embryologic developmental programs, and might result from Wnt-ligand-independent, constitutive β-catenin activation induced by extracellular matrix components, such as SPARC and FnEDA, acting in a self-amplifying autocrine feedback loop.107,116
The ability of canonical Wnts to induce fibroblast activation and progenitor cell differentiation in vitro, coupled with evidence of chronic Wnt-ligand-independent β-catenin signaling in lesional tissues, supports an important role for this developmental pathway in SSc. Blocking Wnt–β-catenin signaling with currently available drugs, such as the PPAR-γ ligand thiazolidinediones, the vitamin D derivative paricalcitol,117 the antihelminthic drug pyrvinium, or novel small molecules,108,118 represents a potential antifibrotic therapeutic approach deserves investigation.
Effect of matrix stiffness on fibrogenesis
The mechanical properties of the extracellular matrix environment influence the behavior of resident mesenchymal cells. Because substrate stiffness modulates fibroblast morphology, migration, proliferation, differentiation and biosynthetic activity, the stiffness of an affected tissue is not just an outcome of fibrosis, but is itself responsible for amplifying and sustaining fibrogenesis.119 Following injury, a rapid increase in the stiffness of the affected organ occurs, which might even precede the appearance of morphologic hallmarks of fibrosis.120 Fibrosis in the skin, lungs and other organs is associated with sometimes striking increases in matrix stiffness. Plating of explanted fibroblasts on substrates of increasing stiffness causes their activation, reflecting the activation of matrix-bound latent TGF-β by biomechanically stressed fibroblasts.121 Other studies have shown that biomechanical strain generated by stiff matrix suppresses cyclooxygenase 2 expression and production of prostaglandin E2, an autocrine inhibitor of fibroblast activation.122 Fibroblasts sense matrix stiffness via integrins and changes in cytoskeletal tension mediated by focal adhesion kinase (FAK) or Rho-associated kinase (ROCK).
The implication of these findings is that matrix stiffening during fibrogenesis itself drives further fibroblast activation and matrix deposition, creating a self-amplifying feed-forward loop. Targeting the activity of the collagen cross-linking enzyme lysyl oxidase and blocking integrin-mediated biomechanical signaling in fibroblasts represent potential therapeutic strategies to interfering with the vicious cycle of stiffness-driven fibrogenesis.
Integrin signaling and TGF-β crosstalk
By binding to the extracellular matrix outside the cell and to the cytoskeleton intracellularly, the integrin family of transmembrane receptors integrate the extracellular environment with cellular behavior, and are essential for adhesive signaling.123,124 Several integrins also control TGF-β localization and activation. All integrins consist of α and β subunits. The β1 integrin subunit mediates fibroblast attachment to type I collagen and fibronectin, and has long been known to have a role in tissue repair. Its expression is induced by TGF-β, but its role in mediating TGF-β activation is not entirely clear. By contrast, β1 integrin activation results in integrin-linked kinase-mediated fibroblast adhesion to the matrix, and is essential for matrix contraction. Mice with fibroblast-specific conditional β1 integrin knockout show defective wound healing, and are protected from bleomycin-induced skin fibrosis.125,126
Extensive bidirectional crosstalk exists between inte-grin and TGF-β signaling in fibrosis, with TGF-β inducing integrin expression and integrins modulating TGF-β activation and signaling.123 The αVβ6 integrin, expressed on injured epithelial cells, is a potent inducer of latent TGF-β activation.127 Like other TGF-β-binding integrins, αVβ6 interacts with the RGD motif on the latent TGF-β complex, causing its spatially restricted activation.128 Constitutive αVβ6 expression in the epidermis in transgenic mice is associated with enhanced TGF-β activation and cutaneous fibrosis.129 Mice with deleted β6 integrin are protected from lung fibrosis and, moreover, blocking β6 with an antibody protected mice from developing experimentally induced pulmonary fibrosis.63 Idiopathic and SSc-associated pulmonary fibrosis are accompanied by markedly increased expression of αVβ6 integrins on epithelial cells at fibrotic foci, with consequent liberation of activated TGF-β in the microenvironment.63 Inhibition of profibrotic TGF-β signaling restricted to sites of β6-integrin-mediated TGF-β activation might, therefore, be a strategy to bypass global TGF-β inhibition that could potentially be complicated by autoimmunity.
The αVβ5 and αVβ3 integrins are expressed on fibroblasts, and activate latent TGF-β at the cell surface. The expression of these integrins is significantly increased on SSc fibroblasts, which might directly contribute to their activated phenotype.61,62,130 Patients with stiff skin syndrome, a rare autosomal-dominant, inherited scleroderma-like condition, have mutations in the fibrillin-1 gene (FBN1) that disrupt αVβ3 integrin function and, subsequently, the ability of fibroblasts to interact with the surrounding matrix, resulting in increased TGF-β accumulation and activity in the dermis.131 Thus, in light of their extensive crosstalk with profibrotic TGF-β signaling, integrins provide attractive therapeutic targets for interfering with TGF-β signaling.
Autoantibodies
Patients with SSc have unique autoantibodies that are not associated with other autoimmune diseases. SSc-specific antibodies against ubiquitous autoantigens, such as topoisomerase I and the centromere, accurately identify distinct disease subsets, and their titers might correlate with disease severity and activity. In contrast to their well-recognized diagnostic utility, however, the contribution of SSc-specific autoantibodies in pathogenesis remains uncertain. One study showed that antitopoisomerase I antibodies bind to topoisomerase I on the cell surface, resulting in enhanced monocyte adhesion to fibro-blasts.132 Additional autoantibodies against cell-surface epitopes (anti-fibroblast antibodies), receptors (such as PDGF), extracellular matrix components (such as fibril-lin 1) or matrix-degrading metalloproteinases (such as MMP-3) have been described in patients with SSc, and might have a direct role in disease pathogenesis.133
Anti-fibroblast antibodies—reportedly present in up to 40% of patients with SSc—were shown to induce ICAM1 expression and stimulate the production of IL-6 and profibrotic chemokines in normal dermal fibro-blasts.54 Antibodies against fibrillin 1, reported in one study to occur in a majority of North American patients with SSc, were shown to stimulate collagen gene expression in dermal fibroblasts via endogenous TGF-β signaling.134 However, a subsequent study in European patients with SSc failed to detect anti-fibrillin 1 auto-antibodies.135 Another study described the presence of circulating antibodies that bind to the PDGF receptors on normal dermal fibroblasts and induce their activation, resulting in ROS generation, enhanced collagen synthesis and myofibroblast differentiation.136 While stimulatory anti-PDGF-receptor antibodies were detected in all 46 patients with SSc examined in this report, subsequent studies have failed to reproduce these findings.137,138
Vascular injury and oxidative stress
The double insults of vascular injury and matrix deposition in SSc give rise to vascular rarefaction and tissue hypoxia, which in turn is itself a potent stimulus for the synthesis of collagen and its cross-linking enzyme lysyl hydroxylase, fibronectin and fibrogenic cytokines.138 Hypoxia can also drive EMT via autocrine TGF-β signaling or hypoxia-inducible factor 1.31,139 Thus, hypoxia resulting from capillary rarefaction directly contributes to propagation of a vicious cycle of fibrogenesis. Vascular injury is associated with activation of the coagulation cascade and accompanying generation of thrombin. Thrombin acts as a potent stimulus for myofibroblast differentiation, and blocking its activity with dabigatran ameliorates experimental lung fibrosis in mice.140 Thrombin also promotes platelet activation, which is a prominent feature of SSc. Activated platelets release PDGF, a potent mitogen; trans-genic mice expressing a constitutively active PDGF receptor α develop skin and lung fibrosis.141 Activated platelets also secrete serotonin, which stimulates fibroblast matrix synthesis via 5-HT2B receptors.142
Oxidative stress due to excessive generation of oxygen free radicals and related ROS is prevalent in SSc, and contributes to the progression of tissue damage.143 In fibroblasts, TGF-β induces the NADPH oxidase enzyme NOX4, which catalyzes the reduction of oxygen to ROS.144 In turn, ROS act as signals to induce fibroblast activation and myofibroblast differentiation. Indeed, ROS seem to be required for the execution of the profibrotic effects of TGF-β. Pharmacological targeting of NOX4 protects mice from experimental fibrosis.144 Fibrotic tissues demonstrate elevated NOX4 expression, and explanted SSc fibroblasts spontaneously generate ROS.144,145 Spontaneous ROS production might contribute to an autocrine amplification loop and persistent activation of these cells.
Bioactive lipids
Bioactive lipids are potent modulators of fibroblast function, and some prostanoids have been shown to directly or indirectly inhibit fibrotic responses.146 By contrast, prostaglandin F2α (PGF2α) stimulates collagen production and fibroblast proliferation, and is elevated in fibrotic lungs.147 Lysophosphatidic acid (LPA), generated at sites of injury by hydrolysis of membrane phospholipids, exerts multiple biological activities via G-protein coupled receptors.148 LPA induces fibroblast activation and migration, and mice with genetic deletion of the LPA receptor 1 (LPAR1) are protected from bleomycin-induced lung and skin fibrosis.149,150 Moreover, by stimulating αVβ6-integrin-mediated TGF-β activation in epithelial cells, LPA might contribute to sustained autocrine TGF-β signaling and fibrosis.151 Small molecules that block LPAR1 signaling are in preclinical development as antifibrotic therapies.
PPAR-γ: endogenous fibrosis inhibitor
The progression of pathological fibrosis in SSc is exacerbated by the failure of intrinsic mechanisms that normally constrain fibroblast activation. An important component of the antifibrotic defense armamentarium is the nuclear receptor PPAR-γ.152 Originally identified in adipose tissue, PPAR-γ is known to be broadly expressed and have fundamental roles in glucose and lipid metabolism, energy homeostasis and inflammation. Endogenous and diet-derived fatty acids and prostanoids, such as 15d-prostaglandin J2 (15d-PGJ2), are natural PPAR-γ agonists, whereas the thiazolidinedione class of insulin-sensitizing drugs (for example, rosiglitazone or pioglitazone) are potent synthetic agonists.
An entirely novel function for PPAR-γ in connective tissue homeostasis has emerged during the past 10 years. In normal fibroblasts, PPAR-γ ligands abrogate TGF-β-induced fibrotic responses.152–154 Moreover, PPAR-γ blocks EMT and the differentiation of preadipocytes into fibro-blasts.33 Specific deletion of PPAR-γ in mouse fibroblasts or hair follicle stem cells resulted in markedly exaggerated skin fibrosis and spontaneous development of scarring alopecia.155,156 On the other hand, pharmacologic activation of PPAR-γ signaling attenuated bleomycin-induced scleroderma in mice.157 A decrease in PPAR-γ expression in target tissues accompanies progression of fibrosis in experimental models of lung, liver and kidney fibrosis, and in normal aging.158–161 These studies suggest a key physiologic function of PPAR-γ signaling as an endogenous mechanism to prevent excessive fibrogenesis following injury. We observed reduced PPAR-γ expression, and an inverse correlation between PPAR-γ expression and TGF-β signaling, in skin biopsies from a subset of patients with SSc.161 Moreover, serum levels of adipo-nectin, a sensitive marker of PPAR-γ activity, were reduced in patients with diffuse cutaneous SSc, and showed an inverse correlation with skin score.
Several factors implicated in the pathogenesis of fibrosis—TGF-β, Wnt proteins, IL-13, hypoxia, LPA, CTGF, leptin and miR-27—are known to suppress PPAR-γ activity or expression.113 Thus, aberrant expression or activity of these profibrotic mediators might be responsible for impaired PPAR-γ function in SSc, which in turn contributes to fibrosis progression. The use of synthetic agonists to induce the activation of PPAR-γ signaling, or pharmacological enhancement of defective PPAR-γ tissue expression, might be effective novel approaches to the treatment of fibrosis.
Conclusions
Loss of the functional integrity of vital organs caused by progressive fibrosis accounts for the late mortality in patients with SSc. Because immunomodulatory therapies have not been proven to be effective in controlling fibrosis, therapeutic progress depends on the emergence of new paradigms for its pathogenesis. An explosion of information from recent studies raises many new questions, challenges accepted wisdom, and provides myriad novel opportunities for further investigation. Cellular plasticity, aberrant recapitulation of embryological genetic programs, altered epigenetic modifications and miRNA regulation, and the occurrence of self-amplifying feed-forward loops driven by hypoxia, matrix stiffness and tissue damage, all contribute to an abnormal tissue repair process decoupled from the primary injury that, if left unchecked, progresses to intractable fibrosis. Many potential opportunities exist for interfering with the process, and there is now a rich pipeline of biologic agents with which to neutralize the principal extra-cellular signals driving fibroblast activation (individually or in combination); agents to attenuate oxidative stress and reduce matrix stiffness and selectively shape the immune response; and small molecules to disrupt intra-cellular signaling pathways linked to fibrotic outcomes. Moreover, existing drugs approved for other indications show antifibrotic activity, and could be ‘repurposed’ as therapies for patients with fibrotic conditions. A critical challenge going forward will be to develop robust pathomechanism-based patient classifications so that a particular intervention can be targeted to the most appropriate subset of patients. The success of clinical trials of antifibrotic therapies will be critically dependent on bio-markers that reflect fibrogenic activity and cumulative fibrotic burden in clinically meaningful ways.
Key points.
Fibrosis represents a deregulated and uncontrolled repair process that recapitulates features of embryonic development and normal wound healing
Fibrosis in systemic sclerosis (SSc) shares mechanisms that underlie organ-based fibrosing disorders such as idiopathic pulmonary fibrosis
Transforming growth factor β seems to be a master regulator of both physiological and pathological matrix remodeling, and might be responsible for maintaining the activated fibroblast phenotype in SSc
The Wnt–β-catenin, Hedgehog–Patched and Jagged–Notch pathways are important in embryological development, and seem to be aberrantly activated in some patients with SSc
Mesenchymal cells are chronically activated via self-amplifying loops, resulting in a vicious cycle of progressive fibrogenesis
Selective targeting of molecules and pathways involved in fibroblast activation, singly or in combination, offers new approaches to the treatment of fibrosis
Review criteria.
MEDLINE and PubMed databases were searched for original articles focusing on fibrosis published between 2006 and 2011. The search terms used were “systemic sclerosis”, “fibrosis”, “scleroderma” and “fibroblast activation”. All papers identified were English-language, full text papers. The reference lists of identified articles were searched for further papers.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (AR 42309) and the Scleroderma Research Foundation. We are grateful to Carol Feghali-Bostwick, Warren Tourtellotte, Monique Hinchcliff, Robert Lafyatis, Michael Whitfield, Cara Gottardi, Carol Artlett, Maria Trojanowska and members of the Northwestern Scleroderma Program for valuable discussions.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed equally to researching data, discussing content, writing, and review/editing of the manuscript before subscription.
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–166. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:134–144. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 4.Wei J, Bhattacharyya S, Tourtellotte WG, Varga J. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun Rev. 2011;10:267–275. doi: 10.1016/j.autrev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quillinan NP, Denton CP. Disease-modifying treatment in systemic sclerosis: current status. Curr Opin Rheumatol. 2009;21:636–641. doi: 10.1097/BOR.0b013e3283310d57. [DOI] [PubMed] [Google Scholar]
- 6.Distler J, Distler O. Novel treatment approaches to fibrosis in scleroderma. Rheum Dis Clin North Am. 2008;34:145–159. doi: 10.1016/j.rdc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Trojanowska M, Varga J. Molecular pathways as novel therapeutic targets in systemic sclerosis. Curr Opin Rheumatol. 2007;19:568–573. doi: 10.1097/BOR.0b013e3282e6f495. [DOI] [PubMed] [Google Scholar]
- 8.van der Slot AJ, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23:251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Higgins DF, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner H, et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 2006;54:1961–1973. doi: 10.1002/art.21894. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield ML, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci USA. 2003;100:12319–12324. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milano A, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan FK, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006;45:694–702. doi: 10.1093/rheumatology/kei244. [DOI] [PubMed] [Google Scholar]
- 14.Hsu E, et al. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroy EC. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972;135:1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeRoy EC. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974;54:880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihn H. Autocrine TGF-β signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci. 2008;49:103–113. doi: 10.1016/j.jdermsci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh AK, Mori Y, Dowling E, Varga J. Trichostatin A blocks TGF-β-induced collagen gene expression in skin fibroblasts: involvement of Sp1. Biochem Biophys Res Commun. 2007;354:420–426. doi: 10.1016/j.bbrc.2006.12.204. [DOI] [PubMed] [Google Scholar]
- 19.Bechtel W, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 21.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Pandit KV, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer B, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, et al. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc Natl Acad Sci USA. 2002;99:3908–3913. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol. 2009;29:4325–4339. doi: 10.1128/MCB.01776-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmatazad H, et al. Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum. 2009;60:1519–1529. doi: 10.1002/art.24494. [DOI] [PubMed] [Google Scholar]
- 28.Kaimori A, et al. Histone deacetylase inhibition suppresses the transforming growth factor β1-induced epithelial-to-mesenchymal transition in hepatocytes. Hepatology. 2010;52:1033–1045. doi: 10.1002/hep.23765. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-β1-induced fibroblast–myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajkumar VS, et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou G, et al. Hypoxia-induced alveolar epithelial–mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1120–L1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Jimenez SA. Protein kinase Cδ and the c-Abl kinase are required for transforming growth factor β induction of endothelial–mesenchymal transition in vitro. Arthritis Rheum. 2011;63:2473–2483. doi: 10.1002/art.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan X, Dagher H, Hutton CA, Bourke JE. Effects of PPARγ ligands on TGF-β1-induced epithelial–mesenchymal transition in alveolar epithelial cells. Respir Res. 2010;11:21. doi: 10.1186/1465-9921-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reka AK, et al. Peroxisome proliferator-activated receptor-γ activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol Cancer Ther. 2010;9:3221–3232. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du F, et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70:10080–10089. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dees C, et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011;63:1396–1404. doi: 10.1002/art.30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavian N, et al. Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum. 2010;62:3477–3487. doi: 10.1002/art.27626. [DOI] [PubMed] [Google Scholar]
- 38.Greenbaum LE. Hedgehog signaling in biliary fibrosis. J Clin Invest. 2008;118:3263–3265. doi: 10.1172/JCI37189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, et al. Induction of interferon-α by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-α activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- 41.York MR, et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and Toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 42.Duan H, et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 2008;58:1465–1474. doi: 10.1002/art.23451. [DOI] [PubMed] [Google Scholar]
- 43.Assassi S, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62:589–598. doi: 10.1002/art.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eloranta ML, et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69:1396–1402. doi: 10.1136/ard.2009.121400. [DOI] [PubMed] [Google Scholar]
- 45.Dieude P, et al. Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: a new perspective for pulmonary fibrosis. Arthritis Rheum. 2009;60:225–233. doi: 10.1002/art.24183. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal SK, et al. Toll-like receptor 3 upregulation by type I interferon in healthy and scleroderma dermal fibroblasts. Arthritis Res Ther. 2011;13:R3. doi: 10.1186/ar3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farina GA, et al. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130:2583–2593. doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyde DM, Giri SN. Polyinosinic-polycytidylic acid, an interferon inducer, ameliorates bleomycin-induced lung fibrosis in mice. Exp Lung Res. 1990;16:533–546. doi: 10.3109/01902149009068825. [DOI] [PubMed] [Google Scholar]
- 49.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 51.Arslan F, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 52.Muro AF, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seki E, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 54.Fineschi S, et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 2008;58:3913–3923. doi: 10.1002/art.24049. [DOI] [PubMed] [Google Scholar]
- 55.Csak T, et al. Deficiency in myeloid differentiation factor-2 and Toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–G441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pulskens WP, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21:1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell MT, et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2009;168:e61–e69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He Z, Zhu Y, Jiang H. Inhibiting Toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir Res. 2009;10:126. doi: 10.1186/1465-9921-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617–622. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Galdo F, Wermuth PJ, Addya S, Fortina P, Jimenez SA. NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69:2024–2033. doi: 10.1136/ard.2010.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Increased expression levels of integrin αvβ5 on scleroderma fibroblasts. Am J Pathol. 2004;164:1275–1292. doi: 10.1016/s0002-9440(10)63215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asano Y, et al. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 63.Horan GS, et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 64.Varga J, Pasche B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sargent JL, et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawakami T, et al. Increased expression of TGF-β receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-β signaling to scleroderma phenotype. J Invest Dermatol. 1998;110:47–51. doi: 10.1046/j.1523-1747.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- 67.Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-β receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 68.Pannu J, Gardner H, Shearstone JR, Smith E, Trojanowska M. Increased levels of transforming growth factor β receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum. 2006;54:3011–3021. doi: 10.1002/art.22063. [DOI] [PubMed] [Google Scholar]
- 69.Ihn H. Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr Rheumatol Rep. 2005;7:156–162. doi: 10.1007/s11926-005-0069-9. [DOI] [PubMed] [Google Scholar]
- 70.Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor β signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor β receptors. Arthritis Rheum. 2001;44:474–480. doi: 10.1002/1529-0131(200102)44:2<474::AID-ANR67>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum. 2008;58:577–585. doi: 10.1002/art.23146. [DOI] [PubMed] [Google Scholar]
- 72.Jun JB, et al. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J Invest Dermatol. 2005;124:298–303. doi: 10.1111/j.0022-202X.2004.23559.x. [DOI] [PubMed] [Google Scholar]
- 73.Pannu J, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–2537. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharyya S, et al. Smad-independent transforming growth factor-β regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol. 2008;173:1085–1099. doi: 10.2353/ajpath.2008.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen SJ, et al. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad 3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- 77.Hong M, et al. Non-Smad TGF-β signaling regulated by focal adhesion kinase binding the P85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 2011;286:17841–17850. doi: 10.1074/jbc.M111.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi-wen X, et al. Requirement of transforming growth factor β-activated kinase 1 for transforming growth factor β-induced α-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234–241. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- 79.Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 80.Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxford) 2006;45:157–165. doi: 10.1093/rheumatology/kei124. [DOI] [PubMed] [Google Scholar]
- 81.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7–Smurf-mediated negative regulation of TGF-β signaling in scleroderma fibroblasts. J Clin Invest. 2004;113:253–264. doi: 10.1172/JCI16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya S, et al. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor β. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- 84.Daniels CE, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhattacharyya S, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-β via c-Abl and EGR-1: selective modulation by imatinib mesylate. Oncogene. 2009;28:1285–1297. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajkumar VS, et al. Platelet-derived growth factor-β receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Distler JH, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 88.Vittal R, et al. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/ mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321:35–44. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- 89.Daniels CE, et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 90.Bhattacharyya S, et al. Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol. 2011;30:235–242. doi: 10.1016/j.matbio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu M, et al. Essential roles for early growth response transcription factor EGR-1 in tissue fibrosis and wound healing. Am J Pathol. 2009;175:1041–1055. doi: 10.2353/ajpath.2009.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen SJ, et al. The early-immediate gene EGR-1 is induced by transforming growth factor-β and mediates stimulation of collagen gene expression. J Biol Chem. 2006;281:21183–21197. doi: 10.1074/jbc.M603270200. [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharyya S, et al. The transcriptional cofactor Nab2 is induced by TGF-β and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS ONE. 2009;4:e7620. doi: 10.1371/journal.pone.0007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharyya S, et al. EGR-1 induces a profibrotic injury/repair gene program associated with systemic sclerosis. PLoS ONE. 6:e23082. doi: 10.1371/journal.pone.0023082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leask A. Towards an anti-fibrotic therapy for scleroderma: targeting myofibroblast differentiation and recruitment. Fibrogenesis Tissue Repair. 2010;3:8. doi: 10.1186/1755-1536-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sonnylal S, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 98.Fonseca C, et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N Engl J Med. 2007;357:1210–1220. doi: 10.1056/NEJMoa067655. [DOI] [PubMed] [Google Scholar]
- 99.Xu SW, et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- 100.Abraham DJ, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151:831–841. [PMC free article] [PubMed] [Google Scholar]
- 101.Shi-wen X, et al. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor β in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 102.Schroll S, et al. Improvement of bleomycin-induced pulmonary hypertension and pulmonary fibrosis by the endothelin receptor antagonist Bosentan. Respir Physiol Neurobiol. 2010;170:32–36. doi: 10.1016/j.resp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 103.King TE, Jr , et al. BUILD-3: A randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 104.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 105.Vlad A, Röhrs S, Klein-Hitpass L, Müller O. The first five years of the Wnt targetome. Cell Signal. 2008;20:795–802. doi: 10.1016/j.cellsig.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 106.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PLoS ONE. 6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang W, et al. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of β-catenin. J Biol Chem. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thorne CA, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei J, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42:21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 111.Bayle J, et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 112.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chilosi M, et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konigshoff M, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam AP, et al. Nuclear β-catenin is increased in SSc pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2010-0113OC. http://dx.doi.org/10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed]
- 116.Bielefeld KA, et al. Fibronectin and β-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J Biol Chem. 2011;286:27687–27697. doi: 10.1074/jbc.M111.261677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henderson WR, Jr, et al. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georges PC, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 121.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu F, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Margadant C, Sonnenberg A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eckes B, et al. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol. 2000;19:325–332. doi: 10.1016/s0945-053x(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 125.Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A. Loss of β1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60:2817–2821. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 126.Liu S, et al. Expression of integrin β1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 127.Munger JS, et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 128.Worthington JJ, Klementowicz JE, Travis MA. TGFβ: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 129.Hakkinen L, et al. Increased expression of β 6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loeys BL, et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. 2010;2:23–20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henault J, Robitaille G, Senecal JL, Raymond Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheum. 2006;54:963–973. doi: 10.1002/art.21646. [DOI] [PubMed] [Google Scholar]
- 133.Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276–281. doi: 10.1016/j.autrev.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 134.Zhou X, et al. Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-β pathway to recapitulate the “scleroderma phenotype”. J Immunol. 2005;175:4555–4560. doi: 10.4049/jimmunol.175.7.4555. [DOI] [PubMed] [Google Scholar]
- 135.Brinckmann J, et al. Absence of autoantibodies against correctly folded recombinant fibrillin-1 protein in systemic sclerosis patients. Arthritis Res Ther. 2005;7:R1221–R1226. doi: 10.1186/ar1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baroni SS, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 137.Classen JF, et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum. 2009;60:1137–1144. doi: 10.1002/art.24381. [DOI] [PubMed] [Google Scholar]
- 138.Loizos N, et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor α in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum. 2009;60:1145–1151. doi: 10.1002/art.24365. [DOI] [PubMed] [Google Scholar]
- 139.Distler JH, et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56:4203–4215. doi: 10.1002/art.23074. [DOI] [PubMed] [Google Scholar]
- 140.Bogatkevich GS, et al. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 2011;63:1416–1425. doi: 10.1002/art.30255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dees C, et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. J Exp Med. 2011;208:961–972. doi: 10.1084/jem.20101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 144.Hecker L, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Servettaz A, et al. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann Rheum Dis. 2007;66:1202–1209. doi: 10.1136/ard.2006.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang SK, Peters-Golden M. Eicosanoid lipid mediators in fibrotic lung diseases: ready for prime time? Chest. 2008;133:1442–1450. doi: 10.1378/chest.08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oga T, et al. Prostaglandin F2α receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-β. Nat Med. 2009;15:1426–1430. doi: 10.1038/nm.2066. [DOI] [PubMed] [Google Scholar]
- 148.Rancoule C, et al. Lysophosphatidic acid-1-receptor targeting agents for fibrosis. Expert Opin Investig Drugs. 2011;20:657–667. doi: 10.1517/13543784.2011.566864. [DOI] [PubMed] [Google Scholar]
- 149.Tager AM, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 150.Castelino FV, et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu MY, et al. Lysophosphatidic acid induces αvβ6 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gαq. Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei J, Bhattacharyya S, Varga J. Peroxisome proliferator-activated receptor γ: innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr Opin Rheumatol. 2010;22:671–676. doi: 10.1097/BOR.0b013e32833de1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghosh AK, et al. Peroxisome proliferator-activated receptor-γ abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 2009;23:2968–2977. doi: 10.1096/fj.08-128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghosh AK, et al. Disruption of transforming growth factor β signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor γ. Arthritis Rheum. 2004;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 155.Kapoor M, et al. Loss of peroxisome proliferator-activated receptor γ in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 2009;60:2822–2829. doi: 10.1002/art.24761. [DOI] [PubMed] [Google Scholar]
- 156.Karnik P, et al. Hair follicle stem cell-specific PPARγ deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu M, et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng F, et al. Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am J Physiol Renal Physiol. 2002;282:F639–F648. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- 159.Miyahara T, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 160.Culver DA, et al. Peroxisome proliferator-activated receptor γ activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 2004;30:1–5. doi: 10.1165/rcmb.2003-0304RC. [DOI] [PubMed] [Google Scholar]
- 161.Wei J, et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS ONE. 2010;5:e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fang F, et al. The early growth response gene EGR2 (alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Pathol. 2011;178:2077–2090. doi: 10.1016/j.ajpath.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kubo M, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jinnin M, Ihn H, Mimura Y, Asano Y, Tamaki K. Involvement of the constitutive complex formation of c-Ski/SnoN with Smads in the impaired negative feedback regulation of transforming growth factor β signaling in scleroderma fibroblasts. Arthritis Rheum. 2007;56:1694–1705. doi: 10.1002/art.22588. [DOI] [PubMed] [Google Scholar]
- 165.Bu S, et al. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 2010;62:2117–2126. doi: 10.1002/art.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parapuram SK, et al. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J Invest Dermatol. doi: 10.1038/jid.2011.156. http://dx.doi.org/10.1038/jid.2011.156. [DOI] [PubMed]