Abstract
Neurons in the upper lumbar spinal cord project axons containing gastrin-releasing peptide (GRP) to innervate lower lumbar regions controlling erection and ejaculation. This system is vestigial in female rats and in males with genetic dysfunction of androgen receptors, but in male rats, pharmacological stimulation of spinal GRP receptors restores penile reflexes and ejaculation after castration. GRP offers new avenues for understanding potential therapeutic approaches to masculine reproductive dysfunction.
GRP, a member of the bombesin-like peptide family1, is distributed widely in the central nervous system and gastrointestinal tract of mammals2,3. GRP and neuromedin B (NMB), the mammalian counterpart of bombesin, play a role in many physiological processes, including itch4, circadian rhythms5, food intake6 and fear memory consolidation7,8. In mammals, bombesin-like peptides act through a family of at least three G protein–coupled receptors: GRP-preferring receptor (GRP-R), NMB-preferring receptor (NMB-R) and bombesin receptor subtype-3 (BRS-3)9.
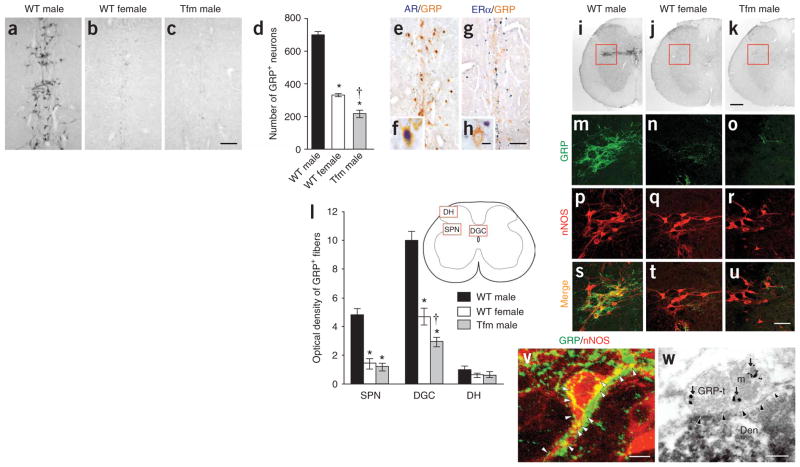
Using immunocytochemistry (ICC) directed at GRP, we found a group of neurons within a region previously dubbed the ‘spinal ejaculation generator’ because toxins that selectively lesion galanin-containing neurons there virtually eliminate ejaculation in rats10. These galaninergic neurons project to the thalamus10, but it had been unclear whether there are also direct connections between this center and the lower spinal cord regions that directly trigger ejaculation11. The separate, GRP-containing neurons that we found within the center projected axons to more caudal spinal regions and were much more prominent in wild-type (WT) males than in WT females (Fig. 1a,b; Supplementary Fig. 1 online) (n = 5, F2,12 = 299.9, P < 0.001). Semiquantitative reverse transcription (RT)-PCR confirmed more pre-pro Grp transcripts in this region of males than of females (Supplementary Fig. 2 online). To test whether androgen receptors direct sexual dimorphism of these neurons, we examined genetically male (XY) Long-Evans rats carrying the testicular feminization mutation (Tfm) of the androgen receptor gene Ar. These rats develop testes embryologically and secrete testosterone pre- and postnatally but, because their androgen receptor protein is dysfunctional, develop a wholly feminine exterior, including a clitoris rather than a penis. The spinal cord of Tfm rats was hyperfeminine, having even fewer GRP-positive neurons in this region than did WT females (P <0.001) (Fig. 1c,d). In normal males, GRP-expressing neurons also expressed androgen receptor (96.1 ± 1.7%; n = 4 WT males), but not estrogen receptor alpha (ERα) (Fig. 1e–h). Because androgens such as testosterone augment ejaculation in male rats and humans12, the presence of androgen receptor in the GRP-positive neurons of the ejaculation center offers a locus for androgenic modulation of ejaculation and other sexual reflexes.
Figure 1.
Origin, targets and androgen dependence of GRP neurons in the lumbar spinal cord. (a–d) More GRP-immunoreactive neurons were found in upper lumbar spinal cord of WT males (a) than females (b). Tfm males were feminine in this regard (c), with significantly fewer GRP+ neurons in this region than WT females (d; means ± s.e.m.). (e–h) In WT males, nearly every GRP-immunoreactive neuron also contained androgen receptor (e,f), but these neurons did not contain ERα (g,h). (i–l) In lower lumbar spinal cord autonomic nuclei, males (i) had more GRP-immunoreactive fibers in the SPN (red inset) and the DGC than did females (j; quantitation in l). Androgen receptor–lacking Tfm males were feminine in this regard (k). GRP fibers in the non-autonomic dorsal horn region of the spinal cord (DH) were equivalent across groups (l). (m–u) GRP-containing fibers closely apposed cell bodies and proximal dendrites of nNOS+ neurons in the SPN. Left column, WT male; center, WT female; right, Tfm male. (v) Possible synaptic sites (arrowheads). (w) Electron micrographs confirmed GRP-immunoreactive presynaptic boutons innervating nNOS-containing dendrites in the SPN. Arrows, GRP-immunoreactive secretory granules; arrowheads, synapse. GRP-t, GRP terminal; m, mitochondrion; Den, nNOS-immunoreactive dendrite. *P < 0.001 compared with WT males; †P < 0.001 compared with WT females (one-way (d) or two-way (l) analysis of variance; significant effects followed by post hoc Bonferroni tests). Scale bars: c (also applies to a,b) and g (applies to e), 100 μm; h (applies to f), 10 μm; k (applies to i,j), 200 μm; u (applies to m–t), 50 μm; v, 10 μm; w, 200 nm. All animal procedures were approved by institutional review boards of Kyoto Prefectural University of Medicine and/or Michigan State University. For methodological details, see Supplementary Methods online.
ICC revealed that these GRP neurons in the upper lumbar spinal cord projected their axons to the lower lumbar and upper sacral spinal cord, presenting a sexually dimorphic pattern of fibers innervating autonomic centers that regulate male sexual reflexes11. These fibers were much more prominent in male rats than in females in the sacral parasympathetic nucleus (SPN) (n = 5, F2,12 = 42.2, P < 0.001) and the dorsal gray commissure (DGC) surrounding the central canal (F2,12 = 53.9, P < 0.001) (Fig. 1i–l), both of which provide autonomic preganglionic fibers to genitalia13. Mirroring the results in the upper spinal cord, the lumbosacral cords of Tfm rats showed an entirely feminine pattern of GRP+ fibers in the SPN autonomic nucleus and a hyperfeminine pattern in the DGC (P <0.001) (Fig. 1i–l). Double ICC confirmed that GRP-containing fibers surrounded neurons expressing neuronal nitric oxide synthase (nNOS; Fig. 1m–u), a marker for autonomic preganglionic neurons14 and an enzyme involved in erection and ejaculation12. Electron micrography demonstrated GRP-immunoreactive presynaptic boutons innervating nNOS-positive dendrites in these autonomic nuclei of the lumbosacral spinal cord (Fig. 1v,w). In males, GRP-immunoreactive fibers also extended to the spinal nucleus of the bulbocavernosus (Supplementary Fig. 3 online), which innervates striated muscles attached to the penis and is important in copulatory behavior. There was no sex difference in GRP fibers in the dorsal horn (F2,12 = 0.8) (see Fig. 1l), which presumably processes non-autonomic sensory stimuli such as itch4.
Castration of adult male rats reduced the intensity of GRP-immunoreactive fibers in the SPN (n =4, F2,9 = 15.6, *P =0.001, †P <0.001) and DGC (F2,9 = 10.1, *P = 0.003, †P = 0.004) (but not in the dorsal horn; F2,9 = 0.3) of the lower spinal cord 28 d later, a reduction that was averted by androgen replacement (Supplementary Table 1 online). Ovariectomy had no effect on GRP expression in females, and androgen treatment of ovariectomized females increased GRP-immunoreactive fibers to only a modest degree (Supplementary Table 1). These results suggest that the GRP spinal cord system is masculinized by perinatal androgen yet retains some androgen responsiveness in adulthood.
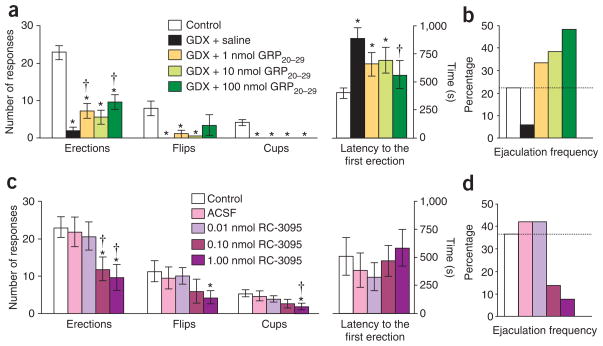
We probed the presence of GRP-R in the lower spinal cord by binding of GRP, RT-PCR, and double ICC, finding heavy concentration of GRP-R on the nNOS-positive neurons of the lumbosacral autonomic nuclei (n ≥4 in each experiment) (Fig. 2). Furthermore, the rat homolog of GRP-R agonists (rat GRP20–29)4,6, which had no effect on pain perception in tail-flick tests (n = 8, F4,28 = 0.8) (Supplementary Fig. 4 online), restored many of the spinal reflexes of the penis that were lost after castration (n = 10; simple erections, F4,36 = 34.0, P < 0.001 compared with saline-injected intact males, P <0.018 compared with saline-injected castrated males; dorsal flips of the penis, F4,36 = 8.1, P <0.003 compared with saline-injected intact males; cup-like flaring erections of the glans, F4,36 = 34.7, P < 0.001 compared with saline-injected intact males; latencies to the first erection, F4,36 = 4.4, P < 0.018 compared with saline-injected intact males, P = 0.013 compared with saline-injected castrated males) (Fig. 3a). Most notably, the agents were particularly effective in restoring ejaculation per se, resulting in a greater frequency of ejaculation in treated castrated rats than in gonadally intact control males (Fig. 3b). We administered RC-3095, a specific GRP-R antagonist8, intrathecally at the level of the fifth lumbar to first sacral vertebrae (L5–S1) in gonadally intact males (n = 7). This treatment suppressed penile reflexes, including erections (F4,24 = 4.4, P < 0.013 compared with uninjected males, P < 0.026 compared with vehicle-injected males), flips (F4,24 = 2.8, P < 0.008 compared with uninjected males) and cups (F4,24 = 2.8, P = 0.007 compared with uninjected males, P = 0.022 compared with vehicle-injected males), without affecting latencies to the first erection (F4,24 = 0.4) and also attenuated the spontaneous ejaculation rate in a dose-dependent manner (Fig. 3c,d), indicating that the GRP/GRP-R system regulates masculine reproductive functions within the spinal cord.
Figure 2.
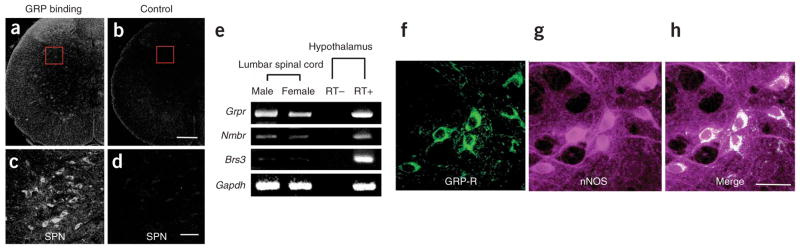
GRP-Rs in autonomic regions of the lower lumbar spinal cord. (a–d) Incubating L5–6 spinal cord sections with biotinylated GRP yielded accumulation of GRP (a), especially among neurons in the SPN (red inset in a, magnified in c). Control incubations with biotinylated GRP and excess free ligand demonstrated the specificity of this binding (b,d). (e) RT-PCR confirmed the presence of Grpr and the related Nmbr, but few or no Brs3 transcripts, in the lumbar spinal cord. All three receptor transcripts were found in hypothalamus, as expected. (f–h) Double ICC localized the spinal GRP-R to cell bodies of nNOS+ neurons in the autonomic SPN. Scale bars: b (applies to a), 200 μm; d (applies to c) and h (applies to f,g), 50 μm.
Figure 3.
Role of spinal cord GRP system in sexual reflexes. (a) Gonadectomy (GDX) of male rats reduced spinal reflexes of the penis, including simple erections, dorsal flips of the penis and cup-like flaring erections of the distal glans. Systemic treatment of GDX males with a specific agonist for GRP-R (rat GRP20–29) restored erections and flips, without affecting cups. *P < 0.05 compared with saline-injected intact males (control); †P < 0.05 compared with saline-injected castrated males (GDX + saline). (b) Spontaneous ejaculations during reflex tests were also reduced after castration, an effect fully reversed, in a dose-dependent manner, by systemic treatment with rat GRP20–29. (c) In gonadally intact males, these penile reflexes were suppressed by intrathecal injection of a specific antagonist for GRP-R (RC-3095). *P < 0.05 compared with uninjected males (control); †P < 0.05 compared with vehicle-injected males (ACSF). (d) Spontaneous ejaculations were attenuated by intrathecal RC-3095 in intact males. Significant effects in analyses of variance were followed by post hoc Bonferroni test. Data are means ± s.e.m. (a,c) or percentages (b,d).
Expression of spinal Grpr mRNA is also sexually dimorphic (Fig. 2e; Supplementary Fig. 5 online), and GRP-R is localized not only in the SPN but also in the spinal nucleus of the bulbocavernosus, suggesting that GRP simultaneously stimulates autonomic preganglionic neurons (see Fig. 2c) and somatic motor neurons controlling the penis (Supplementary Figs. 6 and 7 online), consequently augmenting penile reflexes and ejaculation (Supplementary Fig. 8 online). Thus, reduced GRP expression in the spinal cord of castrated rats may mediate their reduced male copulatory behavior.
It is unclear how the previously identified galanin-containing neurons interact with these GRP-containing neurons within the ejaculation generator10. Some neurons may contain both galanin and GRP, but the distributions of somata containing the two peptides differed somewhat (Supplementary Figs. 9 and 10 online). Double ICC showed many GRP-immunoreactive, galanin-negative neurons just dorsal to the central canal (Supplementary Fig. 10a–d). Notably, most GRP-containing neurons lateral to the central canal and in the medial portion of lamina VII also expressed galanin (Supplementary Fig. 10e–h). Indeed, in the lower lumbar spinal cord (L5–6 level), the ventral portion of the GRP-immunoreactive fibers projecting to the SPN also contained galanin, whereas the dorsal portion contained GRP only (Supplementary Fig. 10i–l). Thus, both GRP- and galanin-containing neurons within the ejaculation center project axons into the SPN through DGC (see Supplementary Fig. 8), and these two populations are at least partially independent.
The galanin-containing neurons, which are required for ejaculation10, project to the thalamus, so either information from the thalamus returns to the ejaculation center to trigger the GRP neurons or the two populations within the center interact more directly15. We conclude that the sexually dimorphic GRP system in the lumbar spinal cord is crucial in male sexual function, revealing a hitherto unrecognized role for GRP in mammalian reproductive behavior. It is of particular note that GRP replacement alone can restore reproductive functions in the absence of androgen. To our knowledge, this is the first neural pathway known to restore ejaculation in castrated male rats. Because this system relies on a specific peptide, it may offer an avenue for pharmacological interventions to reverse male reproductive disorders.
Supplementary Material
Acknowledgments
We thank A. Takara for technical assistance. Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology, Japan (H.S., K.-I.M. and M.K.) and US National Institutes of Health grant NS28421 (S.M.B.).
Footnotes
AUTHOR CONTRIBUTIONS
H.S., K.-I.M. and M.K. designed experiments and conducted data analysis. H.S. and H.H. performed histological experiments. H.S. and K.-I.M. performed molecular experiments. H.S. and D.G.Z. performed behavioral experiments. C.L.J., S.M.B., E.W. and K.W. provided Tfm rats, advice, antibodies and equipment. H.S., S.M.B. and M.K. wrote the paper. All authors discussed the results and commented on the manuscript.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Anastasi A, Erspamer V, Bucci M. Experientia. 1971;27:166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 2.Battey J, Wada E. Trends Neurosci. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- 3.Panula P, Nieminen O, Falkenberg M, Auvinen S. Ann NY Acad Sci. 1988;547:54–69. doi: 10.1111/j.1749-6632.1988.tb23875.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun YG, Chen ZF. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara K, Tominaga K, Isobe Y, Inouye ST. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladenheim EE, Taylor JE, Coy DH, Moore KA, Moran TH. Am J Physiol. 1996;271:R180–R184. doi: 10.1152/ajpregu.1996.271.1.R180. [DOI] [PubMed] [Google Scholar]
- 7.Shumyatsky GP, et al. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 8.Roesler R, et al. Eur J Neurosci. 2004;19:1041–1045. doi: 10.1111/j.0953-816x.2004.03175.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen RT, Battey JF, Spindel ER, Benya RV. Pharmacol Rev. 2007;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truitt WA, Coolen LM. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- 11.Carro-Juarez M, Rodriguez-Manzo G. Brain Res Brain Res Rev. (in the press) [Google Scholar]
- 12.Meston CM, Frohlich PF. Arch Gen Psychiatry. 2000;57:1012–1030. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- 13.Morgan C, Nadelhaft I, de Groat WC. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 14.Vizzard MA, Erdman SL, de Groat WC. J Neurosci. 1995;15:4033–4045. doi: 10.1523/JNEUROSCI.15-05-04033.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoli M, Agnati LF. Prog Neurobiol. 1996;49:363–380. doi: 10.1016/0301-0082(96)00020-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.