Abstract
Rhizobia and legumes are able to interact in a symbiotic way leading to the development of root nodules. Within nodules, rhizobia fix nitrogen for the benefit of the plant. These interactions are efficient because spectacularly high densities of nitrogen fixing rhizobia are maintained in the plant cells. DNF2, a Medicago truncatula gene has been described as required for nitrogen fixation, bacteroid’s persistence and to prevent defense-like reactions in the nodules. This manuscript shows that a Rhizobium mutant unable to differentiate is not sufficient to trigger defense-like reactions in this organ. Furthermore, we show that the requirement of DNF2 for effective symbiosis can be overcome by permissive growth conditions. The dnf2 knockout mutants grown in vitro on agarose or Phytagel as gelling agents are able to produce nodules fixing nitrogen with the same efficiency as the wild-type. However, when agarose medium is supplemented with the plant defense elicitor ulvan, the dnf2 mutant recovers the fix− phenotype. Together, our data show that plant growth conditions impact the gene requirement for symbiotic nitrogen fixation and suggest that they influence the symbiotic suppression of defense reactions in nodules.
Introduction
Legumes and rhizobia form symbiotic interactions leading to the development of a symbiotic organ (the nodule) in which bacteria fix atmospheric nitrogen for the benefit of the plant. Legume nodules are massively colonized by rhizobia and endosymbionts (bacteroids) accumulate into so called symbiotic cells. Despite this infection level, nodules do not display defense reactions suggesting that the innate immunity is suppressed in this organ in order to allow chronic infection by rhizobia [1]. This hypothesis is supported by the observation that when compared to the infected cells, uninfected nodule cells express a relatively high number of genes that can be associated with biotic stress or defense responses against pathogenic microbes [2]. Now that the transfer of symbiotic capacity to non-legume plants is considered as a feasible challenge [3], understanding how bacteroids are maintained at a high density in symbiotic cells during the rhizobium/legume symbiosis is crucial. Bacteroid maintenance defect is associated with two physiological processes; i) premature senescence of nodules that is frequently associated with nitrogen fixation defect and ii), defense-like reactions in the nodules. In the later situation, which is caused by either plant or bacterial mutants or inappropriate plant growth conditions for nodule development [4], it is unclear whether the defense-like reactions are the cause or the consequence of the symbiotic defect.
A key step in the comprehension of the plant tolerance to the massive and chronic rhizobia infection is the identification of plant genes involved in this process. Recently, a gene involved in bacteroid maintenance has been identified in Medicago truncatula (one of the favorite models used to study rhizobium/legume interactions) [5]. This gene, DNF2, encodes a phosphatidyl-inositol specific phospholipase C X domain containing protein [5]. The dnf2 mutants are competent for the initial steps of the symbiosis but deficient for nitrogen fixation (nod+ fix−) [5]. In mutant nodules the bacteria are released into the plant cells where they rapidly loose viability [5]. Nodules of dnf2 mutant display typical features of defense reactions such as phenolics accumulation [5], [6], and induction of a PR10 gene, accompanied with necrosis [4], [5]. Furthermore, bacteroids terminal differentiation triggered in M. truncatula by antimicrobial nodule cysteine rich (NCR) plant peptides [7] is altered in the dnf2 mutant [5]. It is not clear whether defense-like reactions in the dnf2 mutant are a cause or a consequence of bacteroid differentiation defect.
The relationship between plant defenses and rhizobium/legume symbiosis has been previously investigated. However, all the studies were focused on the early steps of the symbiotic process [4], [8], [9], [10], [11], [12], [13], [14], [15] or done in cell-culture systems [10]. The potential effect of plant growth condition on the symbiotic control of endosymbiont persistence and of plant defenses after the internalization step of the symbiosis is not well understood [16]. In contrast, in the plant/pathogen interactions, the effect of (biotic) environment is well known to modulate plant defenses [10], [17], [18]. Here we present data indicating that plant growth conditions determine the DNF2 requirement for chronic infection by rhizobia and nitrogen fixation and that no defense-like reactions are elicited in nodules of dnf2 mutant growing under permissive conditions.
Materials and Methods
Plant and Bacterial Cultures
Medicago truncatula ecotypes R108 [19] and A17 [20] and the derived mutants [5], [21], [22], [23] were grown in vitro on Buffered Nodulation Medium (BNM) [24] supplemented with 1 µM AVG [L-a-(2-aminoethoxyvinyl)-Gly] and solidified with gelling agents as indicated. Agar HP 696–7470 was from Kalys (Bernin, France; http://www.kalys.com/), Bacto Agar from Difco (Sparks, USA), Agarose GEPAGA0765 from Eurobio (Courtaboeuf, France; http://www.eurobio.fr) and Phytagel from Sigma Aldrich (http://www.sigmaaldrich.com/). Agar and bactoagar were used at 2%, Phytagel and agarose at 0.8%. Sinorhizobium meliloti strain Rm41 [25], strain SM1021 [26] and bacA [27] and strain Sinorhizobium medicae strain WSM419 [28] were cultivated in YEB medium [29], Pseudomonas fluorescens Q2-87 [30] in King’s B medium [31] and Bradyrhizobium sp. ORS285 [32] on YM medium [33]. All these bacteria were cultivated at 30°C with shaking. Escherichia coli K12 was cultivated in LB medium at 37°C with shaking.
Plant Inoculation
M. truncatula seeds were surface sterilized as previously described [5] and vernalized for at least 48 h at 4°C in the dark on water agarose (1%) plates. Seeds were then germinated by incubating them at 24°C for 36 h before being transferred to 12 cm square plates containing BNM. For single inoculation, overnight cultures of S. medicae were pelleted and washed in sterile water. OD600 nm was then adjusted to 0.1 in water by re-suspension. Eight seedlings per plate were together inoculated with 1 mL of S. medicae cell suspensions. For co-inoculation experiments, S. medicae strain WSM419 and either one of the following strain: E. coli K12 or Bradyrhizobium sp. ORS278 or P. fluorescens Q2–87 were pelleted separately, washed in sterile water, re-suspended in water and the OD600 nm were adjusted to 0.4 for WSM419 and 0.2 for other bacteria. Suspensions of S. medicae and one of the other strains were then mixed in a 1∶1 ratio (V/V) and the resulting suspension was used to inoculate seedlings as described above for single inoculation.
Microscopy
For all microscopy imaging presented in this study, nodules were prepared as described in [5], [7]. They were either embedded into technovit matrix or in agarose (6%). From technovit embedded material thin sections (7 µm) were prepared using a microtome and stained with 0.02% toluidine blue. Agarose sections were made using a vibratome. For β-glucuronidase activity detection, roots were vacuum-infiltrated with X-gluc and treated as described [34]. Sections were observed and photographed with a Leica DMI 6000B inverted microscope. Phenolic compounds were revealed as described previously [35]. Agarose sections were observed using a macroscope Nikon AZ10.
Acetylene Reduction Assays (ARA)
ARA were conducted on single plants using a protocol derived from [36]. Briefly, a single nodulated plant was placed into a 10 ml glass vial sealed with a rubber septum. 250 µl of acetylene were injected per vial. Plants were incubated for at least one hour at room temperature and gas samples (200 µl) were analyzed by gas chromatography using the 7820A Gas Chromatograph from Agilent Technologies (Santa Clara, USA) equipped with a flame ionization detector and a GS-Alumina column (50 m×0.53 mm) with hydrogen as carrier gas. Column temperature and gas flow rate were 120°C and 7.5 mL/min, respectively.
Molecular Biology
RNAs were extracted using the RNeasy Plant Mini Kit (Qiagen, http://www.qiagen.com/). For cDNA synthesis, RNA samples were treated with RNase-free DNAse I to remove traces of genomic DNA. Reverse transcription (RT) was then performed from 1 µg of total RNA using a poly-T primer and the First Strand cDNA Synthesis Kit from Fermentas (http://www.fermentas.de) and RT-qPCR was performed as described in [5]. MtACTIN2 was used as a reference gene. Primers are listed in Table S1.
Statistical Analysis
All statistical analyses were performed on R2.14.2 (R-Development-Core-Team 2012).
Results
Lack of Bacteroid Differentiation is not Sufficient to Trigger Defense-like Reactions in Nodules
In order to better characterize the dnf2 defense-like reactions phenotype, we examined the expression of defense related genes in dnf2 nodules by RT-qPCR. In addition to PR10 (TC94217/MTR_2g035150, [37]), expression of phenylalanineamonialyase (PAL, TC106667, [38]), chitinase (Medtr3g118390, [39]), non-race specific disease resistance 1 (NDR1, Mtr.40876.1.S1_at, TC108235) [40], and vesicle storage protein (VSP, TC93960, [38]) was evaluated. Out of the five defense markers evaluated, four were found to be induced more than two fold in the dnf2–4 nodules when compared to the wild-type (WT) nodules (Figure 1a). dnf2 develops defense–like reactions and displays a bacteroid differentiation defect. In order to determine if differentiation defect triggers defense-like reactions, phenolics accumulation and defense-related gene expression were examined in nodules induced by the S. meliloti bacA mutant (Figure 1). bacA mutant is hypersensitive to NCRs [41] and rapidly degenerate inside symbiotic cells [42]. As a consequence, the bacA mutant does not undergo terminal differentiation and does not fix nitrogen. In contrast to what is observed in dnf2–4 nodules (Figure 1c), WT nodules hosting bacA mutant did not accumulate phenolics (Figure 1d) similar to that of M. truncatula WT nodules induced by the WT bacteria (Figure 1b). Furthermore, defense related genes were not induced in the nodules induced by the bacA mutant (Figure 1a). These data indicate that lack of differentiation is not sufficient to elicit defense-like reactions in nodules.
Figure 1. Bacteroid differentiation defect is not sufficient to trigger defense-like reactions in dnf2 nodules.
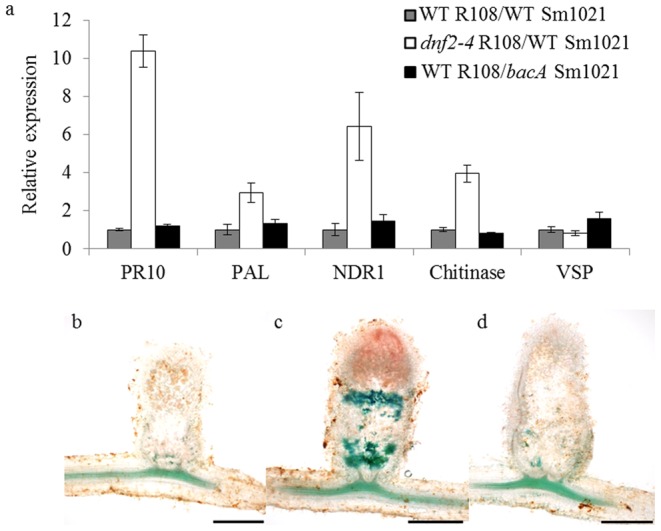
Panel a: Expression of defense markers was evaluated by RT-qPCR using cDNA prepared from 14 dpi nodules. The y axis represents fold induction/WT. Panel b: 25 dpi nodules of R108 WT induced by Sm1021 WT. Panel c: 25 dpi nodules of dnf2–4 induced by Sm1021 WT. Panel d: 25 dpi nodules of R108 WT induced by a SM1021 bacA derivative. Nodules in b, c and d were stained for phenolics using potassium permanganate toluidine blue (scale bars 500 µm).
Plant Growth Conditions Impact the dnf2 Phenotype
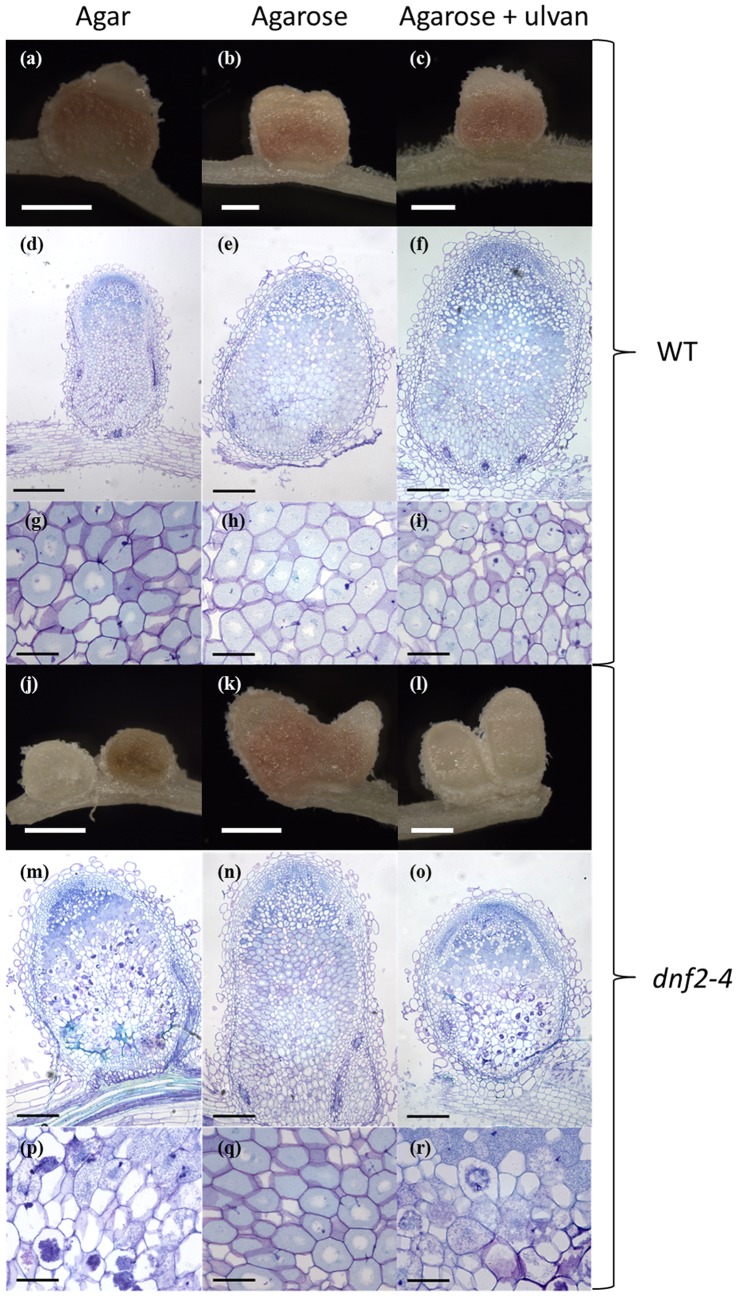
We evaluated whether the plant growth condition had an impact on the dnf2 mutant phenotype. For this we compared dnf2–4 and wild-type nodules of plants developed on media solidified with either agar or agarose. Both agar and agarose are solidifying agents extracted from algae but agarose has a higher purity level, does not contain agaropectins and harbors a reduced level of undefined trace elements. In contrast to white and necrotic nodules previously observed on agar-conditions [5], when agarose was used to solidify the medium, dnf2–4 plants produced a mixture of white and pink nodules (Figure 2k). The pink nodules were indistinguishable from nodules on WT plants (Figure 2b). Nodule occupancy and zonation were studied by imaging nodule sections of plants that were grown on different substrates. In contrast to the phenotype observed on plants cultivated on agar medium ([5]; Figure 2), on agarose-based medium nodule structures (Figure 2e,n) and infected cells (Figure 2h,q) were similar among WT and dnf2–4 plants. These observations suggested that the dnf2–4 mutant is a conditional mutant and the genetic requirement to prevent defense-like reactions, to accumulate leghemoglobin and to produce normally infected nodules can be modulated by the plant substrate.
Figure 2. Plant growth conditions impact the dnf2 phenotype.
Nodules were harvested 18 days post inoculation with Rm41. a-i, Wild-type nodules. j-r, dnf2–4 nodules. Left panels, middle panels and right panels represent nodules grown on agar-, agarose- and agarose-based BNM supplemented with 1% ulvan respectively. Panels a, b, c, j, k and l (scale bars 500 µm) illustrate whole nodules, panels d, e, f, m, n and o (scale bars 200 µm except for d 500 µm) are thin sections of whole nodules and panels g, h, i, p, q, r (scale bars 50 µm) are enlargement of the zone III of panels d, e, f, m, n and o, respectively.
Plant Growth Conditions Determine the DNF2 Requirement for Nitrogen Fixation
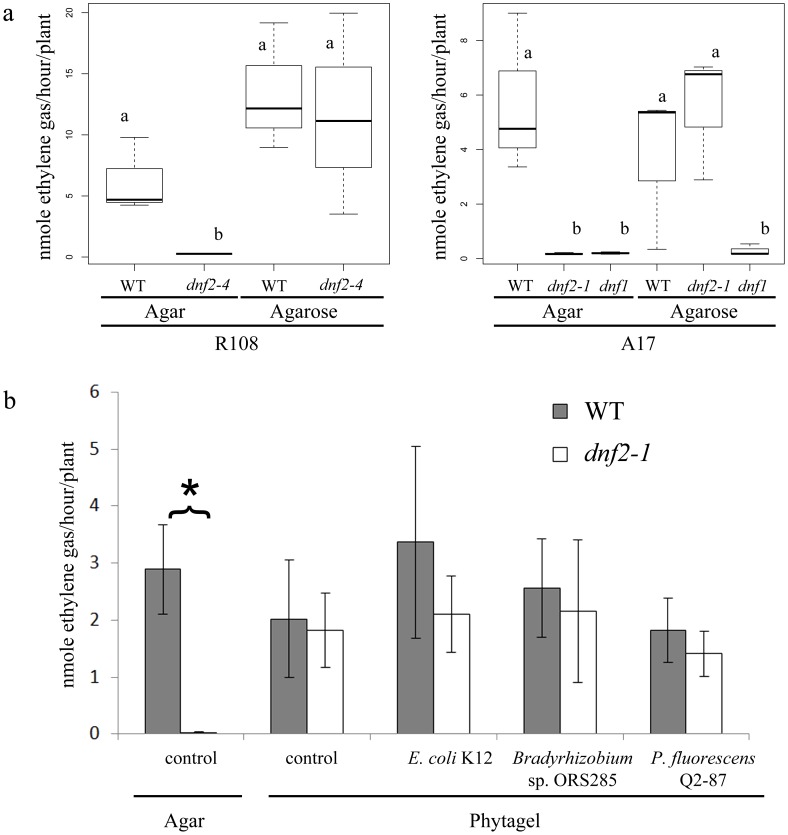
In order to determine if dnf2–4 plants producing pink pigmented nodules correspond to symbiotically efficient nodules, we performed acetylene reduction assay (ARA) on WT and dnf2–4 plants grown on agar- and on agarose-based media. The assays were performed using the S. medicae strain WSM419 which, in contrast to the RM41, is able to fix nitrogen in nodules of both A17 and R108 ecotypes of M. truncatula. The results showed that the agarose-based medium restored the capacity to reduce acetylene in the dnf2–4 mutant (Figure 3a). As the dnf2–4 allele is in the ecotype R108 background, we also evaluated the acetylene reduction activity in the M. truncatula A17 background with the dnf2-1 mutant derived from A17. This dnf2-1 line also recovered its nitrogen fixation capacity when grown on agarose based-medium (Figure 3a) demonstrating that the conditional phenotype of dnf2 is not restricted to dnf2 mutants of the R108 ecotype.
Figure 3. Plant growth conditions determine the DNF2 requirement for symbiosis.
a) Acetylene reduction assays were conducted on M. truncatula WT ecotype R108 and A17 and dnf2–4 (R108 genetic background), and dnf2-1, dnf1 mutants (A17 genetic background) inoculated with S. medicae strain WSM419. A Kruskal-Wallis one-way ANOVA test and a post-hoc Tukey’s test were performed and statistically identical values were attributed identical letters (n = 3). (b) Acetylene reduction assay was conducted on M. truncatula WT ecotype A17 and dnf2-1 mutant inoculated with strain WSM419 alone (control) or in combination with E. coli K12, Bradyrhizobium sp. ORS285 or P. fluorescens Q2–87. Plants were grown under in vitro conditions on BNM supplemented with either agar or agarose and analyzed at 14 dpi (a) or with Agar and Phytagel and analyzed at 25 dpi (b). A Mann-Whitney test was performed between WT and dnf2-1 mutant for each condition. The star indicates a significant difference (p-value <0.05) (n = 4) Error bars represent standard errors.
In order to determine if the conditional phenotype is a general feature of fix− mutants, the previously described A17 dnf1 fix− line [21], [23] was tested for its symbiotic capacity on agarose-based medium. dnf1 remained unable to reduce acetylene on agarose-based medium (Figure 3a) indicating that the observed conditional phenotype of dnf2 is not a general feature of fix− mutants. Additional analyses revealed that nitrogen fixation is also restored in dnf2 when plants are cultivated on medium solidified with Phytagel, another agar-substitute, extracted from bacteria (Figure 3b). It should be noted that the rescue of dnf2 nitrogen fixation varies from one experiment to the other and the presented data indicate that in some conditions, dnf2 symbiotic capacity are similar to the WT. Together, these results indicate that plant growth conditions, represented here by the different types of solidifying agents, can overcome the requirement of DNF2 for nitrogen fixation in R108 and A17 ecotypes.
dnf2 Functional Nodules Correctly Express Symbiotic Markers and do not Express Defense Genes
In order to determine if nitrogen fixation rescue is associated with the loss of defense-like reactions and with the recovery of normal nodulin expression in the dnf2 functional nodules, RT-qPCR analysis were performed. Expression of defense markers, PR10, PAL, chitinase, NDR1, VSP, and of Leghemoglobin and NCR001, NCR006, NCR094, NCR096, NCR109 and NCR121 were evaluated in dnf2 necrotic nodules developed on agar-based media and in dnf2 functional nodules developed on Phytagel-based media as well as in WT nodules grown in the same conditions. Defense markers were not induced as compared to the WT in dnf2 nodules developed under permissive condition (Figure S1a) and expression of the symbiotic markers was normal (Figure S1b,c). In contrast, in dnf2 nodules developed under restrictive conditions, defense markers were induced as compared to the WT whereas Leghemoglobin, as well as of all tested NCR genes expressions were drastically reduced (Figure S1) when compared to WT nodules. Together, these data suggest that WT phenotype is completely recovered in functional dnf2 pinkish nodules developed under permissive condition.
Plant Growth Effect on the dnf2–4 Phenotype is Transient
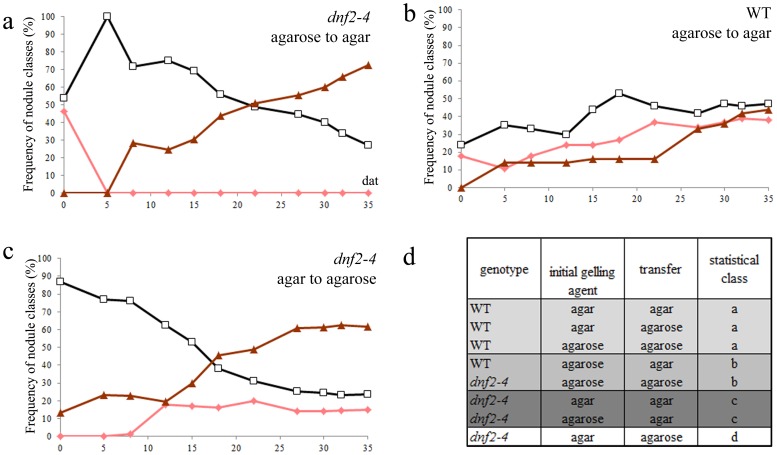
In order to determine if plant substrates provoke the irreversible inability to fix nitrogen in dnf2, S. medicae strain WSM419-inoculated WT and dnf2 plants were cultivated on agar- and agarose-based media and transferred once the first mature nodules were observed (14 dpi) on agar- or agarose-based medium. The frequencies of the different classes of nodules (white, pink, brown) were then monitored (Figure 4, Figure S2). The transfer from agarose to agar medium results in an increase in the formation of necrotic nodules irrespective of the genetic background (Figure 4a and b). WT plants transferred from agarose to agar based medium also triggered the formation of brownish nodules (Figure 4b). This suggests that the growth on agar medium is more stressful and that plants grown on agar need to adapt to this condition. This is not observed when WT plants were transferred to identical medium, indicating that plants are already adapted (Figure 4d). No pink nodules developed after the transfer of dnf2–4 plants to agar-based medium, regardless of the initial gelling agent (Figure 4a, Figure S2). The pink nodules initially formed by the dnf2–4 mutant on agarose based medium turned to white or brownish within the first five days after transferred to agar-based medium (Figure 4a). In contrast, the appearance of pink nodules only occurred when dnf2–4 plants were transferred onto agarose-based medium regardless of the initial growth medium (Figure 4c, Figure S2). These results show that the plant substrate effect on dnf2 is reversible and can trigger the conversion of fixing to non-fixing plants and vice-a-versa.
Figure 4. Influence of the plant growth conditions on dnf2 phenotype is transient.
(a–c) Frequencies of nodule classes after transfer to agar or agarose medium. M. truncatula dnf2–4 and WT plants (n = 24 for every conditions) inoculated with S. meliloti Rm41 were cultivated in vitro on BNM using agar or agarose as a gelling agent for 14 days and transfer to new medium with the same or different gelling agent. Pink nodules are represented by diamonds, white nodules by open squares and brownish nodules by triangles. The experiment was repeated three times with similar results. (d) analysis of the distribution of nodule classes at 35 days after transfer. Statistically identical distribution are attributed identical letters (Chi-Square Test of Homogeneity with Bonferroni correction, p-value = 2.2–16).
Plant Substrate Effect on the dnf2–4 Phenotype Acts at Distance
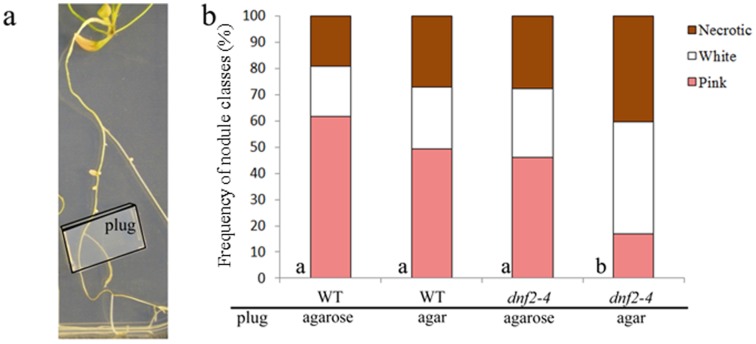
To determine if the agar-based medium can trigger the fix− phenotype at distance, WT and dnf2–4 plants were cultivated on both agar- or agarose-based BNM medium. 15 days post inoculation, agar- or agarose-based BNM medium plugs were placed onto root systems of the wild-type and dnf2–4 plants at a distance from the nodules (Figure 5a). The number and the color of the nodules were then monitored (Figure S3). At 35 days after placing the plugs, an agarose-based BNM plug did not stop the development of pink nodules in the dnf2–4 mutant grown on agarose-based medium (Figure 5b). In contrast, an agar-based BNM plug significantly modified the distribution with a decrease in the number of pink nodules and an increase of the brownish nodules for dnf2–4 plants grown on agarose-based medium (Figure 5b). 35 days after placing the agarose or agar plugs, the proportion of white, brown and pink nodules were similar in the WT plants grown on agarose. These results indicated that the plant growth condition effect on the dnf2–4 fix− phenotype results from a signal that can act distantly.
Figure 5. The plant substrate triggers dnf2 fix− phenotype at distance.
dnf2–4 and WT plants nodulated by S. meliloti Rm41 were grown on agarose-based BNM. Agar- or agarose-based plugs (1.5×1×0.5 cm) were laid onto root systems at 15 dpi and the color and numbers of nodules produced by 24 plants were monitored. The experimental set up is illustrated in (a). (b) Distribution of nodule classes 35 days after plug addition for WT and dnf2–4 grown on agarose based medium. The experiment was repeated three times with similar results. Statistically identical distribution are attributed identical letters (Chi-Square Test of Homogeneity with Bonferroni correction, p-value = 1.32e-06).
The dnf2 Fix− Phenotype is not Restored by Addition of Standard Defense Priming Agents
Based on the above results we hypothesize that component(s) present in the agar but absent in the agarose might trigger or prime defense reactions in the dnf2–4 nodules. In order to determine if the addition of molecules known to trigger defense reactions can restore the dnf2 fix− phenotype on permissive conditions we supplemented the medium with plant defense elicitors or phytohormones involved in defense reactions. The pattern-triggered immunity (PTI) activator, Flg22, and the systemic acquired resistance messengers, salicylic and jasmonic acids (SA and JA, respectively) did not restore the fix− phenotype of dnf2–4 on agarose-based medium (Table S2). In addition to these two molecules, we aimed at creating a more complex environment potentially priming or triggering defenses in dnf2 nodules. To do this, the presence of yeast extract or simple artificial microflora during the symbiotic interaction was also evaluated. To test the effect of simple microflora, co-inoculations of S. medicae WSM419 with the enteric bacteria E. coli K12, the rhizobium Bradyrhizobium sp. ORS285 [32] and the induced systemic resistance (ISR) inducing bacterium Pseudomonas fluorescens Q2–87 [30] were performed. None of these strains restored the dnf2 fix− phenotype as determined by nodule color or ARA measurements at 25 dpi (Figure 3b, Table S2) in the agarose grown dnf2–4 plants. Similar results were observed when the medium was supplemented with yeast extract that contain the PTI activator chitin (Table S2).
The dnf2 Fix− Phenotype is Triggered by the Complex Defense Priming Agent Ulvan
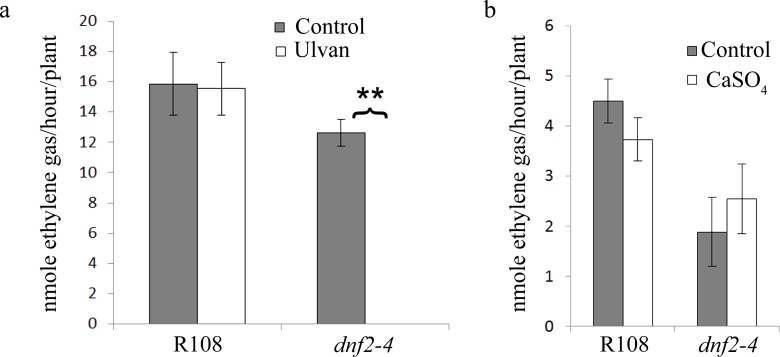
Ulvan is a complex polysaccharide extracted from algae known to prime plant defenses in Arabidopsis and M. truncatula [43], [44]. We tested its capacity to restore the fix− phenotype of dnf2 on agarose grown plants. Addition of 1% ulvan in the agarose-based medium did not trigger any detectable change in the symbiotic capacity of the WT plants based on nodule histology and acetylene reduction capacity (Figure 2c,f,i; Figure 6a; Figure S4). In contrast, 1% ulvan restored the dnf2 phenotype (fix− nodules) in the dnf2–4 mutant grown on agarose-based medium (Figure 2l,o,r; Table S2). Further, using ARA assay, we showed that addition of ulvan in the agarose-based medium triggered dnf2 fix− phenotype in dnf2–4 mutant grown on permissive condition (Figure 6a).
Figure 6. Ulvan abolishes dnf2 nitrogen fixation on permissive condition.
M. truncatula WT R108 and dnf2–4 plants were cultivated on agarose BNM supplemented or not with 1% ulvan. Acetylene reduction assays were conducted on plants 27 dpi with S. medicae WSM419 (n = 8) (a). M. truncatula WT R108 and dnf2–4 plants were cultivated on Phytagel BNM supplemented or not with 10 mM CaSO4. Acetylene reduction assays were conducted on plants 14 dpi with S. medicae WSM419 (n = 5) (b). A Mann-Whitney test was performed between WT and dnf2-1 mutant for each condition. Stars indicate significant differences (** p-value <1e-03) Error bars represent standard errors.
In order to determine if DNF2 expression is regulated by the plant growth conditions, we analyzed DNF2 expression in the nodules developed on R108 plants cultivated on permissive agarose- and Phytagel-based BNM or restrictive agar-based BNM and agarose-based BNM supplemented with ulvan using RT-qPCR analysis. No significant differences were observed in the abundance of DNF2 transcript between the tested conditions (Figure S5a). To determine if DNF2 expression pattern is modified by the permissive/restrictive conditions, R108 transgenic plants expressing the β-glucuronidase under the promoter of DNF2 were used [5]. Nodules from plants cultivated on agarose- and agar–based medium were analyzed. DNF2 expression was essentially detected in the infection zone in both conditions (Figure S5b,c).
In contrary to agarose and Phytagel, a high content of sulfate is a striking feature common to agar and ulvan. This difference in the sulfate content [45] or the presence of sulfate ions in the medium could be responsible for a different response of the mutant to the different compounds. To test this possibility, a Phytagel based medium was supplemented with 10 mM CaSO4 (a concentration similar to that found in ulvan [46]). However, the dnf2 mutant did not recover its fix− phenotype in presence of 10 mM CaSO4 (Figure 6b), indicating that high sulfate concentration alone is not enough to trigger the dnf2 fix− phenotype. Altogether these results suggest that DNF2 expression is not significantly modified by plant growth conditions and that the fix− phenotype of the dnf2 mutant is triggered by ulvan treatment through defense priming.
Discussion
M. truncatula dnf2 mutant lines were isolated during independent genetic screens as fix− mutants [5], [21], [22], [23], [47]. In the dnf2 alleles, the nodule organogenesis is not altered and the symbiotic process is blocked only after bacterial release from the infection thread [5]. Defense-like reactions in the nodules and defect in bacteroid differentiation were reported in these mutants [5], [6]. It remained unclear whether differentiation defect is a cause or a consequence of defense-like reactions. Here we report that the bacA mutant does not trigger defense-like reactions (Figure 1). Furthermore, we show that dnf2 KO mutants can form a functional symbiosis when grown in vitro on agarose- and Phytagel-based media (Figure 3a,b). We show that this trait is not a general feature of the fix− mutants as the dnf1 line remains unable to reduce nitrogen under dnf2 permissive conditions (Figure 3a).
Our study further shows that addition of ulvan converts the condition from permissive to restrictive for the dnf2–4 symbiotic phenotype on agarose and Phytagel-based media. Ulvan is a complex carbohydrate polymer extracted from the green algae Ulva spp. [46]. In M. truncatula, it induces the expression of a PR10 gene as well as a broad range of defense-related genes, notably genes involved in phytoalexin biosynthesis and cell wall proteins [43]. Interestingly, we found that the PR10 gene was expressed at high level in M. truncatula roots grown on agar-based BNM medium and this expression was strongly reduced in wild-type nodules while in nodules of the dnf2–4 mutant, the PR10 gene was significantly expressed [5]. Ulvan activates defense priming through the JA pathway on both M. truncatula and Arabidopsis thaliana [44] and induces resistance against the pathogenic fungus Colletotrichum trifolii [43]. In wheat and barley ulvan also acts as a priming agent allowing resistance against Blumeria graminis [48]. The ulvan effect observed here on dnf2 phenotype thus confirms the role of DNF2 in symbiotic repression of plant defenses at the stage of the bacterial internalization.
Our study also shows that agar triggers transiently and at distance the inability of the dnf2 mutants to reduce nitrogen (Figure 4,5). The compound(s) in agar, responsible for the dnf2 fix− phenotype, can act at a distance and only transiently (Figure 4,5). This suggests that in planta the signal triggering the fix− phenotype (including defense-like reactions) is mobile or alternatively the agar mobile element could be a small molecule released into the medium or the plant atmosphere. It also suggests that DNF2 prevents the fix− phenotype triggered by a mobile signal. This observation is reminiscent of defense priming.
The way how plants perceive microbial invaders and the signaling cascades controlling the activation of defense reactions are now relatively well characterized in Arabidopsis [49], [50]. It is known that microbial associated molecular patterns (MAMPs) such as Flg22, the flagellin active epitope, are able to trigger defense reactions on a wide variety of plants [51]. Hormone signaling enhancing resistance to a variety of pathogens has also been described and JA and SA act in these processes [18] referred to as induced systemic resistance (ISR) and systemic acquired resistance (SAR), respectively. However, we found that neither Flg22, SA, JA nor ISR were able to switch the condition from permissive to restrictive for dnf2. Considering the ulvan effect and its presumed mode of action through the JA pathway, it is unexpected that JA addition does not restore the dnf2 fix− phenotype under permissive conditions. This could reflect difference(s) in the regulatory network in M. truncatula in which, in contrast to Arabidopsis, knowledge on signaling cascades leading to plant defenses remains poorly understood. Genetic tools will help elucidate these potential differences.
Determining the biochemical function of DNF2 will be a key step in the comprehension of the mechanisms allowing plant tolerance to rhizobia. DNF2 displays similarity with phosphatidyl-inositol phospholipase C (PI-PLC) X-domain [5]. In addition to the X-domain, experimentally described plant PI-PLCs harbor a calcium binding domain and a so called Y-domain containing residues of the catalytic site [52]. The DNF2 atypical structure makes difficult to predict its biochemical function despite that bacterial PI-PLCs containing only the X-domain were shown to be functional and to be able to cleave phosphatidyl-inositol and/or GPI anchors [53]. A recent study suggests that human PI-PLCXD containing protein also displays phospholipase activity while laking the Y and Ca2+ binding domains [54], leaving open the possibility that PI-PLCXD containing proteins, amongst which is DNF2, play a role in phospholipid cleavage. Without any demonstrated biochemical activity, for now, it is only possible to speculate why DNF2 is unnecessary under permissive condition. It seems resonnable to speculate that the DNF2 substrate or ligand is absent in the cells under permissive conditions and that this substrate or free ligand is responsible for the plant defense activation. Irrespective of the DNF2 biochemical function, homologues of this protein are present in all plant species (including Arabidopsis and major crop species) [5] suggesting a more general role in plants than its action during symbiosis. The potential involvement of these proteins in the tolerance to endophytic microbia remains to be investigated. In this respect, the identification of several indigenous rhizobia in Arabidopsis root microbiota should make it possible to investigate this proposed function [55], [56].
From the work presented here, using artificial in vitro growth conditions, we evidenced a mechanism that allows the development of a functional nodule in the dnf2 mutant. We propose a hypothetical model for the action of DNF2 in the control of bacteroids persistence in the symbiotic organ. In this hypothetical model presented in Figure S6, we propose that the DNF2 restrictive conditions prime defense reactions in the plant but this elicitation is counteracted by the action of DNF2. This would explain the reason why when plants are cultivated under these conditions, the rhizobial infection triggers defense-like reactions in the dnf2 mutant but not in the WT. In this hypothetical model, under priming conditions, in absence of DNF2 the rhizobial infection results in defense elicitations and death of the rhizobia. In growth conditions which do not prime the defense reactions (i.e. permissive conditions), the effect of the rhizobial infection alone is not able to reach the threshold that results in defense reactions and the symbiotic interaction can take place. Determining the DNF2 biological function will suggest validity of this model.
Supporting Information
dnf2–4 pink nodules correctly express symbiotic markers and do not express defense genes. Expression of defense (panel a) marker and of symbiotic markers (panels b and c) were evaluated by qRT-PCR in dnf2–4 nodules (21 dpi) induced by S. medicae strain WSM419. Data were normalized with MtACTIN expression and reported to the expression in WT nodules developed in the same conditions.
(TIF)
Plant growth conditions effect on dnf2 plants is reversible. (a–e) Frequencies of nodule classes after transfer to agar or agarose media. M. truncatula dnf2–4 and WT plants (n = 24 for every conditions) inoculated with S. meliloti Rm41 were cultivated in vitro on BNM using either agar or agarose as a gelling agent for 14 days and transfer to new medium with the same or a different gelling agent. Pink nodules are represented by diamonds, white nodules by open squares and brownish nodules by triangles. The experiment has been repeated three times with similar results. (f) % of nodule classes at 35 days after transfer.
(TIF)
Plant substrates effect on dnf2 can act at distance. M. truncatula R108 and dnf2–4 plants (n = 24 plants for every condition) nodulated with S. meliloti Rm41 were grown on agarose based BNM. Agar- or agarose-based BNM plugs (1.5×1×0.5 cm) were laid onto root systems of the plants 15 dpi. The y-axis represents the % of nodule classes. Abscises represent days after addition of the plug. The experiment has been repeated three times with similar results.
(TIF)
Gelling agents do not alter WT nitrogen fixation capacity. Acetylene reduction assays were conducted on M. truncatula WT R108 plants cultivated on BNM solidified with the indicated gelling agents, 21 dpi with S. medicae WSM419. A Kruskal-Wallis one-way ANOVA test did not show significant differences between conditions (p-value = 0.3349).
(TIF)
DNF2 expression is not controlled by DNF2 requirement conditions. DNF2 expression level and expression pattern were investigated in nodules of M. truncatula WT R108 (A) plants or transgenic WT pDNF2::Gus (B,C). The plants were cultivated on BNM solidified with the indicated agent and DNF2 expression level was followed by RT-qPCR using Actin as a reference (A). Results are expressed as ratio versus expression on Phytagel based BNM. Error bars represent the standard error on three biological repetitions. Transgenic plants expressing the reporter construct were cultivated on Agar- (B) and agarose-BNM (C), scales bars represent 100 µm.
(TIF)
Hypothetical model for the effect of growth conditions on DNF2 requirement for symbiosis. In M. truncatula WT and dnf2 nodules from plants cultivated on non-defense priming environments (agarose- and Phytagel-based media), the defense elicitation does not reach the threshold for defense reactions and the symbiosis is efficient (central part of the figure). When plants are cultivated on defense priming environments (agar- and ulvan supplemented agarose-media) elicitation reaches the threshold for defenses (left and right part of the figure) but, in the WT nodules, DNF2 (represented by a green box) prevents defense reactions to a large extent. In contrast, the dnf2 mutant nodules develop defense reactions.
(TIF)
List of primers used during this study.
(TIF)
Ulvan triggers the DNF2 requirement for symbiosis.
(TIF)
Acknowledgments
We are grateful to Dr. Djamel Gully (Laboratoire des Symbioses Tropicales et Méditerranéennes, IRD, Montpellier, France) for help with acetylene reduction assays and to Dr. Peter Kalo (Agricultural Biotechnology Center, Gödöllö, Hungary) for WSM419 strain and to Sylvie Salamagne (Biotech Marine, France) for providing concentrated ulvan extract. The authors thank Dr. Anne Francez-Charlot, Andreas Kaczmarczyk (Institute of Microbiology, ETH, Zurich, Switzerland), Dr. Peter Mergaert, Dr. Benoit Alunni (Institut des sciences du vegetal, Gif sur Yvette, France), Dr. Sylvain Raffaele (Laboratory of Plant-Microbe Interactions, Castanet Tolosan, France), Pr. Kiran S. Mysore (Noble Foundation, USA) and Dr. Bénédicte Lebouteiller for critical comments and edition of the manuscript.
Funding Statement
This work has benefited from a French State grant (reference ANR-10-LABX-0040-SPS) managed by the French National Research Agency under an Investments for the Future program (reference n°ANR-11-IDEX-0003-02). This work was supported by the Centre National de la Recherche Scientifique (CNRS) and the grant Agence Nationale de la Recherche (ANR) Blanc International SVSE 6.2010.1 (LEGUMICS) to PR. MB was supported by a PhD fellowship from the French Ministry of Research. This work has benefited from the facilities and expertise of the Imagif Cell Biology Unit of the Gif campus (www.imagif.cnrs.fr) which is supported by the Conseil Général de l’Essonne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, et al. (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165: 683–701. [DOI] [PubMed] [Google Scholar]
- 2. Limpens E, Moling S, Hooiveld G, Pereira PA, Bisseling T, et al. (2013) Cell- and tissue-specific transcriptome analyses of Medicago truncatula root nodules. PLoS One 8: e64377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charpentier M, Oldroyd G (2010) How close are we to nitrogen-fixing cereals? Curr Opin Plant Biol 13: 556–564. [DOI] [PubMed] [Google Scholar]
- 4.Bourcy M, Berrabah F, Ratet P, Gourion B (2013) Failure of self-control: Defense-like reactions during legume/rhizobia symbiosis. Plant Signal Behav 8. [DOI] [PMC free article] [PubMed]
- 5. Bourcy M, Brocard L, Pislariu CI, Cosson V, Mergaert P, et al. (2013) Medicago truncatula DNF2 is a PI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol 197: 1250–1261. [DOI] [PubMed] [Google Scholar]
- 6. Pislariu CI, Dickstein R (2007) An IRE-like AGC kinase gene, MtIRE, has unique expression in the invasion zone of developing root nodules in Medicago truncatula . Plant Physiol 144: 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, et al. (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327: 1122–1126. [DOI] [PubMed] [Google Scholar]
- 8.Gough C, Jacquet C (2013) Nod factor perception protein carries weight in biotic interactions. Trends Plant Sci. [DOI] [PubMed]
- 9. Rey T, Nars A, Bonhomme M, Bottin A, Huguet S, et al. (2013) NFP, a LysM protein controlling Nod factor perception, also intervenes in Medicago truncatula resistance to pathogens. New Phytol 198: 875–886. [DOI] [PubMed] [Google Scholar]
- 10. Zamioudis C, Pieterse CM (2012) Modulation of host immunity by beneficial microbes. Mol Plant Microbe Interact 25: 139–150. [DOI] [PubMed] [Google Scholar]
- 11. Peleg-Grossman S, Golani Y, Kaye Y, Melamed-Book N, Levine A (2009) NPR1 protein regulates pathogenic and symbiotic interactions between Rhizobium and legumes and non-legumes. PLoS One 4: e8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peleg-Grossman S, Melamed-Book N, Levine A (2012) ROS production during symbiotic infection suppresses pathogenesis-related gene expression. Plant Signal Behav 7: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Gomez M, Sandal N, Stougaard J, Boller T (2012) Interplay of flg22-induced defence responses and nodulation in Lotus japonicus . J Exp Bot 63: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stacey G, McAlvin CB, Kim SY, Olivares J, Soto MJ (2006) Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula . Plant Physiol 141: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mithöfer A (2002) Suppression of plant defence in rhizobia-legume symbiosis. Trends Plant Sci 7: 440–444. [DOI] [PubMed] [Google Scholar]
- 16. Reguera M, Bonilla I, Bolanos L (2010) Boron deficiency results in induction of pathogenesis-related proteins from the PR-10 family during the legume-rhizobia interaction. Journal of Plant Physiology 167: 625–632. [DOI] [PubMed] [Google Scholar]
- 17. Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17: 478–486. [DOI] [PubMed] [Google Scholar]
- 18. Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64: 839–863. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann B, Trinh TH, Leung J, Kondorosi A, Kondorosi E (1997) A new Medicago truncatula line with superior in vitro regeneration, transformation, and symbiotic properties isolated through cell culture selection. Mol Plant Microbe Interact 10: 307–315. [Google Scholar]
- 20. Young ND, Debelle F, Oldroyd GE, Geurts R, Cannon SB, et al. (2011) The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitra RM, Long SR (2004) Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol 134: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pislariu CI, Murray JD, Wen J, Cosson V, Muni RR, et al. (2012) A Medicago truncatula tobacco retrotransposon insertion mutant collection with defects in nodule development and symbiotic nitrogen fixation. Plant Physiol 159: 1686–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Starker CG, Parra-Colmenares AL, Smith L, Mitra RM, Long SR (2006) Nitrogen fixation mutants of Medicago truncatula fail to support plant and bacterial symbiotic gene expression. Plant Physiol 140: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrhardt DW, Atkinson EM, Long SR (1992) Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256: 998–1000. [DOI] [PubMed] [Google Scholar]
- 25. Kondorosi E, Banfalvi Z, Kondorosi A (1984) Physical and genetic analysis of a symbiotic region of Rhizobium meliloti: Identification of nodulation genes. Mol Gen Genet 193: 445–452. [Google Scholar]
- 26. Galibert F, Finan TM, Long SR, Puhler A, Abola P, et al. (2001) The composite genome of the legume symbiont Sinorhizobium meliloti . Science 293: 668–672. [DOI] [PubMed] [Google Scholar]
- 27. Ferguson GP, Roop RM 2nd, Walker GC (2002) Deficiency of a Sinorhizobium meliloti bacA mutant in alfalfa symbiosis correlates with alteration of the cell envelope. Journal of Bacteriology 184: 5625–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howieson J, Ewing M (1986) Acid tolerance in the Rhizobium meliloti-Medicago symbiosis. Australian Journal of Agricultural Research 37: 55–64. [Google Scholar]
- 29. Krall L, Wiedemann U, Unsin G, Weiss S, Domke N, et al. (2002) Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens . Proc Natl Acad Sci U S A 99: 11405–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller DM, Mavrodi DV, van Pelt JA, Pieterse CMJ, van Loon LC, et al. (2012) Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens . Phytopathology 102: 403–412. [DOI] [PubMed] [Google Scholar]
- 31. King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307. [PubMed] [Google Scholar]
- 32. Molouba F, Lorquin J, Willems A, Hoste B, Giraud E, et al. (1999) Photosynthetic bradyrhizobia from Aeschynomene spp. are specific to stem-nodulated species and form a separate 16S ribosomal DNA restriction fragment length polymorphism group. Appl Environ Microbiol 65: 3084–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giraud E, Hannibal L, Fardoux J, Vermeglio A, Dreyfus B (2000) Effect of Bradyrhizobium photosynthesis on stem nodulation of Aeschynomene sensitiva . Proc Natl Acad Sci U S A 97: 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Welchen E, Gonzalez DH (2005) Differential expression of the Arabidopsis cytochrome c genes Cytc-1 and Cytc-2. Evidence for the involvement of TCP-domain protein-binding elements in anther- and meristem-specific expression of the Cytc-1 gene. Plant Physiol 139: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vasse J, de Billy F, Truchet G (1993) Abortion of infection during the Rhizobium meliloti–alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. The Plant Journal 4: 555–566. [Google Scholar]
- 36. Koch B, Evans HJ (1966) Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol 41: 1748–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samac DA, Penuela S, Schnurr JA, Hunt EN, Foster-Hartnett D, et al. (2011) Expression of coordinately regulated defence response genes and analysis of their role in disease resistance in Medicago truncatula . Mol Plant Pathol 12: 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao LL, Anderson JP, Klingler JP, Nair RM, Edwards OR, et al. (2007) Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula . Mol Plant Microbe Interact 20: 82–93. [DOI] [PubMed] [Google Scholar]
- 39. Nars A, Rey T, Lafitte C, Vergnes S, Amatya S, et al. (2013) An experimental system to study responses of Medicago truncatula roots to chitin oligomers of high degree of polymerization and other microbial elicitors. Plant Cell Rep 32: 489–502. [DOI] [PubMed] [Google Scholar]
- 40. Century KS, Shapiro AD, Repetti PP, Dahlbeck D, Holub E, et al. (1997) NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278: 1963–1965. [DOI] [PubMed] [Google Scholar]
- 41. Haag AF, Baloban M, Sani M, Kerscher B, Pierre O, et al. (2011) Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biology 9: e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glazebrook J, Ichige A, Walker GC (1993) A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes & Development 7: 1485–1497. [DOI] [PubMed] [Google Scholar]
- 43. Cluzet S, Torregrosa C, Jacquet C, Lafitte C, Fournier J, et al. (2004) Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp . Plant Cell Environ 27: 917–928. [Google Scholar]
- 44. Jaulneau V, Lafitte C, Jacquet C, Fournier S, Salamagne S, et al. (2010) Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J Biomed Biotechnol 2010: 525291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Menard R, Alban S, de Ruffray P, Jamois F, Franz G, et al. (2004) Beta-1,3 glucan sulfate, but not beta-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 16: 3020–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lahaye M, Robic A (2007) Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 8: 1765–1774. [DOI] [PubMed] [Google Scholar]
- 47. Tadege M, Wen J, He J, Tu H, Kwak Y, et al. (2008) Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula . Plant J 54: 335–347. [DOI] [PubMed] [Google Scholar]
- 48. Paulert R, Ebbinghaus D, Urlass C, Moerschbacher BM (2010) Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathology 59: 634–642. [Google Scholar]
- 49. Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20: 10–16. [DOI] [PubMed] [Google Scholar]
- 50. Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 51. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276. [DOI] [PubMed] [Google Scholar]
- 52. Rupwate SD, Rajasekharan R (2012) Plant phosphoinositide-specific phospholipase C: an insight. Plant Signal Behav 7: 1281–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heinz DW, Essen LO, Williams RL (1998) Structural and mechanistic comparison of prokaryotic and eukaryotic phosphoinositide-specific phospholipases C. J Mol Biol. 275: 635–650. [DOI] [PubMed] [Google Scholar]
- 54. Gellatly SA, Kalujnaia S, Cramb G (2012) Cloning, tissue distribution and sub-cellular localisation of phospholipase C X-domain containing protein (PLCXD) isoforms. Biochem Biophys Res Commun 424: 651–656. [DOI] [PubMed] [Google Scholar]
- 55. Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, et al. (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488: 91–95. [DOI] [PubMed] [Google Scholar]
- 56. Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, et al. (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
dnf2–4 pink nodules correctly express symbiotic markers and do not express defense genes. Expression of defense (panel a) marker and of symbiotic markers (panels b and c) were evaluated by qRT-PCR in dnf2–4 nodules (21 dpi) induced by S. medicae strain WSM419. Data were normalized with MtACTIN expression and reported to the expression in WT nodules developed in the same conditions.
(TIF)
Plant growth conditions effect on dnf2 plants is reversible. (a–e) Frequencies of nodule classes after transfer to agar or agarose media. M. truncatula dnf2–4 and WT plants (n = 24 for every conditions) inoculated with S. meliloti Rm41 were cultivated in vitro on BNM using either agar or agarose as a gelling agent for 14 days and transfer to new medium with the same or a different gelling agent. Pink nodules are represented by diamonds, white nodules by open squares and brownish nodules by triangles. The experiment has been repeated three times with similar results. (f) % of nodule classes at 35 days after transfer.
(TIF)
Plant substrates effect on dnf2 can act at distance. M. truncatula R108 and dnf2–4 plants (n = 24 plants for every condition) nodulated with S. meliloti Rm41 were grown on agarose based BNM. Agar- or agarose-based BNM plugs (1.5×1×0.5 cm) were laid onto root systems of the plants 15 dpi. The y-axis represents the % of nodule classes. Abscises represent days after addition of the plug. The experiment has been repeated three times with similar results.
(TIF)
Gelling agents do not alter WT nitrogen fixation capacity. Acetylene reduction assays were conducted on M. truncatula WT R108 plants cultivated on BNM solidified with the indicated gelling agents, 21 dpi with S. medicae WSM419. A Kruskal-Wallis one-way ANOVA test did not show significant differences between conditions (p-value = 0.3349).
(TIF)
DNF2 expression is not controlled by DNF2 requirement conditions. DNF2 expression level and expression pattern were investigated in nodules of M. truncatula WT R108 (A) plants or transgenic WT pDNF2::Gus (B,C). The plants were cultivated on BNM solidified with the indicated agent and DNF2 expression level was followed by RT-qPCR using Actin as a reference (A). Results are expressed as ratio versus expression on Phytagel based BNM. Error bars represent the standard error on three biological repetitions. Transgenic plants expressing the reporter construct were cultivated on Agar- (B) and agarose-BNM (C), scales bars represent 100 µm.
(TIF)
Hypothetical model for the effect of growth conditions on DNF2 requirement for symbiosis. In M. truncatula WT and dnf2 nodules from plants cultivated on non-defense priming environments (agarose- and Phytagel-based media), the defense elicitation does not reach the threshold for defense reactions and the symbiosis is efficient (central part of the figure). When plants are cultivated on defense priming environments (agar- and ulvan supplemented agarose-media) elicitation reaches the threshold for defenses (left and right part of the figure) but, in the WT nodules, DNF2 (represented by a green box) prevents defense reactions to a large extent. In contrast, the dnf2 mutant nodules develop defense reactions.
(TIF)
List of primers used during this study.
(TIF)
Ulvan triggers the DNF2 requirement for symbiosis.
(TIF)