Accumulating evidence suggests autophagy, the principle catabolic process for lysosomal degradation of surplus macromolecules (Roy and Debnath 2010) is fundamental to tumourigenesis. Impaired autophagy results in accumulation of cellular breakdown products, increased oxidative stress and neoplastic transformation (Mathew et al. 2009); whilst efficient autophagy facilitates metastatic tumour survival through sustained metabolic activity (Roy and Debnath 2010). Consequently, the currently accepted view is that autophagy suppresses growth early in tumour development but promotes tumour survival at later stages (Garber 2011).
p62 (also known as sequestosome-1/SQSTM1) is a multidomain adaptor protein transporting ubiquitinated proteins during autophagy (Moscat and Diaz-Meco 2009); as such, p62 plays a role in selective autophagic degradation of a number of substrates (Komatsu and Ichimura 2010; Ichimura and Komatsu 2010). Normally, p62 is broken down along with its cargo within the autophagolysosome. However, impaired autophagy is accompanied by p62 accumulation, resulting in large p62/ubiquitinated protein aggregates (Komatsu and Ichimura 2010); a process thought to be a key factor in tumourigenesis (Moscat and Diaz-Meco 2009).
The present biomarker discovery study aimed to define immunohistochemical p62 expression as a prognostic biomarker in a retrospective cohort comprising 29 melanocytic naevi and 121 primary cutaneous melanomas (Supplementary Methods and Table S1). In keeping with the “autophagy paradox”, we hypothesised p62 levels would be elevated in melanoma compared to naevi, with the highest levels detected in early stage disease where conditions represent a pro-tumourgenic environment.
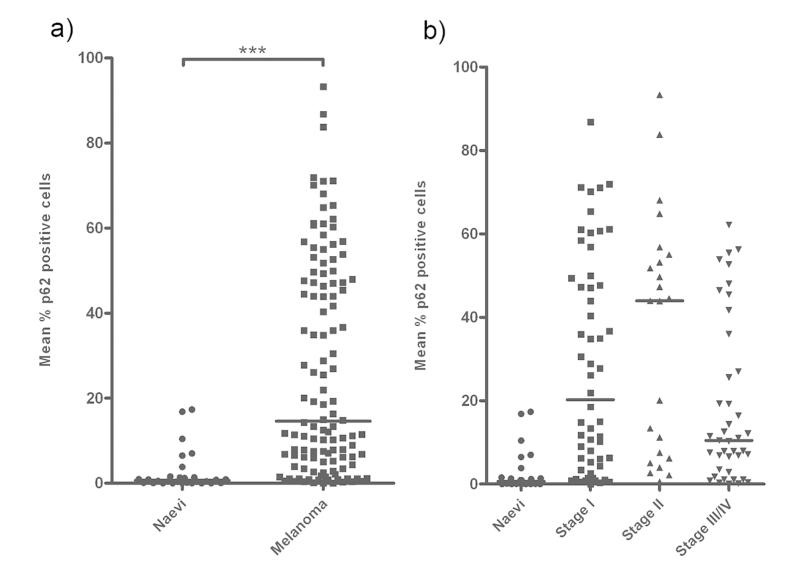
Comparison of median p62 expression levels between naevi and all melanomas revealed a significant increase in expression levels in melanoma (14.82%) compared to naevi (0.51%; Mann-Whitney U, P <0.0001) (Fig 1a), and a step-wise increase in median p62 expression levels with localised tumour development (0.51% in naevi to 44.5% in AJCC II disease, Mann-Whitney P < 0.0001) (Fig 1b). A relative decrease in median p62 expression (to 10.25%) was then observed in primary tumours from patients with metastatic stage III/IV disease (Stage II vs Stage III/IV Mann-Whitney P = 0.005) (Fig 1b). This biphasic expression in primary melanomas thus mirrors the “autophagy paradox”.
Figure 1. p62 expression in melanoma is consistent with the ‘autophagy paradox’ in cancer.
(a) Median p62 expression levels were significantly higher in melanoma compared to benign melanocytic naevi (Mann-Whitney U, P <0.0001). Horizontal lines represent median expression levels. (b) Median p62 expression levels increased between benign naevi and localised melanoma (eventual AJCC stages I and II), but revealed a relative fall in metastatic disease (eventual AJCC stages III and IV) (Kruskal-Wallis P <0.0001). Horizontal lines represent median expression levels.
To determine the efficacy of p62 expression levels at identifying high risk tumours at diagnosis, comparison of localised and metastatic disease (eventual AJCC stages I/II versus stages III/IV) revealed significantly lower median p62 expression in the metastatic cohort (10.25% versus 26.94% in AJCC I/II; Mann-Whitney, P = 0.016). p62 levels were visibly bimodal, with a Wilcoxon signed-rank test confirming that 20% expression was an appropriate cut-point for undertaking survival curve analysis, and as such statistical modelling was based on tumour p62 expression above (“high p62”) or below 20% (“low p62”).
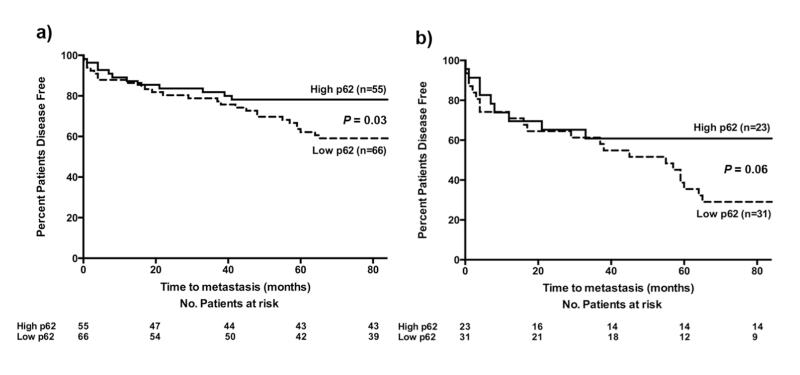
Univariate analysis in all tumours was used to assess disease outcome over a 7-year follow up period and revealed a modest, yet statistically significant, reduction in disease free survival (DFS) in patients with “low p62” tumours (40.9% of patients in the “low p62” group developed metastases compared to 21.8% in “high p62” tumours (Log-Rank (Mantel-Cox) Test P = 0.03, HR 1.66 (95% CI 1.03 – 2.69) (Fig 2a).
Figure 2. p62 as a potential prognostic biomarker in melanoma.
(a) Univariate analysis of p62 expression revealed a significantly increased risk of metastasis in tumours expressing <20% p62 compared to tumours expressing >20% p62 (Log-Rank (Mantel-Cox) P = 0.03, HR 1.66 (95% CI 1.03 – 2.69). (b) Analysis of p62 expression levels after initial stratification by AJCC stage of disease reveals a suggestive increased risk of metastasis in AJCC stage II primary tumours with p62 expression <20% compared to tumours expressing >20% p62 (Log-Rank (Mantel-Cox) P = 0.06, HR 1.70 (95% CI 0.97 – 2.96). The number of patients remaining at risk at 20 month intervals is stated below the x-axis.
Comparable univariate analysis of melanoma specific mortality (MSM) in the whole melanoma cohort revealed a non-significant trend towards an increased MSM in patients with “low p62” tumours (MSM 24.24% “low p62” tumours, versus 14.55% in “high p62” tumours (Log-Rank (Mantel-Cox) Test P = 0.18, HR 1.5 (95% CI 0.83 – 2.74). Univariate analysis of other pre-hypothesised risk factors for disease progression were calculated (Supplementary Methods and Table S2); as expected there was a significant increased risk of metastases with increasing Breslow depth and tumour ulceration in line with AJCC staging criteria (Balch et al. 2009).
Univariate analysis of tumours pre-stratified to AJCC stage II at diagnosis revealed low p62 expression levels were associated with a trend for worse DFS; with 67.74% of patients in the “low p62” cohort developing a metastasis within 7-years compared to only 39.13% in the “high p62” group (Log-Rank (Mantel-Cox) Test) P = 0.06, HR 1.7 (95% CI 0.97 – 2.96) (Fig 2b). A similar trend was seen in AJCC stage I disease, (Log-Rank (Mantel-Cox) Test P = 0.38, HR 1.53 (95% CI 0.58 – 4.09).
MSM followed an identical trend, with “low p62” AJCC stage II tumours resulting in a higher mortality rate compared to “high p62” AJCC II tumours (MSM 35.48% versus 21.74% respectively (Log-Rank (Mantel-Cox) Test P = 0.27, HR 1.51 (95% CI 0.72 – 3.2).
Comparison of mean p62 expression revealed no association with Breslow depth or tumour ulceration (Figure S1) and Cox proportional hazards analysis undertaken to assess whether the predictive effects of p62 may be partially attributable to covariates (Supplementary Methods and Table S3) further supported p62 expression levels as an independent stratifying prognostic variable (p < 9E-7), representing biologically distinct processes to those included in AJCC staging alone.
As a marker of autophagic activity, the p62 status of the current melanoma cohort fits with the “autophagy paradox”; with the highest levels of p62 expression found in early, localised disease (AJCC stages I and II) in keeping with pro-tumourigenic dysfunctional autophagy. However, tumour cells with increased levels of autophagic activity (either through reactivation or retention of this function following tumourigenesis) are more likely to metastasise by harnessing pro-survival autophagy.
Independently of its role in autophagy, p62 expression may be regulated by other signalling mechanisms including via NF-κB activation and interaction with TRAF6 and caspase-8 (Mathew et al. 2009; Komatsu and Ichimura 2010). Therefore, the differential expression of p62 within different AJCC stages of melanoma cannot be assumed to result entirely from impaired autophagic activity. Nevertheless, results from the present study add to the growing body of evidence supporting the introduction of autophagy inhibitors to chemotherapeutic regimes in melanoma, stage I trials of which are currently underway (Komatsu et al 2007), for which p62 expression will likely provide a useful stratification criterion. Crucially however, p62 represents a potential candidate biomarker that may provide additional prognostic information to AJCC disease stage. Further validation in an independent retrospective and ongoing prospective cohort will determine the prognostic significance and effect size of p62 and its application as a biomarker for refining personalised therapies, ultimately translating into improved clinical outcome for melanoma patients.
Supplementary Material
Acknowledgements
This work was supported by the British Skin Foundation (Clinical Research Fellowship, R.A.E, T.N, P.E.L); the Newcastle-upon-Tyne and South Tees Hospitals National Health Service Foundation Trusts (R.A.E), London Research Institute, Cancer Research UK (S.H, S.A.T); the Newcastle Healthcare Charity (J.L.A, P.E.L) the North Eastern Skin Research Fund (R.A.E, J.L.A, P.E.L, N.K) and the Wellcome Trust (Summer Vacation Scholarship, J.L).
Abbreviations
- AJCC
American Joint Committee on Cancer
- DFS
Disease Free Survival
- MSM
Melanoma Specific Mortality
Footnotes
Conflicts of Interest: The authors state no conflict of interest.
References
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Inducing indigestion: companies embrace autophagy inhibitors. J Nat Cancer Inst. 2011;103:708–710. doi: 10.1093/jnci/djr168. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y. Physiological significance of selective degredation of p62 by autophagy. FEBS Letters. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the Crossroads of Autophagy, Apoptosis, and Cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Debnath J. Autophagy and tumorigenesis. Semin Immunopathol. 2010;32:383–396. doi: 10.1007/s00281-010-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.