Abstract
Background
Ischemia/reperfusion (I/R) injury is a major factor leading to intestinal dysfunction or graft loss following intestinal surgery or transplantation. This study investigated the cytoprotective effects and putative mechanisms of IL-13 after intestinal I/R injury in the mouse.
Methods
Mouse warm intestinal I/R injury induced by clamping the superior mesenteric artery for 100 min with tissue analysis at 4- and 24-h post-reperfusion. Treated animals received intravenous recombinant murine IL-13 (rIL-13) and/or anti-IL-13 antibody while controls received saline.
Results
rIL-13 administration markedly prolonged animal survival (100% vs. 50% in saline controls) and resulted in near normal histopathological architecture. rIL-13 treatment also significantly decreased myeloperoxidase (MPO) activity. Mice conditioned with rIL-13 had a markedly depressed TLR4 expression and increased the expression of Stat6, anti-oxidant HO-1, and anti-apoptotic A20, Bcl-2/Bcl-xl, as compared with that of controls. Unlike in controls, the expression of mRNA coding for IL-2/IFN-γ, and IP-10/MCP-1 remained depressed, whereas that of IL-13/IL-4 reciprocally increased in the mice treated with rIL-13. Administration of anti-IL13 antibody either alone or in combination with rIL-13, resulted in outcomes similar to that seen in controls.
Conclusions
This study demonstrates for the first time that IL-13 plays a protective role in intestinal warm I/R injury and a critical role in the regulation of Stat6 and TLR4 signaling. The administration of IL-13 exerts cytoprotective effects in this model by regulating innate and adaptive immunity while the removal of IL-13 using antibody therapy abrogates this effect.
Keywords: Ischemia/reperfusion injury, intestine, IL-13, TLR4, Stat6
Introduction
Ischemia/reperfusion (I/R) injury to the intestine is a major clinical problem resulting in dysfunction, dysmotility, and organ loss. Although the exact mechanisms of intestinal I/R remain unclear, the current research indicates that in the early phases, the production of reactive oxygen species and ATP depletion leads to the initial cellular damage and the release of potent inflammatory mediators such as cytokines, chemokines, adhesion molecules and platelets (1). These mediators may subsequently activate signal transduction pathway that lead to further subacute injury, including cell death by both necrosis and apoptosis (2, 3).
IL-13, a Th2-type cytokine modulates inflammatory responses by down-regulating production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, MIP-1α and MIP-2 (4, 5). In other organ model systems such as the lung (6, 7) and liver (8, 9, 10), the mechanism of the IL-13 anti-inflammatory effects has been linked with activation of signal transducer and activator of transcription-6 (Stat6) (11, 12, 13). Stat6, a rapidly activated transcription factor, plays a key role for the immunoregulatory functions of IL-4 and IL-13 and, conversely, administration of IL-4 and IL-13 results in the activation of Stat6 in liver inflammatory injury induced by I/R (12, 14).
Toll-like receptors (TLRs) belong to the interleukin-1 receptor (IL-1R) family, and their activation results in an innate inflammatory response mediated by macrophages, neutrophils, and complement (15, 16). The resulting production of chemokines and cytokines leads to recruitment of leukocytes to sites of inflammation. TLR4 may have a central role in the induction of a Th1-type cytokine response (17, 18) and imbalances of the Th1/Th2 cytokine profile toward Th1 predominance can contribute to chronic inflammatory states in the intestine (19). We have shown that disruption of TLR4 signaling prevents hepatic I/R injury and inflammation (20), consistent with reduced NF-κB activation in TLR4-deficient system seen in other studies (21). Furthermore, Th2 type cytokines play a regulatory role in the innate TLR expression and function in human intestinal epithelial cells (22). As there have been no published investigations in intestinal I/R injury linking IL-13 and TLR4, we sought to evaluate the putative cytoprotective effects of IL-13 against intestinal inflammatory injury and its link with the innate immune system via TLR4.
RESULTS
rIL-13 treatment prolongs animal survival against intestinal I/R injury
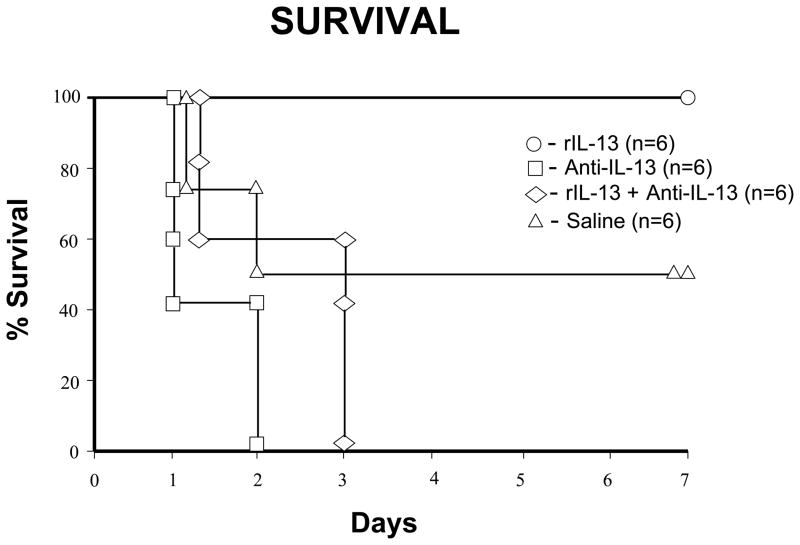
After 100 minutes of warm intestinal ischemia, one hundred percent of rIL-13 survived more than 7 days, as compared with fifty percent of control (p< 0.05; Fig.1). Both of the anti-IL13 groups had no survivors at 7 days. All deaths occurred within 24–72 h of I/R injury and were attributed to I/R injury after necropsy.
Figure 1.
The effects of intestinal I/R injury on mouse survival. C57BL6 wild-type (WT) mice were either treated with rIL-13, anti-IL-13, anti-IL-13+rIL-13, or saline (control). 7 days survival in these groups was 100%, 0%, 0%, and 50%, respectively (n = 6 mice/group).
rIL-13 treatment ameliorates histological signs of intestinal I/R injury and decreases MPO activity
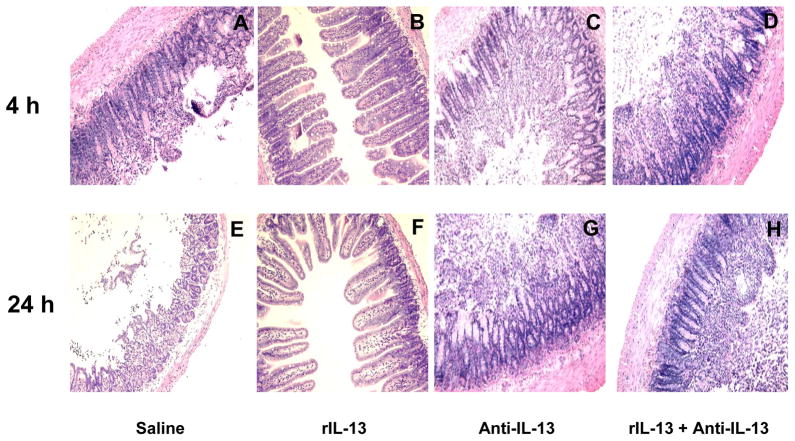
At 4- and 24-h after reperfusion, the severity of I/R injury was blindly evaluated by histopathology. Control had mucosal erosions, severe villous congestion and hemorrhage (Fig. 2A and 2E; score 3.1 ± 0.49 and 3.33 ± 0.52, respectively). In the anti-IL-13 groups, the histopathology resembled that seen in controls (Fig. 2C and 2G; score 3.67 ± 0.4 and 3.75 ± 0.27 (4-h and 24-h, anti-IL-13) even after rIL-13 treatment (Fig. 2D and 2H; score 3.5± 0.45 and 3.83 ± 0.41 (4-h and 24-h, anti-IL-13+rIL-13)). In contrast, rIL-13 showed preserved intestinal architecture and minimal sinusoidal congestion (Fig. 2B and 2F; score, 0.83 ± 0.41 and 1.17 ± 0.41, respectively; p<0.00001).
Figure 2.
Representative histological findings in murine intestines after 100 minutes of warm ischemia followed by reperfusion. The top panels represent the 4-h results and the bottom panels represent the 24-h results. (A and E) saline controls at 4-h and 24-h; (B and F) rIL-13 groups at 4-h and 24-h; (C and G) anti-IL-13 group at 4-h and 24-h; (D and H) anti-IL-13 + rIL-13 at 4-h and 24h; Note, in panels A, C, D, E, G, and H there is mucosal erosion, severe villous congestion and hemorrhage. B and F show preservation of intestinal architecture and minimal sinusoidal congestion.
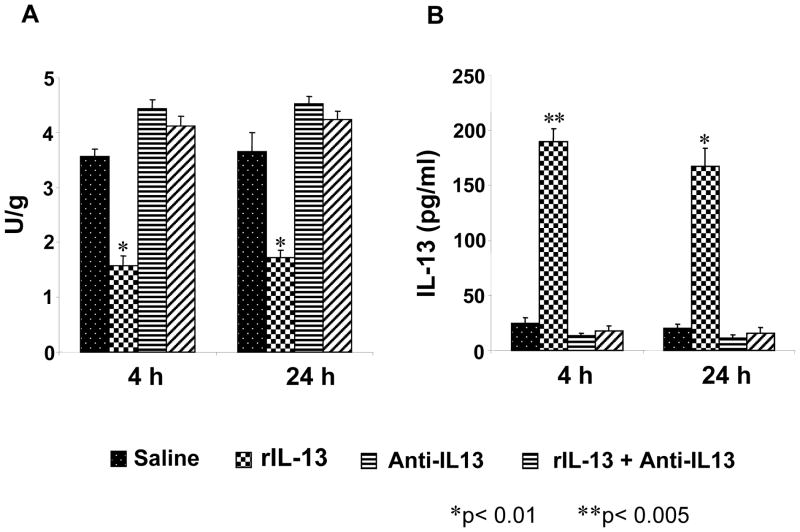
MPO activity at 4- and 24-h post reperfusion is shown in figure 3A. The results in control and anti-IL-13 groups were similar at both 4-h and 24-h (control: 3.57 ± 0.13 U/g (4-h) and 3.65± 0.34 U/g (24-h); anti-IL-13: 4.43 ± 0.17 U/g (4-h) and 4.52 ± 0.14 U/g (24-h); anti-IL-13+rIL-13: 4.12 ± 0.17 U/g (4-h) and 4.23 ± 0.16 U/g (24-h)). In contrast, rIL-13 showed a significant reduction in MPO activity (1.57 ± 0.18 U/g (4-h) and 1.72 ± 0.14 U/g (24-h), respectively, p<0.01 vs. controls).
Figure 3.
A. Intragraft neutrophil accumulation at 4 h and 24 h of reperfusion after 100 minutes of warm ischemia, as analyzed by MPO enzymatic activity (U/gm) are shown. Note: The MPO activity was significantly decreased in the rIL-13 group, as compared to saline control, anti-Il-13, and anti-IL-13+rIL-13 groups. These data represent 3–4 animals for each group. Mean and SD are shown; * p< 0.05 versus control group. B. Protein expression of IL-13 in murine serum by ELISA at 4 h and 24 h perfusion after 100 minutes warm ischemia is shown. Note: IL-13 protein levels (pg/ml) were significantly higher in rIL-13 both 4 h and 24 h groups, as compared with those in saline control, anti-IL-13, and anti-IL-13+rIL-13 groups. Each column represents the mean ± SD (n=3 samples/group; *p<0.005).
rIL-13 treatment results in prolonged increases in serum IL-13 levels
We performed ELISA to assess serum IL-13 protein levels. As shown in Fig. 3B, serum IL-13 levels at 4- and 24-h were significantly higher in rIL-13 (190 ± 11.3 pg/ml (4-h), p<0.005; 168 ± 16.3 pg/ml (24-h) p<0.01), as compared with anti-IL-13 treated groups (13.2 ± 2.1 pg/ml and 11.5 ± 2.35 pg/ml, p<0.01), and controls (10.3 ± 1.13 pg/ml (4-h) and 11.4 ± 1.69 pg/ml (24-h), p<0.01).
IL-13 regulates cytokine and chemokine gene expression in intestinal I/R injury
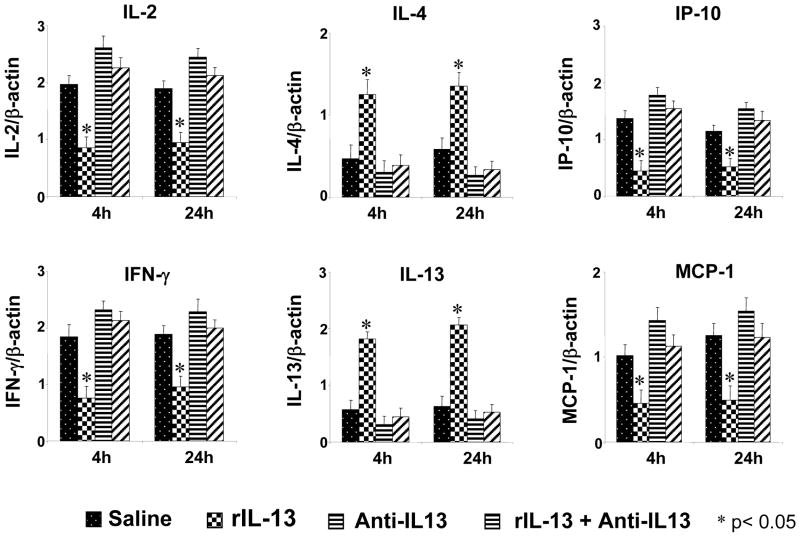
As shown in Fig. 4, at 4- and 24-h after reperfusion, control and anti-IL-13 groups showed significantly increased intestinal mRNA expression of IL-2, IP-10, IFN-γ and MCP-1, as compared with rIL-13 (p<0.05). In contrast, rIL-13 treatment resulted in a significant and progressive increase in mRNA expression for IL-4 and IL-13 (p<0.05), as compared with control and anti-IL-13.
Figure 4.
Competitive template RT-PCR assisted expression of mRNA coding for cytokine IL-2/IFN-γ and IL-4/IL-13, and chemokine IP-10/MCP-1 in ischemic intestines at 4 h and 24 h after 100 minutes warm ischemia. Note: The expression of IL-2, IFN-γ, IP-10 and MCP-1 remained consistently decreased in rIL-13 groups, as compared with saline control, anti-IL-13, and anti-IL-13+rIL-13. In contrast, rIL-13 treatment resulted in a significant and progressive increase in gene transcript levels for IL-4 and IL-13, as compared to saline control, anti-IL-13, and anti-IL-13+rIL-13. Each column represents the mean ± SD (n=3–4 samples/group; *p<0.05).
IL-13 activates Stat6, protective molecules and inhibits TLR4 in intestinal I/R injury
To determine whether Stat6 and TLR4 play a role for the cytoprotective effects of IL-13, Western blots were performed for target gene protein products. As shown in Fig. 5, the expression of Stat6, anti-oxidant HO-1 and anti-apoptotic A20, Bcl-2/Bcl-xl was strongly up-regulated after rIL-13 treatment (2.0–2.2 AU, 1.8–2.0 AU, 1.9–2.1 AU, 1.7–1.9 AU and 1.7–2.0 AU, respectively), as compared with control and anti-IL-13 (0.1–0.2 AU and 0.1–0.3 AU). In contrast, the expression of TLR4 was depressed after rIL-13 treatment (0.3–0.5 AU), as compared with control and anti-IL-13 (2.1–2.3 AU).
Figure 5.
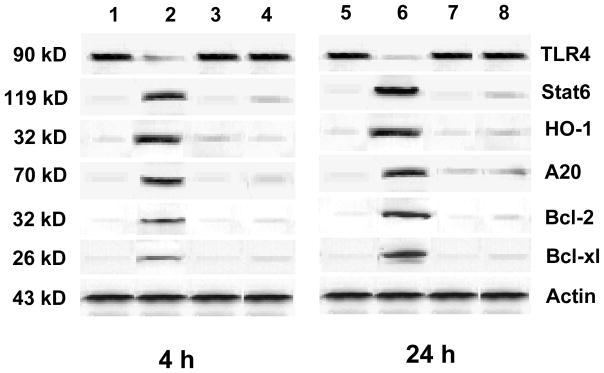
Western blot analysis of TLR4, Stat6, HO-1, A20, Bcl-2 and Bcl-xl gene products in intestinal tissue at 4 h and 24 h of reperfusion after 100 min warm ischemia. Lane 1 – wild type (WT) saline control group at 4-h, Lane 2 – rIL-13 at 4-h; Lane 3 – anti-IL-13 group at 4-h; Lane 4 – anti-IL-13+rIL-13 group at 4-h; Lane 5 - wild type (WT) saline control group at 24-h; Lane 6 - rIL-13 at 24-h; Lane 7 - anti-IL-13 group at 24-h; Lane 8 - anti-IL-13+rIL-13 group at 24-h.
Note: rIL-13 treatment markedly decreased the expression of TLR4 and increased the expression of Stat6, HO-1, A20, Bcl-2 and Bcl-xl in wild type recipients. The data shown are representative of three separate experiments.
DISCUSSION
This is the first published report to demonstrate the protective effects of IL-13 in murine intestinal I/R injury. The data reported here demonstrates that treatment with exogenous rIL-13 prior to I/R injury results in improved animal survival and reduced histopathological tissue injury. These endpoints are associated with reduced tissue MPO activity and inflammatory cytokine production. Exogenous administration of rIL-13 resulted in a cytokine profile switch from a Th1 to Th2 predominant pattern that is potentially mediated through the inhibition of innate TLR4 signaling and activation of Stat6 signaling. These changes resulted in a protective microenvironment characterized by increased expression of HO-1, A20 and Bcl-2/Bcl-xl. To confirm these findings, two control groups were used that involved the administration of an anti-IL-13 antibody in the presence or absence of rIL-13 administration. Indeed, when antibody was administered, the protective effects produced by rIL-13 administration were abrogated. These data suggest that mechanisms of rIL-13 protection may involve multiple inflammatory cascades of events induced by I/R injury.
The genesis of this study lay in our prior intestinal I/R studies regarding P selectin manipulation with either an exogenously administered ligand, rPSGL-Ig or P selectin genetic deficiency (23, 24). Both clearly demonstrated reduce intestinal lymphocyte infiltration with P selectin manipulation as well as a Th1/Th2 cytokine switch. This coupled with data from others regarding the importance of lymphocytes in intestinal I/R injury (27) and our own data regarding Th2 cytokines in hepatic I/R injury (8), led to the hypothesis that, by creating a Th2 microenvironment through the administration of exogenous rIL-13, a protective effect would be seen after intestinal I/R injury. Furthermore, we sought to establish a link between this adaptive immune response manipulation and the innate immune response – neither of which had been previously shown in intestinal I/R models.
One of the major mechanistic points of this study is the fact that exogenous rIL-13 administration was shown to create a Th2 cytokine environment. This statement is supported by both the serum ELISA data and the rt-PCR tissue data. The serum ELISA results showing marked increases in circulating IL-13 at both 4- and 24-h after rIL-13 administration indicate that endogenous production of IL-13 must be responsible for the persistent serum IL-13 levels as the circulating half-life of exogenous IL-13 is too short to account for these findings. Furthermore, the source of the endogenous IL-13 production appears to be the intestine as our rt-PCR data indicates that treatment with exogenous rIL-13 results in increased IL-13 mRNA production within the tissue. Mechanistically, it appears that rIL-13 administration results in further lymphocyte production of IL-13 cytokines thus propagating the Th2 milieu.
The protective role of Th2 cytokine in intestinal I/R injury is controversial as discussed previously (23). Mostly of the published data involves the Th2 cytokine IL-10 with studies reporting both a protective (23, 24, 28, 29, 30) and deleterious effect (31). In support of a protective role is in vitro data demonstrating that IL-13 administration inhibits the production of pro-inflammatory cytokines by monocytes/macrophages (32, 33). Our in vivo data supports these in vitro observations. Indeed, rIL-13 administration markedly suppressed proinflammatory cytokine (IL-1, TNF-α), Th1-type cytokine (IL-2/IFN-γ) and chemokine (IP-10/MCP-1) mRNA expression, whereas significant increases in Th2-type cytokine (IL-4/IL-13) mRNA expression were seen. Inhibition of these pro-inflammatory mediators led to reduced intestinal neutrophil accumulation and prevented the development of intestine injury. Hence, Th2 cytokine switch contributes to the anti-inflammatory effects observed after intestinal warm I/R in this model.
Our study demonstrated that IL-13 exerts cytoprotective function against antigen-independent intestinal I/R injury by activation of Stat6 signaling. This finding is in agreement with other investigations where exogenous murine rIL-13 protects hepatic function and leads to Stat6 activation (12). While not examined in this study, the pro-inflammatory and Th1 cytokine milieu seen in the saline treated controls is known to result from Stat4 signaling. Thus, it is likely that by activating Stat6 signaling, a Th2 protective microenvironment is created (34) whereas by activating a Stat4 signaling pathway, a pro-inflammatory and Th1 microenvironment is created. However, confirmation of the Stat4 signaling hypothesis remains to be examined in this model. Hence, combined with the recent findings (8, 9, 12, 35), our data highlights the anti-inflammatory role of Stat6 signaling, and suggests it operates as a negative regulator in the inflammatory responses induced by I/R.
Our present data also demonstrates that TLR4 activation is involved in the injury resulting from intestinal I/R. rIL-13 treatment was associated with a marked decrease in TLR4 production suggesting that the activation of TLR4 may be related to Stat6 signaling disruption. In support of this observation are studies examining TLR4, LPS signaling, and liver Kupffer cell acitivation during hepatic I/R injury (36). LPS binds to LPS-binding protein produced by hepatocytes which then recognizes and binds to CD14 resulting in Kupffer cell activation, nuclear translocation of NF-κB, and production of pro-inflammatory cytokines (37, 38). We have shown that either TLR4 blockade or TLR4 genetic deficiency prevents hepatic I/R injury, diminished early pro-inflammatory responses, and induced expression of cytoprotective HO-1 (20). Together, these studies underscore the relationship between the innate and adaptive immune response in I/R injury.
Although it is unclear the relationship between innate TLR4 signaling and Stat6 activation in the regulation of intestine inflammatory response, recent studies have shown that chronic intestinal inflammatory disease is associated with a proinflammatory Th1 cytokine profile and enhanced TLR4 expression and signaling (39, 40, 41). TLR4 recognition of LPS leads to transduction of a proinflammatory signal through the adapter molecule myeloid differentiation marker88 (MyD88) (42). While, Stat6 is associated with Th2 cell development and is critical for the immunoregulatory functions of IL-4 andIL-13 in vitro (43, 44), recent studies have suggested that it may also be as a regulator of the acute inflammatory response (12, 13). Our current results demonstrate that Stat6 is associated with IL-13 signaling and reduction in TLR4 coexists in this microenviromental state. These data are consistent with our recent studies showing that disruption of TLR4 signaling or local induction of Th2-type IL-13 decreased inflammatory injury in hepatic I/R (10, 20).
In conclusion, our data demonstrates that: i) Stat6 signaling is induced by rIL-13 treatment and is associated with a Th2 cytokine response and cytoprotection after intestinal I/R injury, and ii) IL-13 plays critical role in regulation of TLR4 signaling and the reduction of TLR4 expression is associated with cytoprotection against inflammatory injury induced by I/R. These findings demonstrate that IL-13 exerts cytoprotective effects in mouse intestinal warm I/R injury by regulating innate and adaptive immunity.
Materials and Methods
Animals
Inbred male C57BL6 wild-type (WT) mice (8–12 weeks of age) were used (Harlan Sprague Dawley, Inc, Indianapolis, IN). Mice were housed in UCLA animal facility under specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” and published by National Institute of Health (NIH publication 86-23 revised 1985). Institutional Review Board approval was obtained prior to initiation of this investigation.
Intestinal I/R injury model
Warm intestinal ischemia was induced as described previously (23). Briefly, under general isoflurane (Forane, Baxter, Deerfield, IL) anesthesia, all animals underwent a midline laparotomy with evisceration of the intestine and isolation of the superior mesenteric and iliocolic arteries. Collateral circulation was eliminated by dividing the mesentery beneath the proximal jejunum and distal ileum. 100 min of ischemia was induced using atraumatic vascular clamps applied to the isolated arteries. The eviscerated bowel was kept moist and the temperature of the animal controlled during ischemia. At the conclusion of ischemia, the clamps were removed and the bowel was returned to its natural intra-abdominal position. Separate survival and analysis groups were performed. In the latter group, animals were sacrificed after 4- and 24-h of reperfusion. Small bowel tissue and blood samples were collected at sacrifice.
Treatment
Mice were set up four groups: (i) received recombinant murine IL-13 (1 μg iv; R&D Systems Inc., Minneapolis, MN) (rIL-13); (ii) received IL-13 antibody (1 μg iv; R&D Systems Inc., Minneapolis, MN) (anti-IL-13); (iii) received rIL-13 plus anti-IL-13 (rIL-13+ anti-IL-13); (iv) received sterile saline (control) into the inferior vena cava prior to the induction of ischemia.
Histopathology
Tissue samples were preserved in 10% neutral-buffered formalin, embedded in paraffin, cut into 4-μm section, and stained with hematoxylin and eosin (H&E). Histological severity of I/R injury was graded using previously reported grading system (23, 24): 0=normal, 1=superficial epithelial/villous tip injury, 2=injury extending through the epithelial and lamina propria, 3=injury extending into the submucosa layer, 4=injury extending into the muscularis propria, and 5=full thickness injury. Six fields were evaluated per tissue section. All data are expressed as mean ± SD.
Myeloperoxidase (MPO) activity assay
The presence of MPO was used as an index of neutrophil accumulation (25). Briefly, liquid nitrogen frozen/stored intestinal tissue was thawed and weighed and placed in 4 ml iced 0.5% hexadecyltrimethyl-ammonium bromide and 50 mmol potassium phosphate buffer solution with the pH adjusted to 5. Each sample was then homogenized for 30 seconds and centrifuged at 15,000 rpm for 15 minutes at 4°C. Supernatants were then mixed with hydrogen peroxide-sodium acetate and tetramethyl-benzidine solutions. The change in absorbance was measured spectrophotometrically at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide per minute at 25°C per gram of tissue.
IL-13 ELISA
ELISA assay to detect IL-13 levels was performed, as described (26). Briefly, serum was collected from animal blood samples. Flat-bottom 96-well microtiter plates were coated with anti-human IL-13 Ab (Pharmingen, San Diego, CA). Nonspecific binding sites were blocked with Blocking Buffer (10% fetal bovine serum, 10% newborn calf serum or 1% BSA in PBS). Plates were rinsed, and serum samples were added, followed by addition of biotinylated mouse anti-human IL-13 Ab (2 μg/ml). After washing, streptavidin-peroxidase conjugate (Pharmingen) was added, and the plates were incubated for color development. Plates were read at 405 nm in ELISA reader. The linear region of IL-13 curves was obtained in a series of eight two-fold dilutions of human IL-13 standard (2000 – 15 pg/ml).
RNA extraction/competitive template RT-PCR
To study cytokine and chemokine gene expression patterns, we used competitive template reverse transcription polymerase chain reaction (RT-PCR), as previously described (26). Briefly, total RNA was extracted from frozen intestinal tissue using RNAse Mini Kit (Qiagen Inc., Chatsworth, CA), and RNA concentration was determined by a spectrophotometer. A total 5 μg of RNA was reverse-transcribed using oligo (dT) primers and superscript reverse transcriptase (GIBCO, Grand Island, NY). According to the varying contents of specific cDNA and amplification efficiencies, PCR was performed by different cycle numbers at the annealing temperature that was optimized empirically for each primer pair: 35, 60° C (IL-2), 40, 62° C (IL-4), 40, 55° C (IL-13), 35, 60° C (IFN-γ), 35, 53° C (IP-10), 35, 55° C (MCP-1), and 35, 63° C (β-actin), respectively. PCR products were analyzed in ethidium bromide-stained 2% agarose gel, and scanned the density using Kodak Digital Science 1D Analysis software (Version 2.0). To compare the relative level of each cytokine in different samples, all samples were normalized against the respective β-actin temple cDNA ratio.
Western blot analysis
Protein was extracted from intestinal tissue with PBSTDS buffer. Proteins (30 μg/sample) in SDS-loading buffer (50 mM Tris, pH 7.6, 10% glycerol, 1% SDS) were subjected to 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The gel was stained with Coomassie blue to document protein loading. The membrane was blocked with 3% dry milk + 0.1% Tween 20 (USB, Cleveland, OH). Polyclonal rabbit anti-mouse TLR4, Stat6, Bcl-2, Bcl-xl, β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Monoclonal mouse anti-human A20 Ab (Imgenex, San Diego, CA), and HO-1 (StressGen Biotech, Victoria, BC, Canada) antibodies were used. The membranes were incubated with Abs, relative quantities of proteins were determined by densitometer, and expressed in absorbance units (AU) (Kodak Digital Science 1D Analysis Software, Rochester, NY).
Statistical analysis
All data are expressed as mean ± SD. Statistical comparisons between groups were analyzed by Student’s t-test. All differences were considered statistically significant at the p-value of <0.05.
Acknowledgments
This work was supported in part by The Roche Surgical Scientist Scholar Award from the American Society of Transplant Surgeons; and The Dumont Research Foundation.
Abbreviations
- rIL-13
recombinant interleukin 13
- ELISA
enzyme-linked immunosorbent assay
- HO-1
hemeoxygenase-1
- I/R
ischemia/reperfusion
- MPO
myeloperoxidase
- Stat6
signal transducer and activator of transcription-6
- TLR4
Toll-like receptor 4
- WT
wild-type
Footnotes
Author contribution:
- Research design: DGF, BK, XDS, FK, RWB, JKW
- Research conduct: DGF, BK, XDS, FK, FG, MJW
- Data analysis: DGF, BK, JKW
- Manuscript preparation: DGF, BK, RWB, JKW
- Authors with conflict of interest: NONE
References
- 1.Farmer DG, Amersi F, Kupiec-Weglinski JW, Busuttil RW. Current status of ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106–126. [Google Scholar]
- 2.Clavien PA, Harvey PRC, Strasberg SM. Preservation and reperfusion injuries in liver allografts: overview and synthesis of current studies. Transplantation. 1992;53:957–978. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Lermasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Ann Rev Pharmacol Toxicol. 1997;37:327–338. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 4.Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 5.Berkman N, John M, Roesems G, Jose P, Barnes PJ, Chung KF. Interleukin 13 inhibits macrophage inflammatory protein-1α production from human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1996;15:382–389. doi: 10.1165/ajrcmb.15.3.8810643. [DOI] [PubMed] [Google Scholar]
- 6.Muchamuel T, Menon S, Pisacane P, Howard MC, Cockayne DA. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: correlation with down-modulation of TNF-α, IFN-γ, and IL-12 production. J Immunol. 1997;158:2898–2903. [PubMed] [Google Scholar]
- 7.Mulligan MS, Warner RL, Foreback JL, Shanley TP, Ward PA. Protective effects of IL-4, IL-10, IL-12, and IL-13 in IgG immune complex-induced lung injury. J Immunol. 1997;159:3483–3489. [PubMed] [Google Scholar]
- 8.Ke B, Shen XD, Lassman CR, et al. IL-13 gene transfer protects rat livers from antigen-independent injury induced by ischemia and reperfusion. Transplantation. 2003;75:1118–1123. doi: 10.1097/01.TP.0000062861.80771.D5. [DOI] [PubMed] [Google Scholar]
- 9.Ke B, Shen XD, Lassman CR, Gao F, Busuttil RW, Kupiec-Weglinski JW. Cytoprotective and antiapoptotic effects of IL-13 in hepatic cold ischemia/reperfusion injury are heme oxygenase-1 dependent. Am J Transplant. 2003;3:1076–1082. doi: 10.1034/j.1600-6143.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 10.Ke B, Shen XD, Gao F, Busuttil RW, Kupiec-Weglinski JW. Interleukin 13 gene transfer in liver ischemia and reperfusion injury: role of stat6 and TLR4 pathways in cytoprotection. Hum Gene Ther. 2004;15:691–698. doi: 10.1089/1043034041361244. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]
- 12.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059–1064. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Regulation of liver inflammatory injury by signal transducer and activator of transcription. Am J Pathol. 2000;157:297–302. doi: 10.1016/S0002-9440(10)64540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmori Y, Hamilton TA. STAT6 is required for the anti-inflammatory activity of interleukin-4 in mouse peritoneal macrophages. J Biol Chem. 1998;273:29202–29209. doi: 10.1074/jbc.273.44.29202. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–387. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Oshikawa T, Ohe G, et al. Severe impairment of anti-cancer effect of lipoteichoic acid-related molecule isolated from a penicillin-killed Streptococcus pyogenes in toll-like receptor 4-deficient mice. Int Immunopharmacol. 2001;1:1789–95. doi: 10.1016/s1567-5769(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 18.Pulendran B, Kumar P, Cutler CW, et al. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–76. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 20.Shen X-D, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplantation. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Gallo DJ, Green AM, et al. Role of toll-like receptors in changes in gene expression and NF-B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J Immunol. 2006;176:5805–5814. doi: 10.4049/jimmunol.176.10.5805. [DOI] [PubMed] [Google Scholar]
- 23.Farmer DG, Anselmo D, Shen X-D, et al. Disruption of p-selectin signaling modulates cell trafficking and results in improved outcomes after mouse warm intestinal ischemia and reperfusion injury. Transplantation. 2005;80:828–835. doi: 10.1097/01.tp.0000174337.53658.b0. [DOI] [PubMed] [Google Scholar]
- 24.Farmer DG, Shen X-D, Amersi F, et al. CD62 Blockade with P-selectin glycoprotein ligand-immunoglobulin fusion protein reduces ischemia-reperfusion injury after rat intestinal transplantation. Transplantation. 2005;79:44–51. doi: 10.1097/01.tp.0000146965.64706.e8. [DOI] [PubMed] [Google Scholar]
- 25.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharm Meth. 1985;4:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 26.Ke B, Ritter T, Kato H, et al. Regulatory cells potentiate the efficacy of IL-4 gene therapy by upregulating Th2-dependent expression of protective molecules in the infectious tolerance pathway in transplant recipients. J Immunol. 2000;164:5739–5745. doi: 10.4049/jimmunol.164.11.5739. [DOI] [PubMed] [Google Scholar]
- 27.Shigematsu T, Wolf RE, Granger DN. T Lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation. 2002;9:99–109. doi: 10.1038/sj/mn/7800126. [DOI] [PubMed] [Google Scholar]
- 28.Zingarelli B, Yang Z, Hake PW, Denenberg A, Wong HR. Absence of endogenous interleukin 10 enhances early stress response during post-ischaemic injury in mice intestine. Gut. 2001;48:610–622. doi: 10.1136/gut.48.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber TS, Gaines GC, Welborn MB, Rosenberg JJ, Seeger JM, Moldawer LL. Anticytokine therapies for acute inflammation and the systemic inflammatory response syndrome: IL-10 and ischemia/reperfusion injury as a new paradigm. Shock. 2000;13:425–434. doi: 10.1097/00024382-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Gloor B, Todd KE, Lane JS, Rigberg DA, Reber HA. Mechanism of increased lung injury after acute pancreatitis in IL-10 knockout mice. J Surg Res. 1998;80:110–114. doi: 10.1006/jsre.1997.5289. [DOI] [PubMed] [Google Scholar]
- 31.Stallion A, Kou TD, Miller KA, Dahms BB, Dudgeon DL, Levine AD. IL-10 is not protective in intestinal ischemia reperfusion injury. J Surg Res. 2002;105:145–152. doi: 10.1006/jsre.2002.6398. [DOI] [PubMed] [Google Scholar]
- 32.Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 33.Berkman N, John M, Roesems G, Jose P, Barnes PJ, Chung KF. Interleukin 13 inhibits macrophage inflammatory protein-1α production from human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1996;15:382–389. doi: 10.1165/ajrcmb.15.3.8810643. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 35.Kato A, Yoshidome H, Miyazaki M, Edwards MJ, Lentsch AB. Reduced hepatic ischemia/reperfusion injury by IL-4: Potential anti-inflammatory role of STAT6. Inflamm Res. 2000;49:275–279. doi: 10.1007/PL00000207. [DOI] [PubMed] [Google Scholar]
- 36.Tsoulfas GT, Takahashi Y, Ganster RW, et al. Activation of the lipopolysaccharide signaling pathway in hepatic transplantation preservation injury. Transplantation. 2002;74:7–13. doi: 10.1097/00007890-200207150-00003. [DOI] [PubMed] [Google Scholar]
- 37.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 38.Su GL, Klein RD, Aminlari A, et al. Kupffer cell activation by lipoplysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 39.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2000;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 40.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infec Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi D, Das J, Das G. Inflammatory bowel disease requires the interplay between innate and adaptive immune signals. Cell Res. 2006;16:70–74. doi: 10.1038/sj.cr.7310009. [DOI] [PubMed] [Google Scholar]
- 42.Fukata M, Michelsen KS, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]
- 44.Ohmori Y, Hamilton TA. STAT6 is required for the anti-inflammatory activity of interleukin-4 in mouse peritoneal macrophages. J Biol Chem. 1998;273:29202–29209. doi: 10.1074/jbc.273.44.29202. [DOI] [PubMed] [Google Scholar]