Abstract
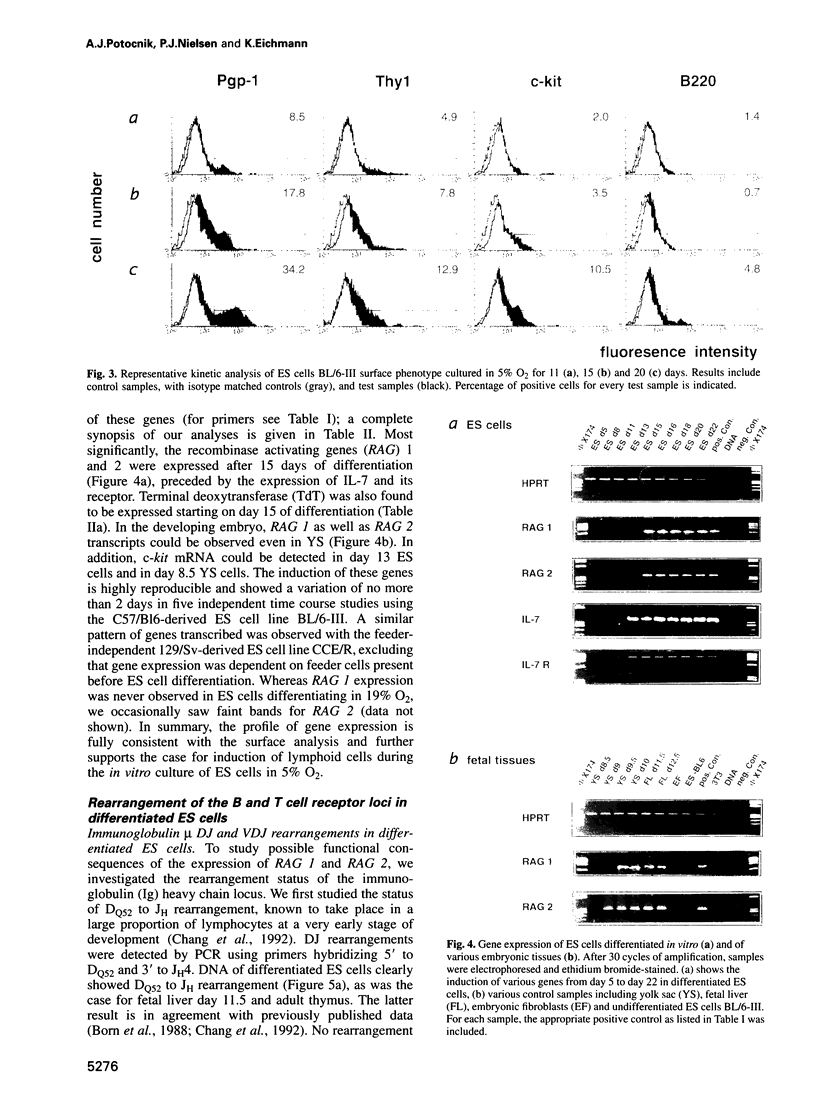
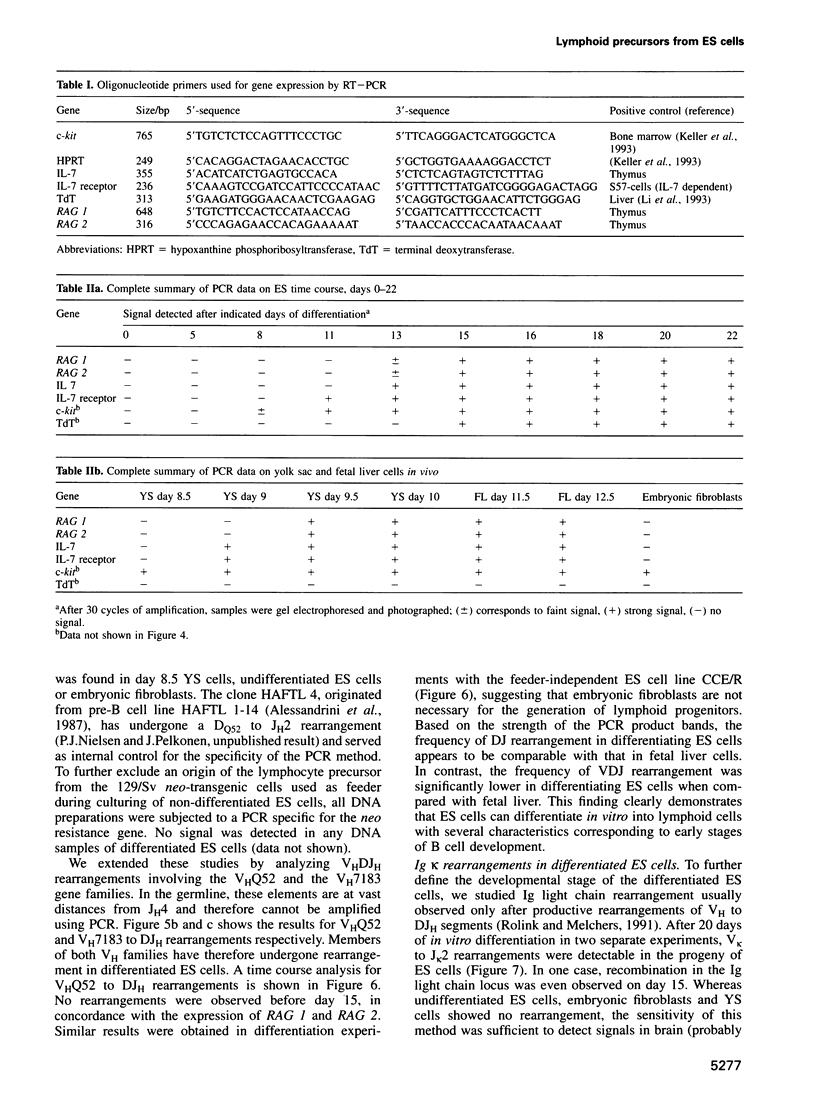
Murine embryonic stem (ES) cells represent a model system for studying certain aspects of hemopoiesis because they can differentiate in vitro into several cell types, including those of the hemopoietic system. We developed cell culture conditions in which ES cells undergo hemopoietic differentiation in a low-oxygen (5% O2) atmosphere without additional exogenous factors. After 15-20 days of culture under these conditions, cells bearing surface markers found on cells of the lymphoid lineage (Thy1+, Pgp-1+, c-kit+ and B-220+) were detected. After 13-15 days, transcripts for the recombinase activating genes (RAG) 1 and 2, interleukin (IL) 7, IL-7 receptor and c-kit were expressed. We also investigated rearrangements of the immunoglobulin (Ig) heavy and light chain and the T cell receptor (TCR) loci. After 15 days of differentiation, we detected DJH gene rearrangement with N-region diversity. Productive VHDJH rearrangements are found after 20 days, paralleled by V Kappa J Kappa recombinations indicating a developmental stage comparable, at least, with that of pre B cells. Rearrangements of TCR gamma as well as delta chain segments were also observed, but no TCR beta chain rearrangement. These results demonstrate that ES cells reproducibly generate lymphoid cells in vitro.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandrini A., Pierce J. H., Baltimore D., Desiderio S. V. Continuing rearrangement of immunoglobulin and T-cell receptor genes in a Ha-ras-transformed lymphoid progenitor cell line. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1799–1803. doi: 10.1073/pnas.84.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. J., Abraham K. M., Nakayama T., Singer A., Perlmutter R. M. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992 Dec;11(13):4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., White J., Kappler J., Marrack P. Rearrangement of IgH genes in normal thymocyte development. J Immunol. 1988 May 1;140(9):3228–3232. [PubMed] [Google Scholar]
- Carding S. R., Kyes S., Jenkinson E. J., Kingston R., Bottomly K., Owen J. J., Hayday A. C. Developmentally regulated fetal thymic and extrathymic T-cell receptor gamma delta gene expression. Genes Dev. 1990 Aug;4(8):1304–1315. doi: 10.1101/gad.4.8.1304. [DOI] [PubMed] [Google Scholar]
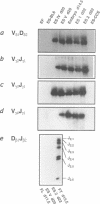
- Chang Y., Paige C. J., Wu G. E. Enumeration and characterization of DJH structures in mouse fetal liver. EMBO J. 1992 May;11(5):1891–1899. doi: 10.1002/j.1460-2075.1992.tb05241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen U., Kosco M., Staerz U. Establishment and characterization of lymphoid and myeloid mixed-cell populations from mouse late embryoid bodies, "embryonic-stem-cell fetuses". Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2541–2545. doi: 10.1073/pnas.89.7.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Furlonger C., Paige C. J. Differentiation and characterization of B-cell precursors detected in the yolk sac and embryo body of embryos beginning at the 10- to 12-somite stage. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6429–6433. doi: 10.1073/pnas.90.14.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S., Dierich A., Lemeur M., Benoist C., Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993 Aug 27;261(5125):1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Goldman J. P., Spencer D. M., Raulet D. H. Ordered rearrangement of variable region genes of the T cell receptor gamma locus correlates with transcription of the unrearranged genes. J Exp Med. 1993 Mar 1;177(3):729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossler A., Doetschman T., Korn R., Serfling E., Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Kitamura D., Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991 Apr 5;65(1):47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Palacios R. In vitro differentiation of embryonic stem cells into lymphocyte precursors able to generate T and B lymphocytes in vivo. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Allison J. P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988 Sep 29;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Hayashida H., Miyazawa S., Kurosawa Y. Only DFL16, DSP2, and DQ52 gene families exist in mouse immunoglobulin heavy chain diversity gene loci, of which DFL16 and DSP2 originate from the same primordial DH gene. Eur J Immunol. 1989 Oct;19(10):1849–1854. doi: 10.1002/eji.1830191014. [DOI] [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E. G., Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T., Wiles M. V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993 Jan;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Ledermann B., Bürki K. Establishment of a germ-line competent C57BL/6 embryonic stem cell line. Exp Cell Res. 1991 Dec;197(2):254–258. doi: 10.1016/0014-4827(91)90430-3. [DOI] [PubMed] [Google Scholar]
- Li Y. S., Hayakawa K., Hardy R. R. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993 Sep 1;178(3):951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum M. H., Grosveld F. An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev. 1990 Dec;4(12A):2075–2085. doi: 10.1101/gad.4.12a.2075. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Auerbach R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development. 1991 Dec;113(4):1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Risser R. G., McKearn J. P. Structure and expression of the T cell receptor gamma locus in pre-B and early hemopoietic cells. Eur J Immunol. 1989 Dec;19(12):2303–2308. doi: 10.1002/eji.1830191219. [DOI] [PubMed] [Google Scholar]
- Müller A. M., Dzierzak E. A. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993 Aug;118(4):1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Kusakabe M., Yoshinaga K., Ogawa M., Hayashi S., Kunisada T., Era T., Sakakura T., Nishikawa S. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991 Aug;10(8):2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisitani S., Tsubata T., Honjo T. Lineage marker-negative lymphocyte precursors derived from embryonic stem cells in vitro differentiate into mature lymphocytes in vivo. Int Immunol. 1994 Jun;6(6):909–916. doi: 10.1093/intimm/6.6.909. [DOI] [PubMed] [Google Scholar]
- Palacios R., Imhof B. A. At day 8-8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Corcoran L. M., Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991 Mar 1;173(3):711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Kiefer M., Dembić Z., Steinmetz M., Karjalainen K. Rearrangements of T cell receptor loci can be found only rarely in B lymphoid cells. Eur J Immunol. 1986 Apr;16(4):430–434. doi: 10.1002/eji.1830160420. [DOI] [PubMed] [Google Scholar]
- Wiles M. V. Embryonic stem cell differentiation in vitro. Methods Enzymol. 1993;225:900–918. doi: 10.1016/0076-6879(93)25057-9. [DOI] [PubMed] [Google Scholar]
- Wiles M. V., Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991 Feb;111(2):259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- van Meerwijk J. P., Blüthmann H., Steinmetz M. T-cell specific rearrangement of T-cell receptor beta transgenes in mice. EMBO J. 1990 Apr;9(4):1057–1062. doi: 10.1002/j.1460-2075.1990.tb08210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]