Abstract
The alpha-2,8-linked sialic acid polymer (PSA) on the neural cell adhesion molecule (NCAM) is an important regulator of cell surface interactions. We have examined the translocation of PSA-NCAM to the surface of cultured cortical neurons and insulin secreting beta cells under different conditions of cell activity. Endoneuraminidase N, an enzyme that specifically cleaves PSA chains, was used to remove pre-existing PSA from the plasma membrane and the re-expression of the molecule was monitored by immunocytochemistry. Punctate PSA immunostaining was restored on the surface of 68% of neurons within 1 h. This recovery was almost completely prevented by tetrodotoxin, suggesting that spontaneous electrical activity is required. K+ depolarization (50 mM) allowed recovery of PSA surface staining in the presence of tetrodotoxin and this effect required the presence of extracellular Ca2+. Rapid redistribution of PSA-NCAM to the surface of beta cells was observed under conditions that stimulate insulin secretion. Ca2+ channel inhibition decreased both PSA-NCAM expression and insulin secretion to control, non-stimulated levels. Finally, subcellular fractionation of an insulin-secreting cell line showed that the secretory vesicle fraction is highly enriched in PSA-NCAM. These results suggest that PSA-NCAM can be translocated to the cell surface via regulated exocytosis. Taken together, our results provide unprecedented evidence linking cell activity and PSA-NCAM expression, and suggest a mechanism for rapid modulation of cell surface interactions.
Full text
PDF
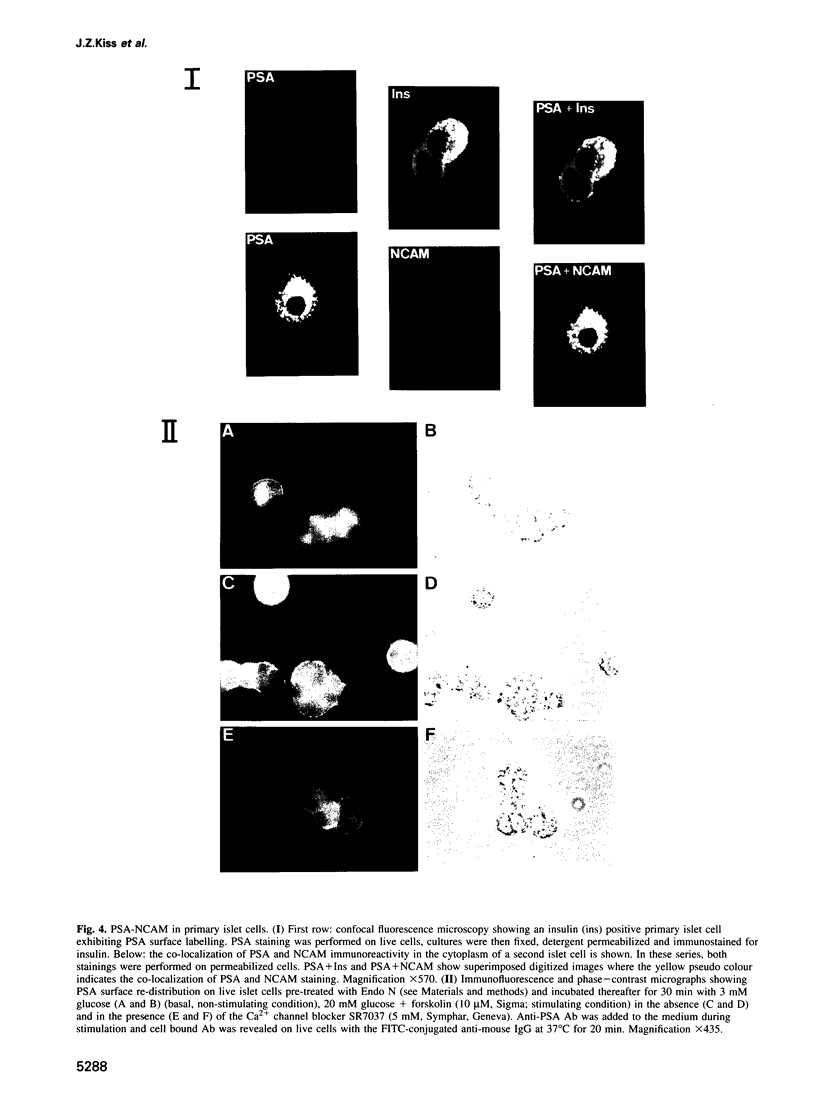
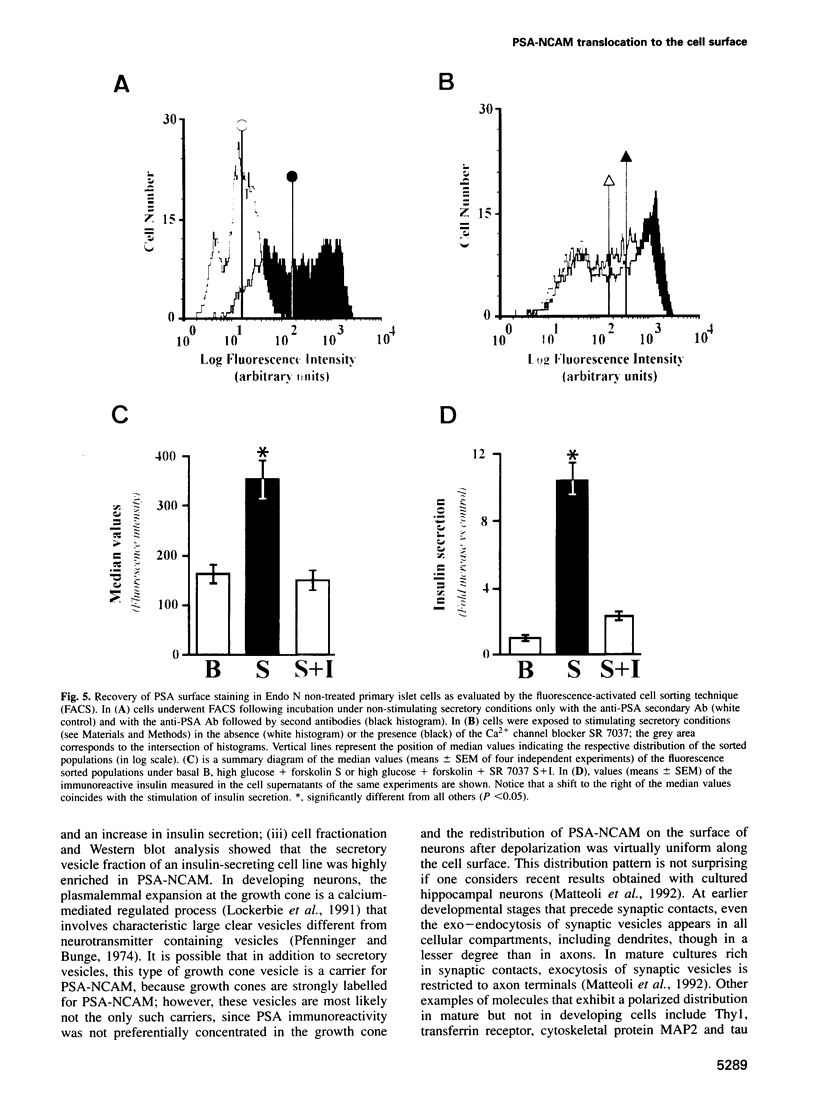



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron L. I., Chesselet M. F. Heterogeneous distribution of polysialylated neuronal-cell adhesion molecule during post-natal development and in the adult: an immunohistochemical study in the rat brain. Neuroscience. 1989;28(3):701–710. doi: 10.1016/0306-4522(89)90015-8. [DOI] [PubMed] [Google Scholar]
- Acheson A., Sunshine J. L., Rutishauser U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol. 1991 Jul;114(1):143–153. doi: 10.1083/jcb.114.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A., Becchetti A., Mannini A., Mugnai G., De Filippi P., Tarone G., Del Bene M. R., Barletta E., Wanke E., Olivotto M. Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J Cell Biol. 1993 Sep;122(5):1131–1143. doi: 10.1083/jcb.122.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfari M., Janjic D., Meda P., Li G., Halban P. A., Wollheim C. B. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992 Jan;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Montarolo P., Chen M., Kandel E. R., Schacher S. Inhibitors of protein and RNA synthesis block structural changes that accompany long-term heterosynaptic plasticity in Aplysia. Neuron. 1992 Oct;9(4):749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwald-Netter Y., Martin-Moutot N., Koulakoff A., Couraud F. Na+-channel-associated scorpion toxin receptor sites as probes for neuronal evolution in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1245–1249. doi: 10.1073/pnas.78.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L., Olive S., Poulain D. A., Theodosis D. T. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience. 1992 Jul;49(2):419–436. doi: 10.1016/0306-4522(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Deery D., Leahy J. L., Weir G. C. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989 Jan;38(1):49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- Breen K. C., Regan C. M. Developmental control of N-CAM sialylation state by Golgi sialyltransferase isoforms. Development. 1988 Sep;104(1):147–154. doi: 10.1242/dev.104.1.147. [DOI] [PubMed] [Google Scholar]
- Cameron P., Mundigl O., De Camilli P. Traffic of synaptic vesicle proteins in polarized and nonpolarized cells. J Cell Sci Suppl. 1993;17:93–100. doi: 10.1242/jcs.1993.supplement_17.14. [DOI] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994 Feb 3;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Disdier M., Morrissey J. H., Fugate R. D., Bainton D. F., McEver R. P. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992 Mar;3(3):309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Cohen J., Walsh F. S. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron. 1990 Aug;5(2):209–219. doi: 10.1016/0896-6273(90)90310-c. [DOI] [PubMed] [Google Scholar]
- Doherty P., Rowett L. H., Moore S. E., Mann D. A., Walsh F. S. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991 Feb;6(2):247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Finne J., Finne U., Deagostini-Bazin H., Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983 Apr 29;112(2):482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Fredette B., Rutishauser U., Landmesser L. Regulation and activity-dependence of N-cadherin, NCAM isoforms, and polysialic acid on chick myotubes during development. J Cell Biol. 1993 Dec;123(6 Pt 2):1867–1888. doi: 10.1083/jcb.123.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Goridis C., Brunet J. F. NCAM: structural diversity, function and regulation of expression. Semin Cell Biol. 1992 Jun;3(3):189–197. doi: 10.1016/s1043-4682(10)80015-7. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Kiss J. Z., Wang C., Rougon G. Nerve-dependent expression of high polysialic acid neural cell adhesion molecule in neurohypophysial astrocytes of adult rats. Neuroscience. 1993 Mar;53(1):213–221. doi: 10.1016/0306-4522(93)90299-u. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Dahm L., Schultz K., Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol. 1988 Dec;130(2):645–670. doi: 10.1016/0012-1606(88)90358-2. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Dahm L., Tang J. C., Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron. 1990 May;4(5):655–667. doi: 10.1016/0896-6273(90)90193-j. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The relationship of intramuscular nerve branching and synaptogenesis to motoneuron survival. J Neurobiol. 1992 Nov;23(9):1131–1139. doi: 10.1002/neu.480230906. [DOI] [PubMed] [Google Scholar]
- Li G. D., Milani D., Dunne M. J., Pralong W. F., Theler J. M., Petersen O. H., Wollheim C. B. Extracellular ATP causes Ca2(+)-dependent and -independent insulin secretion in RINm5F cells. Phospholipase C mediates Ca2+ mobilization but not Ca2+ influx and membrane depolarization. J Biol Chem. 1991 Feb 25;266(6):3449–3457. [PubMed] [Google Scholar]
- Lockerbie R. O., Miller V. E., Pfenninger K. H. Regulated plasmalemmal expansion in nerve growth cones. J Cell Biol. 1991 Mar;112(6):1215–1227. doi: 10.1083/jcb.112.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles J. M., Norrild B., Bock E. Biosynthesis of the D2 cell adhesion molecule: pulse-chase studies in cultured fetal rat neuronal cells. J Cell Biol. 1984 Jun;98(6):2077–2081. doi: 10.1083/jcb.98.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Perin M. S., Südhof T. C., De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992 May;117(4):849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P. Leukocyte interactions mediated by selectins. Thromb Haemost. 1991 Jul 12;66(1):80–87. [PubMed] [Google Scholar]
- Miragall F., Kadmon G., Husmann M., Schachner M. Expression of cell adhesion molecules in the olfactory system of the adult mouse: presence of the embryonic form of N-CAM. Dev Biol. 1988 Oct;129(2):516–531. doi: 10.1016/0012-1606(88)90397-1. [DOI] [PubMed] [Google Scholar]
- Møller C. J., Christgau S., Williamson M. R., Madsen O. D., Niu Z. P., Bock E., Baekkeskov S. Differential expression of neural cell adhesion molecule and cadherins in pancreatic islets, glucagonomas, and insulinomas. Mol Endocrinol. 1992 Aug;6(8):1332–1342. doi: 10.1210/mend.6.8.1406710. [DOI] [PubMed] [Google Scholar]
- Okada Y., Taniguchi H., Schimada C. High concentration of GABA and high glutamate decarboxylase activity in rat pancreatic islets and human insulinoma. Science. 1976 Nov 5;194(4265):620–622. doi: 10.1126/science.185693. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Bunge R. P. Freeze-fracturing of nerve growth cones and young fibers. A study of developing plasma membrane. J Cell Biol. 1974 Oct;63(1):180–196. doi: 10.1083/jcb.63.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G. Heterogeneity in pancreatic beta-cell population. Diabetes. 1992 Jul;41(7):777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Schuit F. C., in't Veld P. A., Maes E., Hooghe-Peters E. L., Van de Winkel M., Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985 Sep;117(3):824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Cameron R. S., Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994 Feb;4(1):63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Rougon G., Dubois C., Buckley N., Magnani J. L., Zollinger W. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986 Dec;103(6 Pt 1):2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougon G., Marshak D. R. Structural and immunological characterization of the amino-terminal domain of mammalian neural cell adhesion molecules. J Biol Chem. 1986 Mar 5;261(7):3396–3401. [PubMed] [Google Scholar]
- Rougon G. Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol. 1993 Aug;61(2):197–207. [PubMed] [Google Scholar]
- Rouiller D. G., Cirulli V., Halban P. A. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991 Nov;148(1):233–242. doi: 10.1016/0012-1606(91)90332-w. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Acheson A., Hall A. K., Mann D. M., Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988 Apr 1;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelman G. Insulin cells of pancreas extend neurites but do not arise from the neuroectoderm. Dev Biol. 1990 Dec;142(2):368–379. doi: 10.1016/0012-1606(90)90357-o. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Lee J. K. Cell lineage analysis of pancreatic islet development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol. 1987 Jun;121(2):454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Teng K. K., Greene L. A. Depolarization maintains neurites and priming of PC12 cells after nerve growth factor withdrawal. J Neurosci. 1993 Jul;13(7):3124–3135. doi: 10.1523/JNEUROSCI.13-07-03124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993 Dec;57(3):501–535. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Rougon G., Poulain D. A. Retention of embryonic features by an adult neuronal system capable of plasticity: polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5494–5498. doi: 10.1073/pnas.88.13.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Lee A. K., Wong J. G., Almers W. A triggered mechanism retrieves membrane in seconds after Ca(2+)-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994 Mar;124(5):667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., McCoy R. D., Vollger H. F., Wilkison N. C., Troy F. A. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Dickson G. Generation of multiple N-CAM polypeptides from a single gene. Bioessays. 1989 Oct;11(4):83–88. doi: 10.1002/bies.950110402. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Zhang H., Miller R. H., Rutishauser U. Polysialic acid is required for optimal growth of axons on a neuronal substrate. J Neurosci. 1992 Aug;12(8):3107–3114. doi: 10.1523/JNEUROSCI.12-08-03107.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop M. J., Dotti C. G. Membrane traffic in polarized neurons in culture. J Cell Sci Suppl. 1993;17:85–92. doi: 10.1242/jcs.1993.supplement_17.13. [DOI] [PubMed] [Google Scholar]