Abstract
Hippocampal neuronal populations exhibit multiple kinds of activity patterns, from the dominant theta rhythm during active exploration to high-frequency ripple-like activity during periods of relative inactivity. In animals, evidence is rapidly accruing that these high-frequency ripple activity patterns subserve retention of spatial learning performance. In a translational effort to address the possible function of offline hippocampal processes in humans, we measured spontaneous gamma activity during an awake rest period within a virtual spatial learning context. Whole-head magnetoencephalographic (MEG) recordings were taken while healthy participants (N = 24) quietly rested (eyes open) between encoding and retrieval phases of a hippocampal-dependent virtual Morris water maze task. Results are that fast gamma activity (80-140 Hz) in the septal or posterior region of the hippocampus (bilaterally) was positively correlated across participants with subsequent within-session spatial learning rate. Fast gamma did not predict initial retrieval performance following rest, failing to provide evidence of a direct link between spontaneous high-frequency activity patterns during awake rest and consolidation of previous spatial memories. The findings nevertheless are consistent with a prospective role for offline human hippocampal processes in spatial learning and indicate that higher spontaneous gamma activity in the septal hippocampal region is related to faster updating of spatial knowledge in familiar virtual surroundings.
Keywords: gamma activity, hippocampus, magnetoencephalography, memory consolidation, spatial learning, virtual reality
1. Introduction
Hippocampal neuronal populations exhibit multiple kinds of coherent activity that support learning and memory. The most studied network pattern is the theta rhythm, which takes the form of sinusoidal-like 4-10 Hz oscillations that are prevalent during exploratory behavior [1]. Elegant empirical and computational studies have provided key insights into theta (reviewed in [2]), including its potential role in regulating place cell firing [3, 4] as well as its link to spatial memory performance in animals [5] and humans [6-9]. In addition to theta, the hippocampus also exhibits rhythmic gamma oscillations (>30 Hz) and irregular sharp-wave activity (i.e., ripples) containing high frequency components (>80 Hz), particularly during non-exploratory behavioral states such as consummation, quiet rest and sleep [10, 11]. These high-frequency activity patterns have garnered significant interest for their possible role in ‘offline’ signaling between the hippocampus and neocortex, a candidate mechanism for long term memory consolidation [10, 12, 13].
Growing evidence indicates that offline high-frequency ripples are critical for learning [14]. Single-unit recordings have revealed that the same hippocampal neuronal populations that are active during spatial exploration show reactivation or ‘replay’ during ripple events after spatial exploration. Replay is thought to strengthen patterned activity and potentially drive hippocampal-neocortical signaling as a mechanism of memory consolidation [12]. Indeed, disrupting ripple activity leads to impaired memory performance [15-17]. Originally associated with sleep [18, 19], recent studies have confirmed that ripple-associated neuronal replay also occurs during awake rest periods [20]. Comparatively little is known about the functional relevance of these offline activity patterns for human learning [21]. Initial findings in epileptic patients are consistent with a role for ripple activity in verbal memory performance [22]. In that study, the number of rhinal cortical ripples, rather than hippocampal ripples, during awake rest was positively associated with subsequent item retrieval. No evidence in humans, however, has been reported in a spatial learning context. This is the ideal starting point for translating findings from animal research to the human hippocampus given that the former traditionally uses spatial learning paradigms to study hippocampal functioning.
We noninvasively studied dynamical brain activity during an awake rest period situated between encoding and retrieval phases of a hippocampal-dependent virtual reality Morris water maze task [23,24]. For these healthy participants, a positive association was previously reported between navigation-related hippocampal theta and task performance [8]. Magnetoencephalographic (MEG) recordings of participants’ quietly resting were used to reconstruct spontaneous hippocampal activity by adaptive beamforming [25, 26], which was correlated with spatial performance. We targeted spontaneous 80-140 Hz activity (‘fast gamma’) to match that used by Axmacher and colleagues [22] for quantifying hippocampal and rhinal cortical ripples from intracranial recordings in humans. We predicted that the overall magnitude of hippocampal region fast-gamma power measured during awake rest would positively correlate with subsequent spatial performance, indicating a possible contribution of offline hippocampal region high frequency activity in human spatial learning.
2. Material and Methods
2.1 Participants
Twenty-five healthy, right-handed adults completed the study and were paid for participation, as previously described [8]. One participant from the original sample was removed from the present analyses because of excessive head movement during the awake rest recording, leaving an N = 24 (12 women; age, mean ± SD = 29 ± 6 y). All participants gave informed consent in writing prior to participation. The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health.
2.2 Task procedure
Participants performed a virtual Morris water maze task, which is also described in Cornwell et al. [8]. Briefly, participants navigated two virtual pools to an escape platform. In one pool, participants were at risk of receiving electric shocks before reaching the platform (threat). In the other pool, they were completely safe from shocks (safe). Participants alternated between pools, performing four trials during each alternation from one of four starting positions (N, S, E, W), randomized without replacement. Other than the distal cues on the surrounding walls that can be used as landmarks for navigation, the pools were structurally identical. The threat manipulation was designed to reveal contributions of navigation-related hippocampal theta (2-8 Hz) to spatial cognition and anxiety [8], but was not relevant to the current goal of linking offline hippocampal gamma activity to subsequent spatial learning performance. Accordingly, navigation data were averaged between the two pool contexts.
Two tasks runs were administered. The first consisted of visible platform trials (encoding phase), with the platform's position fixed. There were 20 visible platform trials completed per pool followed by one probe trial per pool at the end of the run. During the probe trials the platform was removed unbeknownst to the participants. The second task run consisted of 20 hidden platform trials per pool (retrieval phase), with the platform fixed in the same position as during the first task run. During these trials, the platform was initially hidden but became visible after 15 s if it was not found beforehand. Participants were informed that the platform location in each pool context was fixed throughout the task. They were instructed to navigate as quickly and directly as possible to the platform on each trial regardless of whether it was visible or hidden.
In between the two task runs, a single MEG recording was collected while participants relaxed with their eyes open for 5 m. During the awake rest recording, participants were closely monitored by video camera to ensure that they remained awake during this period. They were not given any additional instructions other than to hold still, keep their eyes open and not sleep.
2.3 MEG acquisition
Neuromagnetic activity was measured by a 275-channel whole-head magnetometer (VSM MedTech, Inc., British Columbia, Canada) in a magnetically-shielded room using 3rd-gradient balancing for active noise cancellation. For the awake rest recording, data were collected at 1200 Hz for 5 m with a 0-300 Hz bandpass. Fiducial coils placed at the nasion and preauricular sites were energized during the run to record head position continuously and used for offline coregistration with each participant's anatomical magnetic resonance images (MRI) that were acquired in a separate session. MEG data from the task runs are presented elsewhere [8].
2.4 Resting-state source analyses
A minimum-variance adaptive beamformer algorithm was utilized to estimate regional oscillatory power during rest ([25, 26]; for a similar resting-state analytic approach, see Rutter et al. [27]). Signal covariance across the sensor array was computed from a single 280 s epoch (after removing the first and last 10 s of the 5 m recording). This was done separately for data bandpass filtered in the slow gamma (30-80 Hz) and fast gamma (80-140 Hz) frequency bands. A multi-sphere source space model generated from participants’ MRIs was used for source power estimation (5-mm spatial sampling grid). Band-specific source power at each grid point or voxel was integrated over the entire 280-s epoch and divided by a constant noise estimate, which was derived from the same covariance matrices for each frequency band, to correct for depth biases in beamformer power estimates (pseudo-Z metric). Using Analysis of Functional NeuroImages (AFNI, [28]) individual subject source volumes were within-volume normalized, co-registered to their anatomical MRIs and spatially warped to a Talairach template for group analyses.
Based on our a priori hypothesis, we performed a targeted analysis of bilateral hippocampus. We used the same anatomical masks as in Cornwell et al. [8], which were created with the automated Talairach Atlas Daemon [29] and resampled to the 5-mm grid of the source imaging data. Spontaneous oscillatory power estimates were averaged for voxels in septal (posterior, –45 < y < –29 mm in Talairach space) and temporal (anterior, –9 > y > –20 mm) thirds of the left and right hippocampus and extracted for analyses in SPSS 18. The intermediate third of the hippocampus was not included in our analyses in order to maximize independent septal and temporal hippocampal source power estimates [8]. Figure 1 shows short segments from the entire time courses of fast gamma activity reconstructed from sample voxels located within septal and temporal hippocampal subregions.
Figure 1.
Example virtual sensor time series of spontaneous fast gamma (80-140 Hz) hippocampal activity from two participants. Septal and temporal (Tem.) hippocampal activity (left and right) was reconstructed by adaptive minimum-variance beamformers and source power was integrated over a 280-s window during which participants rested. Brief periods of increased fast gamma power can be observed in the time window displayed (1.53 s), which may be related to hippocampal ripple events observed rodents.
Although the standardized atlas-based masks are centered at the left and right hippocampus, we do not make the strong claim that the source power estimates are exclusively measuring activity of the hippocampus proper. First, through resampling to a 5-mm grid, it is likely that the masks slightly extend 1-2 mm into surrounding parahippocampal cortices in some places. Second, spatial normalization procedures that rely on whole-brain parameters can lead to coregistration errors and noisy regional measurements given inter-subject anatomical variability of medial temporal cortices [30]. Accordingly, the power estimates from septal and temporal subregions likely contain some mixture of source activity from the hippocampus proper and surrounding parahippocampal cortices. Nevertheless, we have shown previously that adaptive beamformers are able to produce sufficiently independent estimates to identify functional differences between septal and temporal thirds of the hippocampus [8]; and through multiple regression analyses here, spatial selectivity can be addressed at an important theoretical level (i.e., subregions along the longitudinal axis) even if hippocampal source activity cannot be completely distinguished from adjacent parahippocampal source activity.
Bivariate correlations between subregion power estimates and performance metrics were first calculated for descriptive purposes. Multiple regression analyses were performed to test for a selective association between septal hippocampal fast gamma power and subsequent spatial performance, controlling for temporal hippocampal gamma power to demonstrate regional specificity and controlling for septal hippocampal slow gamma power (30-80 Hz) to demonstrate spectral specificity (4 regressors of interest). Separate analyses were carried out for the left and right hippocampal spontaneous power estimates to guard against overfitting the regression models. In total, four multiple regression analyses were conducted, and to evaluate the overall model fit in each case, family-wise alpha level was kept at .05 using a modified-Bonferroni correction method [31].
2.5 Whole-brain analysis of correlations
As a secondary analysis, whole-brain images were generated to visualize the volumetric distribution of between-subject correlation coefficients between fast gamma power and spatial learning rate. This analysis was intended to show the degree to which the anatomically-based masks overlap with clusters of high correlation coefficients in the volumetric data for comparative purposes and to validate the region of interest approach used for the main analyses. With high overlap, we can be confident that the masks are selectively capturing signal from the hippocampal region and the data are not being biased by spatial leakage from a large cluster centered elsewhere (e.g., superior temporal cortex). We also computed the Euclidean distance in standardized space between the local maxima within temporal cortices and the left and right hippocampal masks to quantify the amount of disparity. The choice of not using whole-brain analyses to draw our primary inferences was motivated by the substantial loss of statistical power that stems from multiple test correction when the majority of these tests are outside the focus of the present investigation.
2.6 Performance measures
Probe (platform-less) trials were included at the end of the visible platform run to establish that participants had, at least, partially encoded the platform's location in relation to the distal cues in the pools before the awake rest recording. We calculated the percentage of the total trial time spent in the correct quadrant (i.e., where the platform had been located) on these probe trials to identify systematic biases in their search behavior that would be best explained as reflecting accrual of knowledge of the platform's location (>25%).
Heading error on the subsequent retrieval trials in the hidden platform run was used as the dependent variable to address whether spontaneous hippocampal region gamma during awake rest is related to spatial learning performance. These data, which consist of 5 blocks of 4 trials per pool, were analyzed previously to study performance differences between the threat and safe pools (see Figure 2 in [8]). Because of the variable starting positions within each block, mean heading error is sensitive to the extent to which participants use one or more distal cues as landmarks in selecting their paths to the hidden platform.
Figure 2.
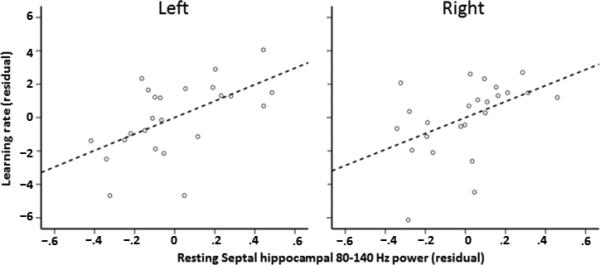
Partial regression plots (with least square lines) show relationships between left and right spontaneous septal or posterior hippocampal fast gamma (80-140 Hz) power and within-session spatial learning rate (1st- 5th block linear trend).
After averaging threat and safe navigation data, we focused on two dependent variables: 1st block mean heading error, which taps initial retrieval performance following rest, and linear change in mean heading error from the 1st to 5th block (using a weighted sum [2, 1, 0, –1, –2] for mean heading error across blocks), which operationalizes within-session spatial learning rate. For this latter measure, positive values reflect better performance as a function of trial block (i.e., within-session spatial learning). While initial retrieval performance is similar to the kind of ‘all-or-nothing’ test commonly used to measure declarative memory performance (e.g., [22]), within-session performance change captures gradual refinement and dynamic updating of a spatial memory that is driven, in part, by new encoding.
3. Results
3.1 Behavioral performance
To determine whether participants had gained knowledge of the platform's location prior to the awake rest period, we quantified their search biases on the final probe (platform-less) trials in the first task run. Averaged across pool contexts, participants spent significantly more time searching in the quadrant in which the platforms had been previously located (35%) than expected by chance (25%), one-sample t(23) = 2.22, p = .037. This result suggests that participants had acquired some knowledge of the platform location before the rest recording. Moreover, mean linear change in heading errors across hidden platform trials in the second task run (after rest) was significantly greater than zero, one-sample t(23) = 3.26, p = .003. This improvement in navigation performance from the 1st to the 5th block of hidden trials indicates that participants gradually refined their knowledge of the platform's location (i.e., within-session spatial learning).
3.2 Spontaneous hippocampal region gamma – spatial performance relationship
Table 1 shows Pearson correlation coefficients for hippocampal region gamma power estimates and heading error performance variables for the hidden platform run. Regression models were run separately for left and right hippocampal region gamma power (see section 2.4) with 1st block mean heading error and linear change in mean heading error. The model fits for 1st block mean heading error regressed on left or right hippocampal region (slow and fast) gamma power estimates were not significant, F's < 1. This outcome indicates that left and right spontaneous hippocampal region gamma during awake rest showed no evidence of being related to retrieval performance on the first block of hidden trials following rest.
Table 1.
Pearson correlation coefficients (N = 24) for spontaneous high-frequency oscillatory power estimated from hippocampal subregions during awake rest and initial spatial retrieval performance (1st block) and subsequent spatial learning rate (linear trend).
| Left hippocampus | Right hippocampus | |||
|---|---|---|---|---|
| Frequency | Anterior | Posterior | Anterior | Posterior |
| 1st block mean heading error | ||||
| 80-140 Hz | −.05 | .03 | .07 | .05 |
| 30-80 Hz | −.08 | −.18 | .27 | −.07 |
| 1st-5th block linear trend | ||||
| 80-140 Hz | .16 | .47 | .14 | .53 |
| 30-80 Hz | .17 | .16 | .10 | .22 |
Note: For 1st block mean heading error, negative coefficients indicate that straighter path trajectories are linked to greater spontaneous power estimates. For 1st-5th block linear trend, positive coefficients indicate that greater linear decreases in error across blocks are related to greater spontaneous power estimates.
For the next two regression analyses, we included 1st block mean heading error as an additional regressor because as a linear change metric, linear change in mean heading error (spatial learning rate) is likely to be partly dependent upon (and thus related to) initial heading error values. The model fit for spatial learning rates regressed on left hippocampal region gamma power was significant, F(5,18) = 3.91, adjusted r2 = .39, p < .05 (corrected). Fast gamma (80-140 Hz) power in the left septal subregion showed a positive association with learning rate, t(18) = 2.37, p = .029, β = .41, partial r = .49, variance inflation factor (VIF) = 1.12 (Figure 2). The model fit for spatial learning rates regressed on right hippocampal region gamma power was also significant, F(5,18) = 4.21, adjusted r2 = .41, p < .05 (corrected). Fast gamma power in the right septal subregion showed a positive association with learning rate, t(18) = 2.46, p = .024, β = .49, partial r = .50, VIF = 1.51 (Figure 2). All other hippocampal-based predictor variables for each model, including slow gamma power estimates, failed to show significant associations with learning rates, all p's > .56.
3.3 Secondary whole-brain analysis of fast gamma – spatial performance correlations
Figure 3 shows whole-brain images of correlation coefficients (Pearson's r) for fast gamma power and linear change in mean heading error, which ranged from –.64 to .63. Clusters of high correlation coefficients can be seen to overlap with the left and right hippocampal masks, particularly around the septal (posterior) subregions. The maximum correlation in the right temporal cortex (xyz = [28, –27, –5], r = .61) was positioned 5 mm rostral to the right hippocampal mask. The maximum correlation in the left temporal cortex (xyz = [–17, –42, 2], r = .63) was positioned 11.2 mm caudomedial to the left hippocampal mask. These observations indicate good correspondence between the whole-brain pattern of correlations and the a priori anatomical masks that were used for extracting hippocampal source power estimates. This outcome provides reasonable assurance that the signal extracted from the anatomically-based masks for the main analyses originated from the hippocampal region rather than being the product of spatial leakage from source(s) centered elsewhere.
Figure 3.
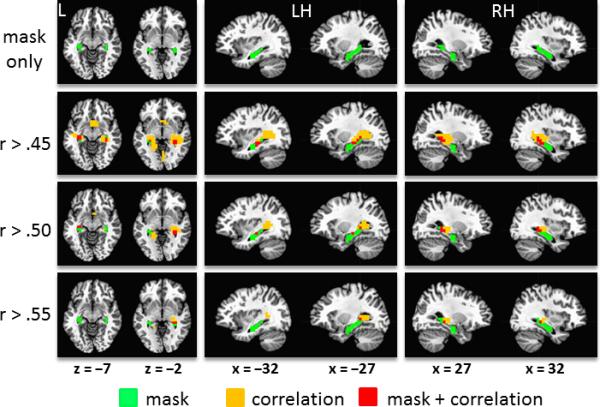
Volumetric distribution of correlation coefficients for fast gamma activity (80-140 Hz) during awake rest and within-session spatial learning rate. The resampled bilateral hippocampal mask (in green) is overlayed on a standardized brain template (top row). The correlation data are also overlayed on a standardized brain template and thresholded at Pearson's r > .45, .50 and .55. (bottom rows). Red voxels are those that contain correlation coefficients that exceed the threshold and are included in the anatomical masks. Orange voxels also contain correlation coefficients that exceed the threshold but are not included in the masks. Images are in neurological orientation (left = left). L = left, LH = left hemisphere, RH = right hemisphere.
4. Discussion
We found that spontaneous fast gamma (80-140 Hz) activity measured during awake rest from the septal (posterior) region of the hippocampus correlates with subsequent spatial learning. Those participants who showed the most within-session learning on the virtual Morris water maze task evidenced greater offline septal hippocampal fast gamma activity than those who showed little change in spatial performance. This finding complements growing evidence in humans that theta activity (4-8 Hz) in the septal hippocampal region during virtual navigation (‘online’) indexes spatial performance level [6-9], suggesting that this structure's functional role in human spatial learning and memory spans periods of active navigation and relative inactivity. Our data demonstrate both regional and spectral specificity in the offline human hippocampal region dynamics that are correlated with spatial learning, extending electrophysiological evidence obtained with invasive measurements in epileptic patients [22].
Consistent with previous work [8], we observed that septal hippocampal activity is particularly linked to superior spatial performance, compared to temporal (anterior) hippocampal activity that does not appear to make a substantial contribution in this regard. This fits with a large corpus of data in animals indicating a predominant role for the septal third of the hippocampus in cognitive processes such as spatial learning and memory [32-34]. While the temporal third of the human hippocampus likely mediates spatial processing to an extent [8, 35], it is believed to also support a wider range of phenomena, including nonspatial processes [36] and affective functions [8, 37]. Unlike our previous MEG work that predominantly implicated online left hippocampal theta in spatial performance, the present data supports the role of bilateral septal hippocampal gamma in offline processes (Table 1). Individually, both left and right septal hippocampal region gamma accounted for significant variance in spatial performance, suggesting that these offline processes do not show a lateralized functional bias.
Fast gamma during awake rest was specifically predictive of spatial learning rate after controlling for the contribution of slow gamma (30-80 Hz) power estimated from the same hippocampal subregions. The selective relationship for spontaneous fast gamma activity is in line with claims of functional heterogeneity of oscillatory activity along the gamma spectrum [38, 39]. Together with evidence that during active, virtual navigation theta oscillatory (4-8 Hz) activity is correlated with spatial performance [6-9], the current findings extend to humans the view that the functional importance of specific hippocampal population dynamics varies by behavioral context [10]. During active exploration, hippocampal place cells discharge systematically in relation to the ongoing theta rhythm [40], such that the relative theta phase of firing of these cell assemblies encodes an animal's location more precisely than their mean firing rate [3, 4]. It is thought that by temporally constraining the firing of sequentially-activated place cell assemblies as an animal navigates through its environment, theta oscillations link these cell assemblies into higher-order dynamic structures embodying spatiotemporal context [2]. Accordingly, these theta-centered dynamics may support spatial encoding.
After exploring an environment, these cell assemblies are known to fire in the same (and also reverse) temporal sequence during sleep and awake rest, a phenomenon called replay [20, 41]. Neuronal replay is embedded in high-frequency ripple events, such that entire sequences of population activity are reenacted in time-compressed fashion. This compression may facilitate spike-timing-dependent plasticity and support memory consolidation by facilitating signal transfer from the hippocampus to the neocortex [42]. Indeed, recent manipulations in animals support a causal role for high-frequency ripple activity during sleep and awake rest in retention of long term memories [15-17]. In humans, similar electrophysiological phenomena in sleep and awake rest have been reported [21, 43], but functional evidence is limited. Axmacher et al. [22] reported that number of ripple events in rhinal cortex recorded invasively during awake rest correlates with subsequent item memory retrieval. Our data extend this work to a possible role for high-frequency activity in human spatial learning.
We found that fast gamma activity predicted the linear decrease in mean heading error across the five hidden trial blocks (within-session learning rate), but did not correlate with performance on the 1st trial block (initial retrieval performance). The lack of a relationship between offline fast gamma activity and initial retrieval performance prevents a straightforward link to be drawn between the former and spatial memory consolidation. Nonetheless, offline hippocampal region fast gamma may function more broadly than simply consolidating past experiences. Notably, hippocampal ripple activity has been implicated in preplay in which novel sequences of population activity are generated that are related to recent experiences but do not specifically reenact prior population activity patterns [44]. It is speculated that preplay may contribute to facilitation of learning when a novel task is introduced gradually [44]. Thus, one possible explanation is that offline hippocampal region fast gamma mediates constructive processes that facilitate the prospective use of distal cues to navigate in a familiar environment when local information (the platform) is no longer available [45]. Spontaneous hippocampal activity may, in short, optimize subsequent integration of new information as spatial knowledge of the environment is dynamically updated, which would be reflected in within-session learning rates.
An alternative hypothesis is that the magnitude of offline fast gamma reflects general integrity of the hippocampus and surrounding cortical regions. Accordingly, the observed relationship could be trait-related in the sense that good spatial learners may generally show greater high-frequency activity compared to poor learners regardless of the experimental context. A second measurement of spontaneous activity taken outside the critical learning context (e.g., before exposure to the task) would address this possibility. Reconfiguring the task procedures may allow for properly testing these hypotheses, and overcome the inferential limitations imposed by the current experimental design. Finally, it should be recognized that high frequency oscillations generally have low amplitudes and reliable estimation of fast gamma from hippocampal sources with MEG may be especially challenging. However, in animals, ripple events can measure several times the amplitude of theta oscillations [11], suggesting that noninvasive MEG may be a promising method of capturing high as well as low frequency hippocampal population activity.
In summary, these findings in humans converge with a growing literature in animals on the role of spontaneous high frequency activity patterns during awake rest and sleep in learning and memory. Using a highly translational model of spatial learning (i.e., Morris water maze), we observed that fast gamma activity that was measured noninvasively with MEG during awake rest from the septal hippocampal region correlates with subsequent spatial learning rate. This complements evidence that theta activity measured during active navigation from the same hippocampal region also contributes to spatial performance [8]. Further specification of the repertoire of hippocampal region dynamics during active and rest periods may inform on their functional importance in mediating learning and memory in humans to keep pace with recent insights from animal models.
Highlights.
Spontaneous hippocampal region gamma correlates with spatial learning in humans
Gamma in the septal hippocampal subregion is associated with superior learning
Fast gamma between 80-140 Hz is especially linked to spatial performance
Acknowledgements
This work was funded by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampalentorhinal system. Nat Neurosci Rev. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 4.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1998;6(2):149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.McNaughton N, Ruan M, Woodnorth MA. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- 6.Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5900. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornwell BR, Salvadore G, Colon-Rosario V, Latov DR, Holroyd T, Carver FW, Coppola R, Manji HK, Zarate CA, Jr., Grillon C. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals. Am J Psychiatry. 2010;167:836–844. doi: 10.1176/appi.ajp.2009.09050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–1859. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N. Movement-related theta rhythm in humans: Coordinating self-directed hippocampal learning. PLoS Biol. 2012;10(2):e1001267. doi: 10.1371/journal.pbio.1001267. doi: 10.1371/journal.pbio.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsaki G. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: Influence of dentate and entorhinal cortical activity. J Neurosci. 2011;31:8605–8161. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;21:452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Rev Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 17.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1457. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–920. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Van Quyen M, Staba R, Bragin A, Dickson C, Valerrama M, Itzhak Fried, Engel J. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30:7770–7782. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 23.Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, Deuschl G, Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2011;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 24.Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20(4):481–491. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- 25.Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25(2):249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 27.Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, Weinberger DR, Coppola R. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 29.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yassa MA, Stark CEL. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Rom DM. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika. 1990;77:663–665. [Google Scholar]
- 32.Morris RGM, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 33.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolbers T, Büchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolley DG, Vermaercke B, de Beeck HO, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. Sex differences in human virtual water maze performance: Novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–414. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Seidenbecher T, Laxmi TR, Stork O, Pape H-C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301(5634):846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 38.Dalal SS, Vidal JR, Hamame CM, Ossandon T, Bertrand O, Lachaux J-P, Jerbi K. Spanning the rich spectrum of the human brain: slow waves to gamma and beyond. Brain Struct Funct. 2011;216:77–84. doi: 10.1007/s00429-011-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaona CM, Sharma M, Freudenburg ZV, Breshears JD, Bundy DT, Roland J, Barbour DL, Shalk G, Leuthardt EC. Nonuniform high-gamma (60-500 Hz) power changes dissociate cognitive task and anatomy in human cortex. J Neurosci. 2011;31:2091–2100. doi: 10.1523/JNEUROSCI.4722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27(14):3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 44.Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–403. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buzsaki G, Lopes da Silva F. High frequency oscillations in the intact brain. Prog Neurobiol. 2012;98:241–249. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


