Abstract
In chronically inflamed tissues, such as those affected by autoimmune disease, activated T helper (Th) cells often colocalize with monocytes. We investigate here how murine Th cells influence the phenotype and function of monocytes. The data demonstrate that Th1, Th2 and Th17 subsets promote the differentiation of autologous monocytes into MHC II+, CD11b+, CD11c+ DC that we call DCTh. While all Th subsets induce the formation of DCTh, activated Th17 cells uniquely promote the formation of IL-12/IL-23 producing DCTh (DCTh17) that can polarize both naïve and Th17 cells to a Th1 phenotype. In the inflamed CNS of mice with Th17-mediated experimental autoimmune encephalomyelitis (EAE), Th cells colocalize with DC, as well as monocytes, and the Th cells obtained from these lesions drive the formation of DCTh that are phenotypically indistinguishable from DCTh17 and polarize naive T-cells toward a Th1 phenotype. These results suggest that DCTh17 are critical in the interplay of Th17- and Th1-mediated responses and may explain the previous finding that IL-17 secreting Th cells become IFNγ secreting Th1 cells in EAE and other autoimmune disorders.
Introduction
Monocytes comprise greater than 10% of circulating leukocytes in humans and approximately 4% in mice (1). These cells rapidly infiltrate inflamed tissues and are renowned for their plasticity (2–4). Upon entering inflamed tissues, monocytes readily differentiate into inflammatory dendritic cells (DC) that share multiple characteristics with conventional DC and tissue-resident macrophages including F4/80 positivity and reduced CD11c and Ly6C expression (5, 6). Our previous studies indicate that inflammatory DCs arise following direct interaction with CD4+ T-helper (Th) cells in humans (7). However, the role these cells play in inflammation has not yet been elucidated. Experimental mouse models of disease are ideally suited to study this question, since the key disease-causing Th cells can be generated in vitro or obtained from inflamed tissue, and tested for their ability to induce the differentiation of monocytes into inflammatory DC. In the current study, we sought to investigate the biology and impact of Th cell driven DC formation in experimental allergic encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS) in which Th cells and circulating monocytes are known to play pathogenic roles.
In EAE, transfer of myelin-reactive Th cells robustly and reliably induces a cascade of events that results in autoimmune demyelination (8). Much is known about the roles of specific Th subsets in both the induction and progression of EAE. Autoreactive IFNγ producing Th1 cells or IL-17 producing Th17 cells can induce disease, while Th2 and Treg cells are believed to be protective (9). Furthermore, mice lacking the Th1 transcription factor, Tbet, or the Th17 transcription factor, RORγt, have reduced EAE symptoms compared to control mice, demonstrating that both the Th1 and Th17 pathways are involved in EAE (10) (11). Interestingly, Hirota et al. recently showed that the majority of IFNγ expressing Th1 cells within the inflamed spinal cord, previously produced IL-17A, demonstrating the plasticity of the Th17 phenotype in EAE (12). However, the cell types responsible for mediating conversion of Th17 cells into Th1 cells in EAE and the significance of this process in disease resolution remain unclear.
In addition to Th cells, monocytes are thought to play an essential role in the development of EAE (13). Indeed, monocytes are often found juxtaposed with Th cells in the CNS of affected mice and the extent of monocyte infiltration correlates strongly with the severity of disease (14). Within the inflamed CNS, activated Th cells express multiple molecules, including CD40L, IFNγ, M-CSF and GM-CSF that enhance the antigen presentation and differentiation potential of monocytes. In fact, bone-marrow chimera mice with myeloid cells deficient in the GM-CSF receptor are completely protected from EAE induction with adjuvants, often referred to as ‘active’ EAE induction (15). Taken together, these findings suggest that factors expressed by CD4+ Th cells act on monocytes and facilitate EAE induction and progression.
Based on our previous observation that activated human Th subsets induce the formation of phenotypically distinct Mo-DC (16), we hypothesized that activated murine Th subsets would behave in a similar manner. We report here that murine Th1, Th2 and Th17 cells are capable of inducing the formation of distinct Mo-DC, termed DCTh1, DCTh2 or DCTh17, respectively. These DCTh subsets have similar cell surface phenotypes, but differ in their cytokine production and T-cell activating ability. Most strikingly, Th17 cells induce the formation of IL-12 producing DCTh17 with potent Th1 polarizing capacity and the ability to convert Th17 cells into IFNγ expressing Th1 cells. Further, CD4+ T-cells taken from the inflamed spinal cords of mice with Th17-mediated EAE also induce the formation of Mo-DC that secrete IL-12p70 and drive Th1 polarization. These findings suggest that such DC serve as a critical link in determining the balance between Th17 and Th1 polarization during immunity and EAE progression.
Materials and Methods
Mice
WT C57BL/6, 2D2, CD40L KO, GM-CSFR KO and IL-12p35 KO female mice were purchased from the Jackson Laboratory and used between 6 and 14 weeks of age. OT-II TCR transgenic Rag2−/− mice were purchased from Taconic. All mice were housed in an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility and maintained in specific pathogen-free conditions. Animal experiments were approved and conducted in accordance with Stanford University APLAC# 13605.
Generation of BM chimeras
6 week-old C57BL/6 B6 mice were placed on antibiotic-supplemented chow. 1 week later they received 5 gy total body irradiation and were injected via tail vein with 10 × 106 syngeneic WT BM or BM from IL-12p35 KO mice. They were allowed to recover for 1 month on antibiotic food before returning to a normal diet. 9 weeks later (13 weeks after irradiation) reconstitution with IL-12p35 KO BM was confirmed by PCR on circulating blood cells.
T-cell polarization and isolation
Splenocytes were depleted with biotinylated anti-CD8α antibody (Biolegend, clone 53.6.7) followed by anti-biotin microbeads (Miltenyi). The remaining cells were cultured in complete media consisting of RPMI media (Gibco) with 100 U/mL penicillin, 100μg/mL streptomycin, 2mM L-glutamine, 50μM 2-ME, 10% fetal calf serum with 1μg/ml anti-CD3 (BD clone 145-2C11) to generate bulk CD4+ T-cells or in the addition of the following polarizing cocktail: Th1 – 10ng/ml IL-12 (R&D Systems); Th2 –20ng/ml IL-4 (R&D Systems), 20μg/ml anti-IL-12 p40 (eBioscience clone C17.8) and 20μg/ml anti-IFNγ (eBioscience clone R4-6A2); Th17 – 1ng/ml rhTGFβ1 (R&D Systems), 20ng/ml IL-6 (R&D Systems) and 10μg/ml anti-IFNγ. T-cells were positively selected from these cultures after 96 hours with Thy1.2 magnetic beads (Miltenyi). In some experiments, IL-17A secreting Th17 cells were sorted to high purity using a FACS Aria II (BD Biosciences) and IL-17A cytokine capture reagents (Miltenyi).
Monocyte, DC and macrophage generation
Bone marrow from femurs, tibias and hips was prepared after cleaning the bones and grinding in a mortar and pestle. Monocytes were negatively selected to ~90% purity via the murine monocyte enrichment kit (Stem Cells Inc). Monocytes were cultured for 5 days in the presence of 50ng/ml GM-CSF (Peprotech) + 20ng/ml IL-4 (Peprotech) to generate DC or in 50ng/ml M-CSF (Sigma) to generate macrophages.
Generation and Activation of DCTh Subsets
Polarized T-cells were purified after 96 hours of culture using Thy1.2 positive selection (Miltenyi) and cultured at a ratio of 1 T-cell: 10 monocytes in the presence of 10ng/ml rmIL-2 (R&D Systems) for 5 days in tissue culture treated plates (Corning). At this point, some cocultures were imaged using a Leica DM IRB inverted microscope and Metamorph software. In some experiments cells were activated via the addition of 1μg/ml standard LPS (TLR 2 and 4 agonist), 1μg/ml ultra-pure LPS (TLR 4 agonist), 200ng/ml PAM3CSK4 (TLR1/2 agonist), 108/ml Heat-Killed Listeria Monocytogenes (mainly TLR2 agonist), 1μg/ml Imiquimod (TLR7 agonist), 1μg/ml Poly IC (TLR3 agonist) or 10μg/ml CpG ODN 2336 (TLR9 agonist) (Invivogen). DC were then purified using CD11c positive selection. Macrophages and monocytes were selected using CD11b positive selection (Miltenyi).
DCTh stimulatory and polarization capacity
Purified DCTh were pulsed with 2.5μg/ml MHC II restricted ISQ peptide (OVA 323–339) (New England Peptides) or 2.5μg/ml MHC II restricted MOG peptide (33–55) in complete media for 90 minutes at 37°C. In some experiments 1μg/ml of LPS was added at the time of pulsing. Cells were then washed twice in PBS and cultured with CD4+ CD62L+ T-cells from OT-II or 2D2 mice purified by CD4 negative selection and CD62L positive selection (Miltenyi). After 3–4 days, cells were pulsed with 1 μCi/well of H3-thymidine and cultured for an additional 18 hours before being harvested by Harvester 400 (Tomtec). Radioactivity was measured by a 1450 MicroBeta counter (LKB Wallac).
Flow cytometry
Cells were blocked with FC block (Biolegend) and then stained with fluorochrome labeled antibodies to: CD4, CD11b, CD11c, Ly6c, Ly6g, CD40, CD86, CD209, MHC II, CD45, Thy1.2, IL-12p40, IFNγ, IL-4 and IL-17A (From Biolegend, BD Biosciences and eBioscience) and analyzed using an LSRII (BD Biosciences) and FlowJo analysis software (Treestar). For intracellular cytokine staining T-cells were stimulated with PMA and ionomycin (Life Technologies) for 4 hours in the presence of brefeldin A (BFA) (BD Biosciences) and then fixed and permeabilized with appropriate buffers (eBioscience).
ELISA
Antibody pairs for IL-12(p70/p40), IL-23(p19/p40), IFNγ, IL-4, IL-17A, IL-17A/F and GM-CSF (eBioscience) were used to detect cytokine in cell-free supernatants.
Differential interference contrast (DIC) and bright field microscopy
DIC images were acquired on cells in cover-slip bottom dishes using a Zeiss LSM 700 using Zen software (Zeiss). Bright field images were acquired on cells in tissue culture treated plates using a Leica DM IRB and Metamorph software (Molecular Devices).
Immunofluorescence
Mice were perfused with PBS and the spinal cords were fixed then snap frozen in O.C.T. compound (Tissue Tek). Slides were cut to 10uM, fixed in acetone and stained overnight with FITC rat anti-mouse CD11b (M1/70 BD), biotin hamster anti-CD11c (HL-3, BD), and rabbit polyclonal anti-CD3 (Vector). After washing, the slides were stained with donkey anti-rat A488, goat anti-hamster A647 or donkey anti-rabbit A594 (Life Technologies). After incubating in DAPI (Life Technologies)/PBS the cells were mounted in permafluor (Thermo Scientific) and imaged with a Leica DM2500 laser scanning confocal microscope and analyzed using Leica LAS AF software.
EAE induction
C57BL/6 female mice were injected with 200μg MOG peptide aa35-55 (Stanford Core Facility) emulsified in complete Freund’s adjuvant (Sigma). Mice were injected on day 0 and day 2 with 400ng pertussis toxin (Sigma). On day 12 after injection, splenocytes and LN cells were frozen. Cells were thawed when needed and cultured in 10μg/ml MOG + 10ng/ml IL-23 for 3 days to skew toward a Th17 phenotype prior to intraperitoneal transfer into naïve mice to induce passive EAE (17). Disease activity was scored on a 0–5 scale using standard methodology (18).
Purification of CD4+ T-cells from spinal cords
At various times after passive EAE induction, mice were euthanized and then perfused with PBS to remove circulating leukocytes from the spinal cord. The spinal cord was then removed and incubated in 2mg/ml collagenase 4 (Worthington) and 10 units/ml DNAse I (Sigma) in PBS for 45 minutes at 37°C. The cells were then layered over a 70% Percoll (GE Healthcare) gradient to enrich for immune cells. To further enrich for Th-cells, a CD4 MACS negative enrichment kit was used (Miltenyi).
Statistical analyses
An unpaired student’s t-test (2-tailed) with a 95% confidence interval was performed in Prism (Graphpad) to analyze all experimental data unless otherwise stated. Error bars represent +/− SEM. P<0.05=*; P<0.01=**; P<0.001=***.
Results
Activated Th cells promote Mo-DC formation in vitro
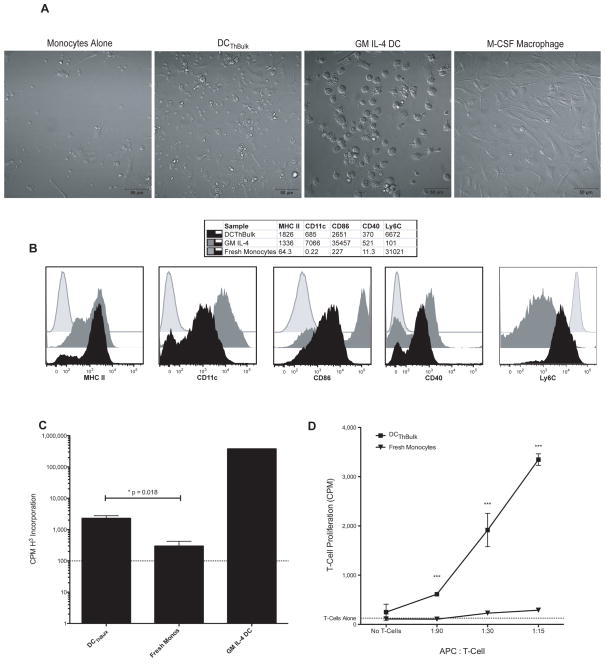
To determine whether activated murine Th cells can induce monocytes to differentiate into DC, we established in vitro monocyte and Th cell cocultures. Bulk CD4+ T-cells were activated with an anti-CD3 antibody for 96 hours (ThBulk), and then isolated with anti-Thy magnetic beads and cultured at a 1:10 ratio with freshly isolated autologous bone marrow monocytes. Monocytes cultured alone, or with exogenous M-CSF or exogenous GM-CSF and IL-4 (GM IL-4 DC), served as controls. After 5 days of coculture, we imaged the cells with DIC microscopy (Fig 1A). Monocytes cultured in media alone displayed poor viability. However, monocytes cultured with activated bulk CD4+ T-cells were viable and showed similarities to both GM IL-4 DC and M-CSF macrophages in their size, shape and granularity. They could easily be distinguished from T-cells based on their larger size and dendritic morphology (Supplementary Movies A). Monocytes cultured with activated T-cells displayed more surface MHC II, CD11c, CD86 and CD40, and less Ly6C, than freshly isolated monocytes (Fig 1B). Based on their morphology and surface phenotype, these ThBulk-induced, monocyte-derived cells resemble DC, and on this basis we refer to them as DCTh.
Figure 1. In Vitro Activated Bulk T-helper Cells Promote the Differentiation of Monocytes into Dendritic Cells.
A) Bone-marrow monocytes were cultured at a ratio of 10:1 with activated bulk CD4+ T-cells (ThBulk) for 5 days in the presence of 10ng/ml IL-2 (DCThBulk), or as controls, monocytes were cultured alone, with GM-CSF and IL-4, or with M-CSF. The cells were then imaged with DIC microscopy. The scale bar represents 50 microns. B) Cell surface phenotype of DAPI−, Thy1.2−, CD11b+ cells after 5 days of culture with GM-CSF and IL-4 (solid gray), with ThBulk cells and IL-2 (solid black) or fresh after isolation (gray line). Median fluorescence intensity (MFI) for each marker is shown in the table above. Data are representative of more than three independent experiments. C and D) DCTh or fresh monocytes were purified, pulsed with MHC II-restricted OVA peptide and 1μg/ml LPS for 90 minutes, washed and then cultured in triplicate with T-cells for 3 days and pulsed with H3-thymidine for the final 18 hours. The dotted line shows the mean proliferation of the T-cells in the absence of APC. The data shown are representative of more than 3 independent experiments. C) DCThBulk, fresh bone marrow monocytes and GM IL-4 DC were pulsed with 2.5μg/ml OVA peptide and cultured at a ratio of 1 APC: 15 naïve T-cells from OT-II RAG KO mice. D) Cells were pulsed with 2.5μg/ml MOG peptide and cultured at various ratios with naïve T-cells from 2D2 mice. Error bars represent SEM.
We next assessed whether DCTh can present antigen and activate T-cells, which is a defining property of DC. DCTh were purified from our cultures using CD11c MACS positive selection, pulsed with an MHC class II restricted OVA peptide in the presence of LPS and cultured with TCR-transgenic responder CD4+ T-cells (OT-II). Consistent with their surface phenotype, DCTh were nearly 10-fold more potent at T-cell activation than fresh monocytes. However, DCTh were far less potent at stimulating T-cell proliferation than GM IL-4 DC (Fig 1C). To verify that DCTh stimulatory ability was not restricted to OVA peptide presentation, we pulsed DCTh and fresh monocytes with an antigen relevant to EAE, myelin oligodendrocyte glycoprotein peptide (MOG aa 35-55), and tested their T-cell activating capacity across much broader APC: T-cell ratios in an antigen-specific T-cell proliferation assay with naïve MOG-reactive T-cells from 2D2 mice (Fig 1D). Under these conditions, DCTh induced T-cell proliferation at low APC: T ratios and were superior to monocytes at all ratios tested. These experiments confirm that activated CD4+ T-cells are capable of inducing the differentiation of monocytes into functional DC.
Th1, Th2 and Th17 cells drive the formation of phenotypically distinct DCTh
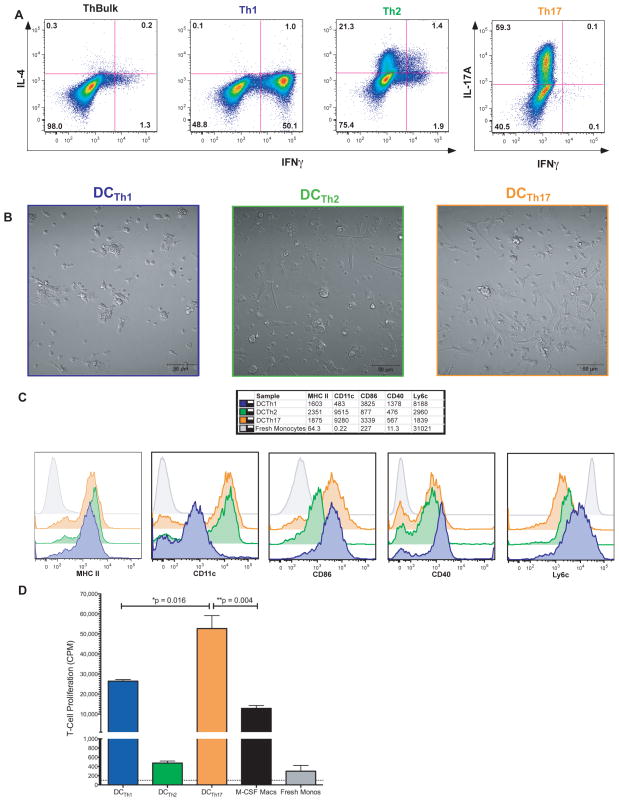
To determine the ability of particular Th subsets to induce DCTh formation, we polarized T-cells into Th1, Th2 or Th17 cells in vitro, using standard combinations of cytokines, blocking antibodies and anti-CD3 activating antibody. T-cell polarization was confirmed by intracellular staining (Fig 2A) and ELISA of culture supernatants (Supplementary Fig A). As expected, Th1 cells expressed high levels of IFNγ, while Th2 cells expressed IL-4 and Th17 cells expressed IL-17A/F. In contrast to IL-17A+ cells in vivo (9) but in accord with IL-17A+ cells generated in vitro (18), our in vitro polarized Th17 cells produced less GM-CSF upon initial polarization and restimulation than the other Th subsets (Supplementary Fig B). Similar to ThBulk cells, activated Th1, Th2, and Th17 cells cocultured with monocytes promoted the formation of cells with dendritic morphology (Fig 2B) (Supplementary Movies B), which we refer to as DCTh1, DCTh2 and DCTh17, respectively. All DCTh subsets upregulated MHC II, CD86 and CD40 compared to fresh monocytes (Fig 2C). Interestingly DCTh2 had lower expression of the costimulatory molecules, CD40 and CD86, as compared to DCTh1 and DCTh17.
Figure 2. Activated Th1, Th2 and Th17 Cells Drive the Formation of Phenotypically Distinct Populations of DCTh.
A) CD8 depleted splenocytes were activated with 1μg/ml αCD3 in the presence of various polarizing cytokines and blocking antibodies to skew the T-cells toward a Th1, Th2 or Th17 phenotype. After 96 hours, the cells were activated and stained intracellularly for IFNγ, IL-4 and IL-17A. Cells shown are gated on Live/Dead Blue−, Thy1.2+ B) Bone-marrow monocytes as used in Figure 1 were cultured 10:1 with Th1, Th2, or Th17 polarized T-cells for 5 days in the presence of 10ng/ml IL-2. The cultures were then imaged with DIC microscopy. C) Cell surface phenotype of DAPI−, Thy1.2−, CD11b+ cells after 5 days of culture with Th1 (blue), Th2 (green), Th17 polarized T-cells (orange), or fresh after isolation. MFI for each marker is shown in the table above. This experiment was performed at the same time and under identical conditions as Figure 1C. Data shown are representative of more than 3 independent experiments. D) DCTh1, DCTh2, DCTh17, M-CSF macrophages or fresh monocytes were purified, pulsed with MHC II-restricted OVA peptide and 1μg/ml LPS for 90 minutes, washed and then cultured at a ratio of 1 APC:15 naïve T-cells from OT-II RAG KO mice in triplicate for 3 days and pulsed with H3 thymidine for the last 18 hours. The dotted line shows the mean proliferation of the T-cells in the absence of APC. Results are representative of 2 independent experiments. Error bars represent SEM.
DCTh1, DCTh2 and DCTh17 were purified, pulsed with OVA peptide and LPS, extensively washed and then cultured with OT-II T-cells to evaluate their functional properties. After 3 days in culture, considerable T-cell activation and proliferation were observed in the DCTh1 and DCTh17 wells, but not in the DCTh2 wells. DCTh17 induced significantly more T-cell proliferation than DCTh1 (Fig 2D), and both DCTh1 and DCTh17 induced significantly more T-cell proliferation than macrophages or fresh monocytes. Although DCTh2 and fresh monocytes induced detectable levels of T-cell proliferation, it was minimal compared to DCTh1 and DCTh17. These observations show that activated Th1, Th2 and Th17 T-cells promote the development of Mo-DC capable of presenting antigen and activating T-cells, but only DCTh1 and DCTh17 are potent APC, as gauged by their ability to induce T-cell proliferation.
Activated DCTh17 produce large amounts of IL-12 in response to TLR stimulation
DC production of IL-12p70 promotes the development of IFNγ producing Th1 cells (19). While a Th1 response can be beneficial in the clearance of tumors and intracellular pathogens, it can be harmful in the setting of autoimmunity (20), (21). In EAE, IL-12p70 and Th1 cells are not required to initiate disease, as IL-12 specific p35 KO mice that cannot produce biologically active IL-12p70 are still susceptible to active EAE induction (22) (23). Nonetheless, IFNγ producing Th1 cells can contribute to inflammation (24) and are sufficient to induce EAE on their own in transgenic T-cell transfer models. Surprisingly, even though Th1 cells can drive inflammation, there is evidence to suggest that a Th1 response is also important for disease resolution in EAE (25).
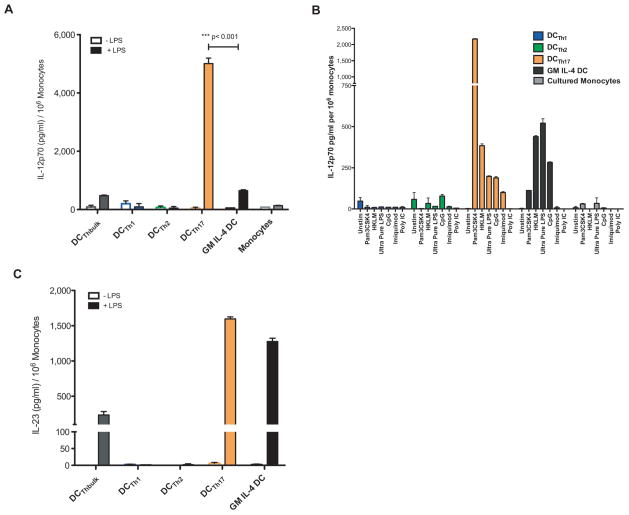
To determine whether the DCTh subsets differ in their capacity to produce IL-12p70, we activated each DCTh subset with standard LPS for 24 hours and then analyzed the supernatants for the presence of this cytokine (Fig 3A). All DCTh subsets produced minimal IL-12p70 in the absence of stimulation. However, after stimulation with LPS, DCTh17 produced significantly more IL-12p70 than either DCTh1 or DCTh2. Remarkably, DCTh17 produced nearly 10 times more IL-12p70 than commonly studied GM IL-4 DC. No intracellular IL-12p40 was detected within the Th17 cells present in the cocultures, indicating that DCTh17 were the sole source of IL-12p70 (Supplementary Fig C).
Figure 3. DCTh17 Produce Large Amounts of IL-12 in Response to TLR Stimulation.
DCTh were generated by monocyte-T-cell coculture for 5 days as in Figure 2. A) On day 5, 1μg/mL of LPS was added or cells were not additionally treated. IL-12p70 in cell-free supernatants collected 24 hours later was measured by ELISA. A representative experiment performed in triplicate is shown. Results are representative of more than 5 similar experiments. B) On day 5, various TLR agonists were added to DCTh1, DCTh2, DCTh17 or control cell cultures. IL-12p70 in cell-free supernatants collected 24 hours later was measured by ELISA. Done in triplicate. C) Supernatants as in A) were tested by ELISA for IL-23. A representative experiment performed in triplicate is shown. Results are representative of 2 similar experiments. Error bars represent SEM.
To address the question of whether stimuli in addition to LPS can induce DCTh17 to produce IL-12p70, we stimulated DCTh1, DCTh2 or DCTh17 cells with a panel of TLR agonists including Pam3CSK4 (TLR1/2), heat-killed Listeria monocytogenes (HKLM) (primarily TLR2), Poly IC (TLR3), ultra-pure LPS (TLR4), imiquimod (TLR7) or CpG ODN2336 (TLR7/9) (Fig 3B). DCTh17 responded robustly to most of these stimuli by producing IL-12p70. Notably, Pam3CSK4 and HKLM, both TLR2 agonists, induced high levels of IL-12p70 secretion. Ultra-pure LPS, CpG and to a lesser extent imiquimod also induced detectable amounts of IL-12p70. Remarkably, the ability to produce IL-12p70 was limited to DCTh17, as DCTh1 and DCTh2 cultured with the same TLR agonists failed to respond by secreting IL-12p70, even though they clearly became activated in response to TLR stimulation (Supplementary Fig D). Thus although all DCTh subsets can respond to TLR stimulation, DCTh17 are uniquely programmed to produce high levels of IL-12p70 and do so in response to a variety of danger signals.
Many recent studies have focused on the role of IL-23 in maintaining the polarization of Th17 cells. In fact, IL-23 is critical for EAE induction in the traditional MOG/CFA model, but is not necessary to maintain disease once EAE has been induced via passive transfer of T-cells (26). DCTh subsets were assessed for IL-23 production by ELISA (Fig 3C). As with IL-12p70, very little IL-23 was detected without stimulation. However, after LPS stimulation both DCTh17 and GM IL-4 DC produced large amounts of IL-23. Taken together, these results demonstrate that DCTh, particularly DCTh17, can respond to many different inflammatory mediators by producing both IL-12 and IL-23.
CD40L and RANKL on activated Th17 cells are required for the formation of IL-12p70 producing DCTh17
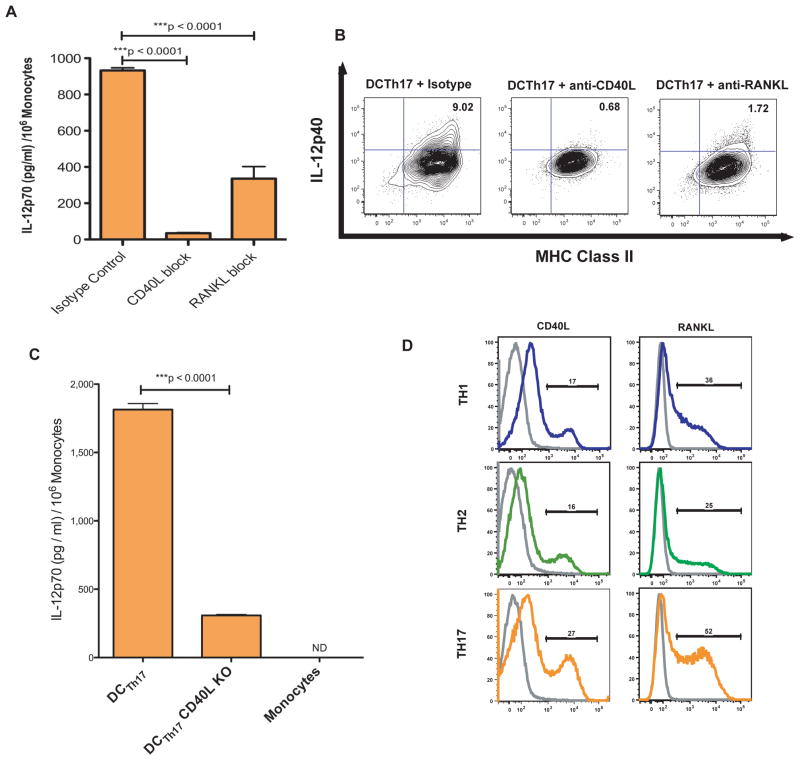
We next wanted to explore why Th17 cells, but not Th1 or Th2 cells, induced DC with the ability to produce large amounts of IL-12p70. Although Th17 cells express a unique set of cytokines and effector molecules such as IL-17A, IL-17F and IL-22, these molecules are not known to enhance IL-12p70 production. In contrast, the TNFα family of proteins contains multiple molecules that influence cytokine production by myeloid cells (27). Two such molecules, CD40L and RANKL, are also highly expressed by activated Th cells (28) (29). We stimulated DCTh17 with LPS in the presence of blocking antibodies to CD40L or RANKL or isotype control antibody (Fig 4A). CD40L blockade resulted in a nearly complete lack of IL-12p70 expression as assayed via ELISA. RANKL blockade resulted in a highly significant partial reduction of IL-12p70 expression. We confirmed these findings with intracellular staining of the same cells using an antibody to IL-12p40 (Fig 4B).
Figure 4. Th17 Cells Induce IL-12 Expression in DCTh17 in a CD40L and RANKL Dependent Manner.
A) Monocytes were cultured for 5 days with Th17 cells in the presence of 10μg/ml of functional grade blocking antibody to either CD40L or RANKL. A coculture with 10μg/ml of each functional grade isotype was included as a control. After 5 days, 1μg/ml of LPS was added to the cultures and the next day the cell free supernatant was harvested for IL-12p70 ELISA. Done in triplicate. B) DCTh17 were prepared as in A), but after 12 hours of stimulation with LPS, the cells were treated with BFA for 4 hours. The cells were then FC blocked and intracellularly stained for IL-12p40. The cells shown were previously gated on live/dead aqua−, Thy1.2−, CD11b+ cells. C) DCTh17 were generated with wild-type Th17 or CD40L KO Th17 cells. After 5 days, the cells were stimulated with 1μg/ml of LPS. 24 hours later, the cell free supernatant was tested for IL-12p70 with ELISA. Done in triplicate. D) Th1, Th2 or Th17 cultures were rested in the presence of IL-2 for 2 days and then restimulated with CD3/CD28 microbeads. 2 hours after restimulation the cells were FC blocked and then stained for CD40L. 6 hours after restimulation the cells were FC blocked and then stained for RANKL. Cells shown were gated on CD4+, Thy1.2+, Dapi− cells. Shown with an isotype control for each antibody and cell type. Representative of 2 independent experiments.
To determine if Th17 cells from CD40L KO mice are also impaired in their ability to generate IL-12p70 competent DCTh17, we generated Th17 cells from wild-type or CD40L KO mice and cultured them with monocytes for 5 days and then stimulated the cocultures with LPS overnight (Fig 4C). In line with data from the antibody blocking experiments, IL-12p70 levels were significantly reduced in the supernatant from monocyte / CD40L KO Th17 cocultures. Similar experiments utilizing Th cells from RANKL KO were considered, but dysregulated lymphoid cell development in these animals would have confounded such experiments (30).
Finally, we wanted to determine whether Th17 skewed cells express more CD40L and/or RANKL compared to the Th1 and Th2 skewed cells. To test this idea we restimulated polarized Th cultures with CD3/CD28 microbeads after a 2-day rest in IL-2, which was necessary to maintain Th1 and Th2 cell viability (data not shown). Th17 cell cultures contained ~50% more CD40L+ T-cells than Th1 or Th2 cultures (Fig 4D). In addition, 52% of the cells in the Th17 cultures were RANKL+ compared to 36% in Th1 cultures and 25% in Th2 cultures. These data demonstrate that Th17 cultures contain large numbers of CD40L and RANKL positive cells and that these molecules are necessary for maximal IL-12p70 expression in T-cell induced monocyte-derived DC.
DCTh17 polarize antigen-specific naïve T cells and previously committed Th17 cells into Th1 cells
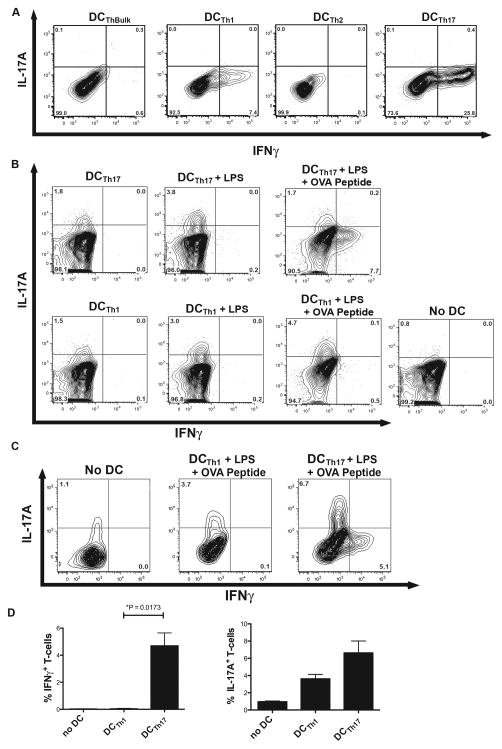
Because stimulated DCTh17 produce large amounts of IL-12p70, we hypothesized that they would be superior at inducing Th1 polarization. To test this, we pulsed the DCTh subsets with OVA peptide and LPS and after extensive washing, cultured purified DCTh subsets at a 1:15 ratio with naïve OT-II T-cells. After 96 hours of culture, intracellular staining for IFNγ, IL-4 and IL-17A was performed (Fig 5A). No IL-4 or IL-17A staining was detected above isotype control levels (data not shown). However, in accordance with the IL-12p70 production data, large numbers of T-cells expressing high levels of IFNγ were seen in the cultures containing DCTh17. IFNγ was detected in a small percentage of the T-cells cultured with DCTh1, but these cells expressed ~2.5 fold less IFNγ on a per cell basis than T-cells cultured with DCTh17.
Figure 5. DCTh17 Polarize Naïve T-cells to Th1 Cells and Can Convert Th17 Cells into Th1 Cells in the Presence of Specific Antigen and LPS.
A) DCTh subsets or control APCs were purified with magnetic microbeads and then pulsed with 2.5μg/ml MHC II-restricted OVA peptide and 1μg/ml LPS for 90 minutes. The cells were washed twice and then cultured at a ratio of 1 APC:15 naïve T-cells from OT-II RAG KO mice. On day 4 of culture, the cells were restimulated with PMA/ionomycin and BFA for 4 hours prior to intracellular staining. Cells shown are gated on live/dead blue−, Thy1.2+ cells. Data are representative of 2 independent experiments. B) Th17 cells were rested in media for 2 days and then cultured 1 DC:5 T-cells with DCTh1 or DCTh17 that had been pulsed with PBS, LPS alone or LPS and MHC II-restricted OVA peptide for 90 minutes. After 3 days of coculture, the T-cells were activated with PMA/ionomycin and BFA for 4 hours and then intracellularly stained for IL-17A and IFNγ. Cells were gated on live/dead blue−, Thy1.2+ cells. One replicate for each condition is shown. Data are representative of 2 independent experiments. C) IL-17A secreting OT-II T-cells were sorted from in vitro Th17 cultures, rested in the absence of IL-2 for 3 days and then cocultured with ISQ and LPS pulsed DCTh1 or DCTh17. After 4 days the ex-IL-17A+ cells were evaluated for IL-17A and IFNγ expression by intracellular cytokine staining. Done in duplicate. D) The percentage of IFNγ and IL-17A single positive T-cells in each condition are shown. Error bars represent SEM.
Under our experimental conditions, DCTh subsets did not induce Th17 polarization from naïve CD4+ T-cells. However, we hypothesized that DCTh may influence the stability of the Th17 phenotype in polarized cells. To investigate this possibility, we generated Th17 cells from OT-II RAG KO mice under polarizing conditions, rested the cells for 2 days in media in the absence of IL-2 and then cultured them with peptide-pulsed or unpulsed DCTh1 or DCTh17. After 4 days, the T-cells were stimulated with PMA and ionomycin in the presence of BFA and then stained intracellularly for IL-17A (Fig 5B and quantified in Supplementary Fig E). Compared to Th17 cells cultured alone, IL-17A production capacity by Th17 cells was better maintained when the Th17 cells were cocultured with DCTh1 or DCTh17, and this effect was further enhanced in cultures treated with LPS. An identical trend was observed when the Th17 cells were rested in the presence of TGFβ, which has been reported to be necessary for Th17 maintenance in vitro (data not shown) (31). Interestingly, the addition of OVA peptide to the DCTh resulted in the appearance of IFNγ-producing T-cells in the DCTh17 culture, but not in the DCTh1 culture, suggesting that DCTh17 can promote reprogramming of Th17 cells into Th1 cells in an antigen-specific manner.
The possibility exists that the IFNγ producing cells in our cocultures were derived from holdover naïve cells that did not become polarized to a Th17 phenotype during the initial coculture. To address this possibility, we polarized OT-II cells toward a Th17 phenotype, and then FACS purified the IL-17A secreting Th17 cells by utilizing cytokine-capture cell sorting. These highly purified cells, which by definition produced IL-17A, were rested and then cultured with antigen and LPS pulsed DCTh1 or DCTh17. In accord with the results from Figure 5B, a significant fraction of these cells began to produce IFNγ when cultured with DCTh17, but not DCTh1 (Fig 5C and 5D). Taken together, these observations indicate that DCTh17, and to a lesser extent DCTh1, promote Th1 polarization from naïve T-cells, but in the presence of an antigen recognized by the T cells, DCTh17 have the unique capacity to convert previously polarized Th17 cells into Th1 cells.
T-cells, monocytes and DC colocalize in EAE lesions
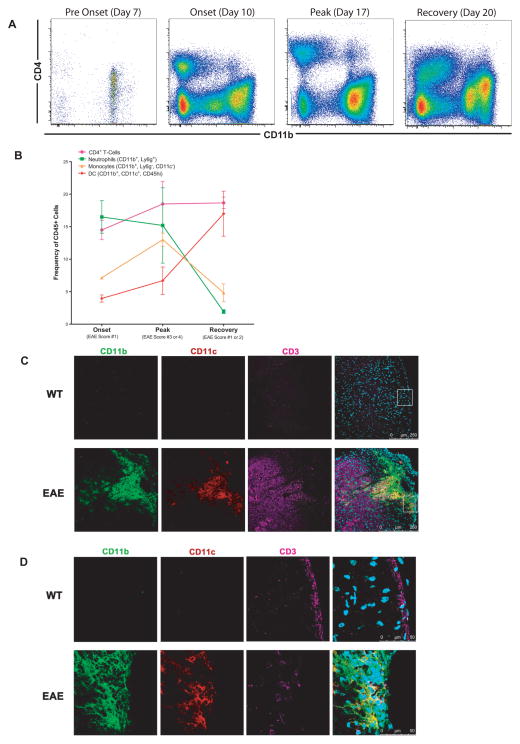
We induced EAE using a Th17-skewed passive-transfer model that enables investigation of the biology of EAE in the absence of confounding factors such as complete Freund’s adjuvant and pertussis toxin. In this model, lymphoid cells from actively immunized mice are cultured in MOG and IL-23 to skew the cells toward a Th17 phenotype (Supplementary Fig F), and then the polarized cells are transferred to naïve recipients to induce EAE. Spinal cords were harvested from diseased mice at various time points, and the surface phenotype of the infiltrating cells was analyzed by flow cytometry. Other than resident microglia, immune cells were largely absent from the spinal cords of presymptomatic mice, but rapidly accumulated at disease onset and remained abundant throughout the course of disease (Fig 6A). As expected, numerous CD4+ T-cells infiltrated the spinal cord at the onset of disease and these cells remained numerous throughout disease progression (Fig 6B). CD45hi, Ly6g-, CD11b+, CD11c-monocytes were present at onset, increased at peak disease and then decreased at recovery. CD45hi, CD11b+, CD11c+ DC increased after onset and remained at high frequency during EAE remission. Interestingly, Ly6g+ CD11b+ neutrophils accumulated with increased disease severity and then rapidly dissipated with recovery.
Figure 6. T-cells, Monocytes and DC Colocalize in EAE Lesions.
A) Leukocytes infiltrating the CNS were obtained by density gradient separation and then analyzed by flow cytometry at the indicated times after passive transfer of Th17 skewed encephalitogenic cells. Representative FACS plots (N= 2–3 mice per group) of DAPI−, CD45+ cells isolated from a single spinal cord are shown. B) Frequency of viable CD4+ T-cells, granulocytes, monocytes and DC from A). Error bars represent SEM. C) Representative spinal cord tissue from a healthy wild-type mouse (top) or an EAE mouse at peak disease with a score of 4 (bottom) was stained for CD11b (green), CD11c (red), CD3 (pink) and DAPI (blue) and the resulting images are shown at low power. D) The tissue outlined by the white box in C) is shown at high power.
To determine if monocytes, DC and T-cells are associated with one another in the EAE lesions, we performed immunofluorescence staining on spinal cord tissue from Th17-skewed passive-transfer EAE at peak disease or healthy control mice (Fig 6C). No immune cells were visualized in the healthy WT control spinal cord tissue. In contrast, multiple perivascular lesions were seen in the white matter of the EAE spinal cords containing CD11b+ cells (granulocytes and monocytes), CD11b+/CD11c+ cells (DC) and CD3+ cells (T-cells). Upon further magnification (Fig 6D), all three cell types could be seen in direct contact with one another. These data show that monocytes, T-cells and DC colocalize in EAE lesions, and are consistent with the hypothesis that activated T-cells drive the formation of DCTh in vivo.
Infiltrating Th cells from EAE lesions drive Mo-DC formation
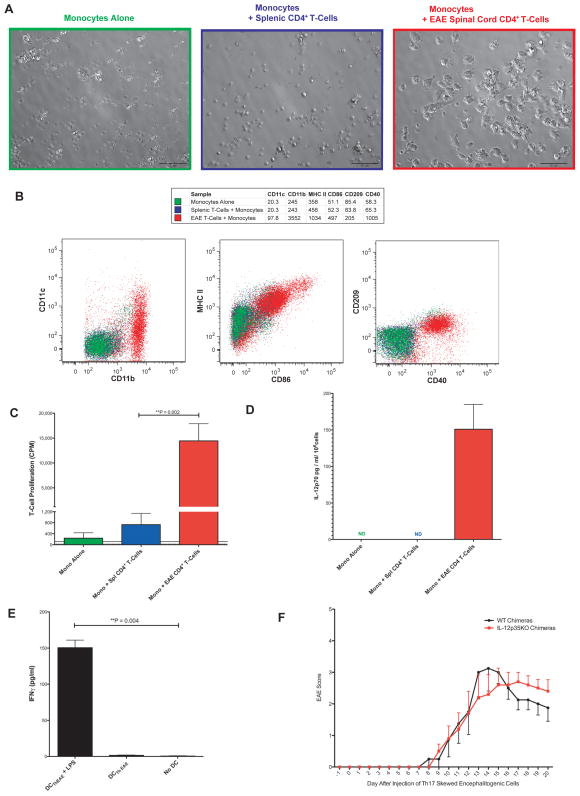
To determine if Th cells from EAE spinal cords can promote DCTh formation, we purified CD4+ T-cells from Th17 passive transfer EAE mice and cultured them with bone marrow monocytes from healthy mice for 4 days. As a control, we used splenic CD4+ T-cells from healthy mice, as almost no T-cells are present in healthy spinal cords and because splenocytes from EAE mice may contain recirculating encephalitogenic T-cells. We performed this coculture in the absence of specific antigen or other stimuli, as the CNS-infiltrating T-cells were already activated. However, as with the in vitro activated Th cells, the addition of exogenous IL-2 was necessary to maintain T-cell survival (data not shown). We observed by microscopy that monocytes cultured alone or with splenic T-cells retained a rounded morphology and displayed poor viability (Fig 7A). In contrast, monocytes cultured with EAE CD4+ T-cells not only remained viable but became considerably larger and more granular and developed numerous active dendrites, reminiscent of GM IL-4 DC and DCTh, that could be visualized with time-lapse microscopy (Supplementary Movie C). The monocytes cultured with EAE CD4+ T-cells also upregulated their surface expression of CD11b, CD11c, MHC-II, CD40 and CD86 compared to monocytes cultured with healthy splenic CD4+ T-cells (Fig 7B). The cells also expressed CD209, a marker of Mo-DC found in inflammatory settings (32). Interestingly, the cells retained high Ly6C expression, reminiscent of Mo-DC recovered from the CNS in transfer experiments (13). This phenotype closely resembled that of DCTh, and on this basis we refer to these EAE Th cell-induced cells as DCThEAE.
Figure 7. Infiltrating EAE Th-cells Induce the Formation of Th1 Skewing Mo-DC.
A) CD4+ T-cells from EAE mice spinal cords or a healthy wild-type spleen cells were cultured 1:10 with monocytes alone or monocytes in the presence of 10ng/ml IL-2 for 4 days and then imaged with bright field microscopy. The scale bar represents 50 microns. The results are representative of 4 independent experiments. B) Cells from A) were gated on CD4−, CD11b+, DAPI− and analyzed by flow cytometry. MFI for each marker is shown in the table above. Results are representative of 3 independent experiments. C) Cells from A) were harvested, pulsed with MHC II-restricted OVA peptide and then cultured in triplicate at a ratio of 1 APC:33 naïve T-cells from OT-II RAG KO mice for 3 days and then pulsed with H3 thymidine for 20 hours. The dotted horizontal line represents the mean proliferation of OT-II T-cells in the absence of APC. The solid horizontal line represents the mean proliferation of the DCThEAE without OT-II T-cells. Cultures were performed in triplicate. D) Cells prepared as in A) were stimulated overnight with 1μg/ml of LPS. The cell-free supernatant was assayed in duplicate by ELISA for IL-12p70. Data shown are representative of 2 independent experiments. ND = Not Detected. E) CD11b+ DCThEAE selected via MACS were pulsed with or without 1μg/ml LPS and 2.5μg/ml OVA peptide for 90 minutes, washed and then cultured at a ratio of 1 APC: 5 naïve T-cells from OT-II RAG KO mice for 5 days. Supernatant was collected and assayed via ELISA for IFNγ. Done in triplicate. F) BM chimeric mice were generated using wild-type or IL-12p35 KO BM. 10 weeks later, the mice were injected with Th17 skewed encephalitogenic cells to induce EAE, and mice were monitored for signs of disease on a daily basis. N = 4–5 mice per group. Data are presented as means ± SEM, with significance determined by a two-tailed Mann-Whitney test. Day 17 p = 0.27, Day 18 p = 0.36, Day 19 p = 0.37, Day 20 p = 0.45
Th cell production of GM-CSF has been implicated as a driver of pathogenesis in EAE (15, 33). In line with these studies, CNS infiltrating Th cells produced GM-CSF ex-vivo when cultured with monocytes and IL-2 (Supplementary Fig G). To determine whether GM-CSF was involved in the formation of DCThEAE, we cultured CNS infiltrating Th cells with monocytes from wild-type or GM-CSFR KO mice. Unexpectedly, signaling through the GM-CSFR on monocytes was not necessary for the induction of a DCTh phenotype (Supplementary Fig H).
In order to determine if DCThEAE, could take up and present antigen to naïve T-cells, we used these cells in an antigen-specific T-cell proliferation assay (Fig 7C). As their phenotype would suggest, Mo-DC generated by EAE Th cells, but not monocytes cultured with splenic Th cells, were capable of robustly stimulating naïve OT-II T-cells when pulsed with OVA peptide.
As this EAE model was induced by Th17-skewed cells, we wanted to determine if DCThEAE behave like DCTh17 in their ability to promote a Th1 response. To address this possibility, we purified DC from monocyte / EAE CD4+ T-cell cocultures and examined IL-12p70 production following stimulation with ultra-pure LPS. Similar to DCTh17, DCThEAE produced substantial amounts of IL-12p70 upon stimulation (Fig 7D). IL-12p40+ DC were also detected in the spinal cords of EAE mice by IC staining in the absence of exogenous stimulation (data not shown). Moreover, when pulsed with LPS and OVA peptide and cultured with naïve OT-II T-cells, DCThEAE polarized naïve T-cells to an IFNγ+ Th1 phenotype as indicated by ELISA (Fig 7E). Neither IL-17A nor IL-17A/F was detected in these same cultures (data not shown). Thus, encephalitogenic CD4+ T-cells from the inflamed spinal cord of Th17-skewed passive transfer EAE mice induce the formation of Mo-DC with Th1-polarizing capacity.
To investigate the role of myeloid cell derived IL-12 in vivo during EAE, we utilized BM chimeric mice. EAE was induced in IL-12p35 KO and WT BM chimeras via passive transfer of Th17 skewed encephalitogenic cells (Fig 7F). Both groups of mice began to show signs of tail paralysis by day 10. However, recipients of IL-12p35 KO BM displayed milder symptoms early (days 13–15), yet were impaired in their ability to recover from peak disease (days 17–20). While these trends did not reach statistical significance due to the low numbers of mice used (n=4–5), the data support the hypothesis that myeloid-derived IL-12 contributes to disease pathology but is also important for suppressing a pathogenic Th17 mediated response in favor of a milder Th1 mediated response.
Discussion
It is well documented that monocytes infiltrate inflamed tissues and differentiate into inflammatory DC (5, 6, 34). However, the physiologic mechanisms responsible for their formation and subsequent function during immunity and inflammatory disease are poorly understood. The present study demonstrates that activated Th cells can govern inflammatory DC formation and function.
The present study also demonstrates that activated CD4+ T-cells found within EAE spinal cords elicit the differentiation of monocytes into DCs, which suggests that this system is active in autoimmune disease. This finding is consistent with published studies of EAE indicating that T-cell-derived molecules including CD40L, GM-CSF, M-CSF, FLT-3L and CCL2 acting on infiltrating myeloid cells are key drivers of disease (15), (13), (35), (36), (37). Further, the phenotype and functions of DCTh are influenced by the type of T-helper cells in the inflammatory milieu. Our data show that DCTh1 and DCTh17 are proficient APCs capable of presenting antigen and stimulating naïve T-cells. Interestingly, while Th2 cells caused the differentiation of monocytes into cells that resembled DCTh, the resulting DCTh2 actually had very little T-cell stimulating capacity, which might be explained by their lower CD86 expression. These findings support previously published work showing that only Th1 and Th17, but not Th2 cells, can induce EAE (9).
The cytokines produced by the various DCTh subsets differed substantially. In contrast to DCThBulk, DCTh17 produced large amounts of IL-12 and IL-23 after LPS stimulation. Importantly IL-12 was secreted from DCTh17 after stimulation with a variety of different TLR agonists, suggesting that these cells produce IL-12 as a generalized response to danger. The induction of IL-12 in DCTh17 was highest when the cells were stimulated with TLR-2 and TLR-4 agonists. This may be important in the context of autoimmunity because multiple endogenous TLR ligands, including products released from necrotic cells, have been shown to signal through these receptors (38) (39).
The capacity of Th17 cells to recruit and activate neutrophils is well established and can lead to the destruction of pathogens as well as injury to peripheral tissue (40). Our time course analysis of the CNS in Th17-skewed passive transfer EAE shows that recovery from disease correlates strongly with a reduction of infiltrating neutrophils. A possible explanation for this reduction is the rise of DCTh17, which through their production of IL-12 would be expected to inhibit the generation of Th17 cells. The rapid shutdown of IL-17 production in Th17 cells after exposure to IL-12 suggests a possible mechanism for limiting this type of pathology (31). Moreover our data show that in the presence of specific antigen, DCTh17, but not DCTh1, can induce already committed Th17 cells to convert into Th1 cells, providing a potential mechanistic explanation for the high frequency of IFNγ-producing Th1 cells that previously produced IL-17 cytokines within the inflamed spinal cord of EAE mice (12). Similar IFNγ-producing T-cells have also been described in other Th17-mediated disease such as colitis (41) and arthritis (42), so it will be important to determine if DCTh17 play a role in these disorders as well.
Our data demonstrate that CD40L and RANKL are necessary for maximal IL-12p70 production by DCTh17. After restimulation, Th17 cells more frequently express these molecules than either Th1 or Th2 cells which may contribute to their ability to enhance IL-12p70 production in DCTh. These molecules are expressed rapidly after T-cell activation, which might be expected to result in the up regulation of IL-12 shortly after a Th17-mediated response. Other molecules differentially expressed by Th17 cells following activation may play a role in endowing Th17 cells with this unique ability to induce IL-12p70 in DCTh.
One clinically important question in MS is the role of Th subsets in established disease. Whereas Th1 cells can clearly cause EAE under experimental conditions, Th17 cells have been implicated as the initiating cells in MS and EAE, based on their unique ability to traffic through the choroid plexus in a CCR6 dependent mannerprior to CNS inflammation (43) (44). Th1 cells are believed to arise later, potentially as a safeguard against excessive Th17-mediated immunity. Th1 cells, which infiltrate the CNS later than Th17 cells, are considered to be more responsive to Treg-mediated immunosuppression than Th17 cells and thus drive less autoimmune pathology (45). This idea is supported by data showing that Th1, but not Th17, cells require IL-2 for their survival (46) (47). Because Treg cells regulate IL-2 levels via expression of the high affinity IL-2 receptor (CD25), they would be poised to control Th1 inflammation, but would be less effective at controlling Th17 inflammation (48).
The findings presented here are consistent with the view that although Th1 cells may contribute to EAE pathology, these cells ultimately have a moderating effect on autoimmune disease. This is supported by the fact that Th1 cells are the major T-cell subset present in the spinal cord just prior to the recovery phase in Th17-initiated EAE (45). Specifically, our findings suggest a model in which Th17 cells initiate disease and then induce the formation of DCTh17 with potent Th1-polarizing capacity. In the presence of antigen and danger signals, these DCTh17 promote the conversion of Th17 cells already present in the inflamed CNS, as well as naïve T-cells, into IFNγ+ Th1 cells. These Th1 cells may contribute to some CNS pathology, before ultimately being suppressed by Tregs.
Our experiment with IL-12p35 BM chimera mice, along with work by others demonstrating that mice lacking IFNγ, IL-12p35 or IL-12Rβ2 develop more severe EAE than control mice, reinforces this hypothesis (49) (50) (51). Because infiltrating DC produce the majority of the IL-12 in the inflamed spinal cord (52), their influence on recovery from EAE may be critical. Additionally, EAE mice lacking the IFNγR in bone-marrow derived cells have persistent neutrophil-rich foci in the CNS, suggesting that sensitivity to IFNγ is necessary for modulating a neutrophil response (53). Most importantly, in an EAE model similar to our own in which disease was induced by passive transfer of IL-23-treated cells, the addition of an IFNγ neutralizing antibody resulted in significantly worse disease (54). Intriguingly, IFNβ, which is widely used in the treatment of MS, requires intact IFNγ signaling for efficacy in EAE (17).
Monocytes and T-cells respond to many of the same chemotactic gradients including CCL2 (55), CXCL9-11 and CCL3-5 (56), so it is not surprising that inflamed tissues in multiple autoimmune diseases contain monocytes and activated T-cells in direct contact (16). The induction of Mo-DC formation by activated Th cells likely has implications that extend beyond CNS inflammation. In this regard, previous work from our group investigating the skin of patients with psoriasis and atopic dermatitis showed that Th cells induce the formation of DCTh from monocytesat sites of inflammation in humans (16). Taken as a whole, our findings raise the exciting possibility that activated T-cells drive the formation of DCTh in a diverse set of autoimmune diseases and that DCTh17 specifically act to bridge Th17 and Th1 responses.
Supplementary Material
Acknowledgments
This study was supported by NIH grants HL075462, DK082537 and DK096038.
We would like to thank Fionna Sun for technical assistance.
Abbreviations used in the article
- DC
dendritic cell
- Th
T-helper
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- RANKL
receptor activator of nuclear factor-kappa B ligand
Footnotes
Author contributions are as follows: M.D., M.A., J.K., M.S. and E.E. were involved in the study design. R.A. and L.S. provided reagents and technical expertise. M.D., M.A., R.Y., J.G. and J.K. performed the experiments. M.D. and E.E. wrote the paper. All authors discussed the results and commented on the manuscript.
Disclosures:
The authors have no financial conflicts of interest to disclose.
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 3.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer seminars in immunopathology. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 4.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells. Semin Immunol. 2005;17:313–318. doi: 10.1016/j.smim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso MN, Wong MT, Zhang AL, Winer D, Suhoski MM, Tolentino LL, Gaitan J, Davidson MG, Kung TH, Galel DM, Nadeau KC, Kim J, Utz PJ, Soderstrom K, Engleman EG. T(H)1, T(H)2, and T(H)17 cells instruct monocytes to differentiate into specialized dendritic cell subsets. Blood. 2011;118:3311–3320. doi: 10.1182/blood-2011-03-341065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson LB, V, Kuchroo K. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–842. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 15.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 16.Alonso MN, Wong MT, Zhang AL, Winer D, Suhoski MM, Tolentino LL, Gaitan J, Davidson MG, Kung TH, Galel DM, Nadeau KC, Kim J, Utz PJ, Söderström K, Engleman EG. T(H)1, T(H)2, and T(H)17 cells instruct monocytes to differentiate into specialized dendritic cell subsets. Blood. 2011;118:3311–3320. doi: 10.1182/blood-2011-03-341065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 20.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 21.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 23.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. The Journal of clinical investigation. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gran B, Chu N, Zhang GX, Yu S, Li Y, Chen XH, Kamoun M, Rostami A. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. J Neuroimmunol. 2004;156:123–131. doi: 10.1016/j.jneuroim.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 27.Summers deLuca L, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nature reviews Immunology. 2012;12:339–351. doi: 10.1038/nri3193. [DOI] [PubMed] [Google Scholar]
- 28.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. The Journal of experimental medicine. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 30.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 31.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheong C, Matos I, Choi J-H, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. Journal of immunology. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 35.Skarica M, Wang T, McCadden E, Kardian D, Calabresi PA, Small D, Whartenby KA. Signal transduction inhibition of APCs diminishes th17 and Th1 responses in experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4192–4199. doi: 10.4049/jimmunol.0803631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard LM, Miller SD. Autoimmune intervention by CD154 blockade prevents T cell retention and effector function in the target organ. J Immunol. 2001;166:1547–1553. doi: 10.4049/jimmunol.166.3.1547. [DOI] [PubMed] [Google Scholar]
- 37.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 38.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? Journal of leukocyte biology. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 39.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nature reviews Immunology. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature reviews Immunology. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 41.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. Journal of immunology. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 45.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 49.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 50.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang GX, Yu S, Gran B, Li J, Siglienti I, Chen X, Calida D, Ventura E, Kamoun M, Rostami A. Role of IL-12 receptor beta 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J Immunol. 2003;171:4485–4492. doi: 10.4049/jimmunol.171.9.4485. [DOI] [PubMed] [Google Scholar]
- 52.Deshpande P, I, King L, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. Journal of immunology. 2007;178:6695–6699. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 53.Lee E, Chanamara S, Pleasure D, Soulika AM. IFN-gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J Neuroinflammation. 2012;9:7. doi: 10.1186/1742-2094-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12-and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. The Journal of experimental medicine. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.