Abstract
Proteases are the most abundant class of proteins produced by mast cells. Many of these are stored in membrane-enclosed intracellular granules until liberated by degranulating stimuli, which include cross-linking of high affinity IgE receptor FcεRI by IgE bound to multivalent allergen. Understanding and separating the functions of the proteases is important because expression differs among mast cells in different tissue locations. Differences between laboratory animals and humans in protease expression also influence the degree of confidence with which results obtained in animal models of mast cell function can be extrapolated to humans. The inflammatory potential of mast cell proteases was the first aspect of their biology to be explored and has received the most attention, in part because some of them—notably tryptases and chymases—are biomarkers of local and systemic mast cell degranulation and anaphylaxis. Although some of the proteases indeed augment allergic inflammation and are potential targets for inhibition to treat asthma and related allergic disorders, they are protective and even anti-inflammatory in some settings. For example, mast cell tryptases may protect from serious bacterial lung infections and may limit the “rubor” component of inflammation caused by vasodilating neuropeptides in the skin. Chymases help to maintain intestinal barrier function and to expel parasitic worms, and may support blood pressure during anaphylaxis by generating angiotensin II. In other life-or-death examples, carboxypeptidase A3 and other mast cell peptidases limit systemic toxicity of endogenous peptides like endothelin and neurotensin during septic peritonitis, and inactivate venom-associated peptides. On the other hand, mast cell peptidase-mediated destruction of protective cytokines, like IL-6, can enhance mortality from sepsis. Peptidases released from mast cells also influence non-mast cell proteases, such as by activating matrix metalloproteinase cascades, which are important in responses to infection and resolution of tissue injury. Overall, mast cell proteases have a variety of roles—inflammatory and anti-inflammatory, protective and deleterious—in keeping with the increasingly well-appreciated contributions of mast cells in allergy, tissue homeostasis, and innate immunity.
Key words/phrases for indexing: Tryptase, chymase, cathepsin G, carboxypeptidase A3, dipeptidyl peptidase I, cathepsin C, mastin, neurolysin, matrix metalloproteinase, tissue-type plasminogen activator, angiotensin, renin, endothelin, neurotensin, proteinase-activated receptor 2, calcitonin gene-related peptide, vasoactive intestinal peptide
INTRODUCTION
Several recent reviews provide in-depth coverage of particulars of mast cell protease biochemistry, genetics and biological function 1–4. The present chapter emphasizes mast cell protease function as it relates to host defense and its frequent by-product, inflammation. The link between mast cell proteases and inflammation is not (as some might assume) automatic; rather, the overall effect can be anti-inflammatory and homeostatic. This chapter cannot do justice to all of the work being done in this field, which has expanded rapidly in the past five years, but it will summarize major recent findings shaping current notions of protease contributions to mast cell function and pathobiology.
PROTECTIVE and ANTI-INFLAMMATORY EFFECTS
Control of neurogenic inflammation: Tryptases, calcitonin gene-related peptide and the triple response of Lewis
A classical response to injury (such as a that created by stroking skin with a blunt instrument) is a red line appearing at the site of injury, followed by transient flare or redness surrounding the region of injury, and a wheal due to edema at and near the site injury. This is the so-called triple response of Lewis. The redness or “rubor” aspect is a cardinal sign of inflammation, along with “calor” (heat) and tumor (swelling). The red line is partly due to the release of histamine from mast cells under the influence of the neuropeptide substance P discharged from sensory nerves stimulated by the injury. The flare is attributed mainly to release of calcitonin gene-related peptide (CGRP) from sensory nerves stimulated by antidromic propagation of sensory nerve signals originating at the site of injury. The transience of CGRP-induced vasodilation is thought to be due to extracellular release of peptidases from mast cells activated by substance P 5. CGRP is hydrolyzed by tryptases, and, kinetically speaking, may be the best natural substrate yet identified for human β-tryptase 6, which inactivates CGRP’s vasodilating actions 7. More recent evidence suggests that primary spinal afferent neurons containing CGRP and substance P also contain protease-activated receptor (PAR)-2 8, which can be activated by tryptases 9–14. Although tryptase is weak compared to trypsin as a PAR-2 activator, mast cells are often near neurons 15, which may be exposed to high concentrations of tryptase during mast cell degranulation. Thus nerves, substance P, CGRP and tryptases may be involved in feedback loops that limit neurogenic inflammation. In effect, tryptases detoxify CGRP, which is perhaps the first-described example of a more general function for tryptases discussed below in connection with venoms and toxic non-neural peptides.
Thresholds for protective nociception and bronchoconstriction: roles of tryptases and PAR-2
Tryptase-activated neural PAR-2 is implicated in the component of itching in atopic dermatitis that is unresponsive to anti-histamines 16, 17. Furthermore, a recently appreciated phenotype of PAR-2-null mice is lowered nociceptive thresholds, such as those involving sensitivity to dermal and visceral pain 18, although a role for tryptases in setting pain thresholds in vivo is partly speculative at this point. Itch and pain are both essentially protective, because they alert the host to the presence of pathogens or impending tissue damage. PAR-2 also is expressed in non-neural tissues and cells, such as airway smooth muscle 11; indeed, isolated bronchi constrict in response to PAR-2 agonists, including tryptases 19–22. Nonetheless, it is not yet clear that the bronchoconstricting activities of tryptases are mediated fully via interactions with PAR-2, especially since most studies find that tryptase, rather than constricting smooth muscle on its own, potentiates the actions of known constrictors, such as histamine, so that they act at lower concentrations and to greater maximum effect 19, 20, 22, 23. Furthermore, PAR-2 agonists acting on epithelium rather than smooth muscle can cause bronchodilation by stimulating epithelial release of PGE2 24. Alternative mechanisms by which tryptase may promote bronchoconstriction include degrading bronchodilating peptides (leaving bronchoconstrictors unopposed) 6, 25–27, spreading degranulation signals 23, untethering muscle from the bronchial wall by cleaving extracellular matrix or activating matrix-cleaving proteases 28, and desensitizing smooth muscle cells to bronchorelaxing β-adrenergic agonists 29. Tryptase’s overall effect is to promote bronchial hyperresponsiveness, a hallmark of asthma. In essence, this is protective, if one accepts that a major purpose of airway smooth muscle is to guard airways from entry of unwanted flora, fauna and other noxious substances, for which mast cells can be sentinels. Surprisingly, the actual function and true benefits of airway smooth muscle are unknown. Clearly, the purpose is not to cause asthma.
Feedback detoxification of neurotensin by neurolysin and carboxypeptidase
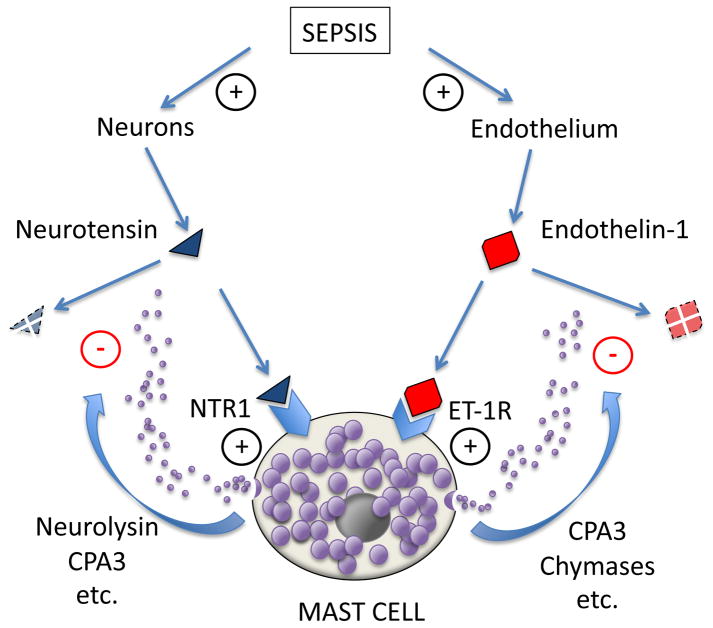
Neurotensin is a 13-residue fragment of a much larger polypeptide that includes the neuromedin N. Neurotensin gains partial protection from degradation by post-translational cyclization of its N-terminal amino acid to pyro-glutamate, thereby eliminating its free amino terminus and reducing its sensitivity to shortening by amino-peptidases. Neurotensin is usually classified as a neuropeptide, since it can originate from neurons. It causes hypotension when injected into mice; moreover, endogenous neurotensin can influence mortality in septic shock, because mice lacking neurotensin are less likely to survive septic peritonitis. Serum levels of neurotensin are elevated in humans with shock and sepsis. The origin of serum and peritoneal neurotensin is suspected to be neural; however, the actual sources in these conditions are not known 30. Intriguing experiments in mice subjected to cecal ligation and puncture, which models septic peritonitis from a ruptured appendix, suggest that mast cells respond to neurotensin in the peritoneum and play a detoxifying role significant enough to affect survival. Although neurotensin is not a strong mast cell degranulator, the detoxifying effect of mast cells depends at least partially on mast cell expression of neurotensin receptor 1, which may signal these cells to express an inactivating metallopeptidase, neurolysin—possibly on the cell surface (see Figure 1). Secreted carboxypeptidase A3 also appears to play a role, presumably by removing residues from neurotensin’s unprotected C-terminus. These findings suggest one explanation for the known benefit of peritoneal and mesenteric mast cells in recovering from cecal ligation and puncture. Whether mast cell-mediated detoxification of neurotensin is important in other types of sepsis and shock—and in humans—remains to be determined.
Figure 1. Mast cell-mediated protection from toxic endogenous peptides in sepsis.
Bacterial sepsis is associated with release of toxic peptides like neurotensin and endothelin-1 from nerves and vascular endothelium, respectively. These peptides are recognized by receptors on mast cells, stimulating release, activation or upregulation of detoxifying peptidases, such as carboxypeptidase A3 (CPA3), chymases, and neurolysin. This is an example of a homeostatic function of mast cells.
Detoxification of endogenous non-neural peptides and proteins
Endothelin
Many endogenous peptides have the potential to harm as well as to help when produced in response to microbial invasion or tissue injury. One particularly closely examined example, intriguing both in regard to its complexity and physiological importance, is provided by endothelins, which are family of peptides produced by endothelial cells in response to injurious stimuli. Mature endothelins act by engaging receptors on the surface a variety of cells, including vascular smooth muscle and mast cells. They can have acute and longer-term effects, including vasoconstriction, mast cell activation, and vascular remodeling. Drugs antagonizing interactions of endothelins with receptors have found a place, for example, in treating idiopathic pulmonary hypertension. Maurer and colleagues 31 described an intriguing nexus between endothelin and mast cells in the peritoneum (see Figure 1). Endothelin injected into the peritoneal cavity of mice is toxic—and, in sufficient doses, lethal—and also has toxic potential when produced endogenously in the context of septic peritonitis. Mouse peritoneal mast cells possess type A endothelin receptors and respond to endothelin by releasing destructive peptidases, which may include chymases and mast cell carboxypeptidase A3. Schneider and colleagues established more recently that removal of a single tryptophan residue from endothelin’s C-terminus is the principal inactivating event 32 in mice. Although other mast cell peptidases, including mouse chymases, can nick endothelin internally, this is not necessarily inactivating, because of stabilizing disulfide bonds. Indeed, the overall contribution of chymases to endothelin homeostasis is uncertain, and may be species-specific, because some chymases can process and activate endothelins from larger, precursor forms. For example, human chymase processes precursor “big” endothelins into a novel, bronchoconstricting form (endothelin [1–31]) 33, whereas rat chymases are less selective and degrade big endothelins as well as endothelin [1–31], which possesses bronchoconstricting, vasoconstricting, and vascular smooth muscle-proliferating activity 33, 34, and thus has potential to contribute to pathological bronchoconstriction in human asthma and vascular remodeling in pulmonary hypertension, systemic atherosclerosis, and re-stenosis after angioplasty. In reference to mice specifically, it should be noted that the overall role of endothelin-1 in asthma-like bronchoconstriction is not clear, since endothelin-1-null mice exhibit airway hyper-responsiveness 35. In humans, then, it appears that mast cells plausibly are involved in activating or inactivating endothelin (or both in succession, since activator chymase and inactivator carboxypeptidase A3 are usually but not inevitably found in the same subsets of mast cells and released together 36–38). Thus, it is reasonable to hypothesize that the role of human mast cells activated by already-mature endothelin is to inactivate the peptide—i.e., to limit toxicity by preventing accumulation and shortening duration of action—but that the role of human mast cells activated by other stimuli, such as allergen, first may be to activate via chymase-mediated processing of precursor big endothelins to smaller active forms, followed perhaps by mast cell carboxypeptidase A3-mediated inactivation.
Cathelicidin
In studies of human lung mast cells, Schiemann and colleagues showed that antibacterial peptide cathelicidin LL-37 (production of which is induced during inflammation) stimulates mast cells to degranulate and secrete β-tryptase, which in turn inactivates cathelicidin 39. This is an example of immunomodulatory negative feedback similar to that described for endothelin. On the other hand, human chymase and cathepsin G in skin mast cells can activate platelet-derived connective tissue activating peptide III to generate neutrophil-activating peptide II, a neutrophil chemokine 40. Therefore, downstream effects of human mast cell degranulation on inflammation will depend on the nature of mast cell stimulus, the protease phenotype of the stimulated mast cell, and the local availability of targets cleavable by the secreted proteases.
Cytokines and interleukins
Mast cell ability to degrade cytokines can be striking. In studies of activated human skin mast cells, exocytosed proteases markedly diminish immunodetection of mast cell-secreted cytokines like IL-6, IL-13 and TNFα, presumably by destroying epitopes recognized by antibodies 41. Inhibitor screens suggest that chymase and cathepsin G are more responsible than are tryptases. These effects are likely to be anti-inflammatory, to the extent that they reduce biological activity as well as immunoreactivity of inflammatory cytokines. Unless countered by inhibitors, this peptidase activity causes considerable underestimation of mast cell production of several cytokines. The in vivo importance of these effects is less clear, because chymases and cathepsin G are released into environments rich in inhibitors, such as α2-macroglobulin and α1-antichymotrypsin 42–44. There are, however, hints from mice that modulation of cytokine activity can be biologically significant in vivo. In septic peritonitis, hydrolytic inactivation of mast cell-derived IL-6 by mMCP-6 tryptase (and perhaps other peptidases) appears to lead to increased mortality 45. This suggests that cytokine-cleaving activities of mast cell proteases can be counterproductive, even while reducing inflammation.
Detoxification of snake and bee venoms
Mast cells have a well-deserved reputation for contributing to severe anaphylactic reactions to bee and wasp stings. Numerous deaths result from systemic release of mast cell mediators of shock and inflammation. Although hymenoptera venoms are complex mixtures of peptides and proteins, some of which directly degranulate mast cells, severe reactions are mediated by mast cell-bound IgE recognizing venom components based on prior sensitization. Because of this association between mast cells and fatal reactions to envenomation, recent evidence 46 that mast cells protect mice from lethality of some venoms is at first glance counter-intuitive. One example was provided by sarafotoxins, a class of venoms produced by snakes related to mole vipers. Sarafotoxins are homologous to endothelins, which, as discussed earlier, activate mast cells to release peptidases. Metz and colleagues 46 showed that mast cell detoxification of sarafotoxins involves similar mechanisms and affords some protection from lethal outcomes of envenomation. As is true for endothelin, carboxypeptidase A3 plays a prominent role in disarming the toxin. It is not yet clear whether this effect applies broadly to other venom peptides and proteins. However, mast cells protect mice from hypothermia and death caused by the venom of two snakes (rattlesnake and copperhead) not containing sarafotoxins. Carboxypeptidase A3 involvement in these cases is not as prominent. Thus, for some venoms, other detoxifying peptidases, like chymases and tryptases, may be important. By unclear mechanisms, mast cells also partially protect mice from effects of honeybee venom 46, which contains mast cell-degranulating apitoxins.
Secretion, activation and destruction of matrix metalloproteinases (MMPs)
MMPs in remodeling and resolution of inflammation
The classic gelatinolytic MMPs produced by inflammatory cells are MMP-2 (gelatinase A) and MMP-9 (gelatinase B). Although mast cells and mast cell lines can secrete MMP-2 and MMP-9 47–51, these enzymes also are produced by a variety of other inflammatory cells, which are likely to be the dominant source in inflamed tissues. MMP-9, for example, is especially abundant in neutrophils, and its levels in inflamed tissues and fluids tend to track with the number of neutrophils. It is not clear whether the well-known and often-assessed capacity of these enzymes to hydrolyze denatured collagen (gelatin) in polyacrylamide gels mirrors or predicts their roles in vivo, for these enzymes cleave a variety of proteins 52, 53 and there is perhaps no equivalent of gelatin in vivo. MMP-9-null mice have phenotypic abnormalities involving long bone angiogenesis 54, but there is no apparent deficit in airway inflammatory angiogenesis and lymphangiogenesis stimulated by mycoplasma infection—and these mice do develop neutrophilic pneumonia 55. Although MMP-2 (which increases in lungs of infected MMP-9-null mice) potentially could compensate for lack of MMP-9, MMP-2/MMP-9 double knockout mice have a neutrophilic pneumonia and airway angiogenesis phenotype similar to that of MMP-9-null mice 55. Data from mice suggest that allergic pulmonary inflammation may differ in this regard 53, 56, 57 because lack of MMP-2 and (especially) of MMP-9 hinders egress of recruited eosinophils and other leukocytes from pulmonary interstitium into the airspaces, from which they would otherwise be cleared via the mucociliary and/or macrophage-mediated apoptotic pathways. This clogs interstitial spaces with leukocytes, impairs gas transfer, and “asphyxiates” the mice. These findings are intriguing because they suggest that MMP-2 and MMP-9 promote resolution of allergic inflammation. Indeed, studies of other types of inflammation also suggest that control of inflammation and associated remodeling may be an important function of these MMPs, as in a model of bronchopulmonary dysplasia, in which transgenic Mmp9 −/− mice have more pulmonary macrophages and alveolar hypoplasia 58.
Mast cells as a source of gelatinolytic MMPs
The most detailed evidence that mast cells produce gelatinolytic MMPs stems from studies of canine, mouse and human mast cells 47, 49, 50, 59. In canine lines, MMP-9 production is regulated by kit ligand and TGFβ 48. MMP-9 appears to be released in a constitutive and regulated manner as a pro-form bound to MMP inhibitor tissue inhibitor of metalloproteinases (TIMP)-1. Thus, MMP-9 is secreted as an inactive, pro-MMP-9/TIMP-1 complex 47, 60. Activation of the mast cell-secreted complex occurs outside of the cell, especially in cells that are stimulated to release contents of serine protease-rich secretory granules. Because mast cell MMP-9 seems to be secreted independently of its serine protease activators, it is likely that the pro-MMP/TIMP-1 complex originates in a compartment separate from the classic secretory granule. Human mast cells also exhibit the interesting property of secreting MMP-9 upon direct contact with T lymphocytes 50.
Activation, disinhibition and destruction of MMPs by chymases
The interactions of mast cell chymases with MMP-9 are biochemically intriguing and possibly unique. Studies of canine mast cell chymase and pro-MMP-9 reveal that chymase activates pro-MMP-9 by selectively hydrolyzing residues in the MMP-9 pro-peptide 47, 60. This yields a large gain in solution-phase proteolytic activity. Beyond this, chymase activates MMP-9 even while bound to TIMP-1 61 and is the only MMP-9 activator shown to possess this capability. Because much extracellular MMP-9 is imbedded in matrix as a TIMP-1-bound pro-enzyme, chymase released from mast cells can bring MMP-9 to life from latency, and launch cascades of MMP activation initiated by MMP-9, which can activate other MMPs. Chymase accomplishes activating TIMP-1-bound MMP-9 by cleaving TIMP-1 itself. Although interactions between chymase and MMP-2 have been studied less intensively, chymase inhibitors are reported to decrease intimal hyperplasia in balloon-injured canine carotid arteries, with an accompanying decrease in activated MMP-2 62. Although the biochemistry of MMP-9 has been explored in greatest detail using canine enzymes and mast cell lines, the phenomenon occurs in other mammals. In mice, for example, the principal pro-mMMP-9-activating chymase is mMCP-4 49, 51, whose actions are hypothesized to be important in tumor growth and invasion in a model of skin cancer 49 as well as in collagenous ear thickening and lung fibronectin accumulation, which are attributed to reduced matrix turnover in mMCP-4-null mice 51, which also suggest that mMCP-4 regulates levels of MMP-2 51. More recently, a study of chymase inhibitors in a colitis model reveals markedly reduced colonic MMP-9 levels in inhibitor-treated mice 63. Similar effects occur in angiotensin II-induced aortic aneurysms 64. Chymase can destroy MMP-9 activity with prolonged incubations, although its preference appears to be for activating cleavages. It should be stressed that the net effect of MMP-9 activation by chymases could be anti-inflammatory, given MMP-9’s involvement in resolution of inflammation.
Mast cell protease-facilitated activation of the renin-angiotensin system
Updating paradigms
Research over the past two decades built a very solid body of evidence supporting physiologically important mast cell peptidase activation of the renin-angiotensin system, whose end product is the vasoactive octapeptide angiotensin II. Several aspects of involvement of mast cell peptidases in generating angiotensin II are at odds with the partly outmoded concept that the key steps in generating angiotensin II occur within vessels, starting with regulated release of the aspartyl peptidase renin into the bloodstream by kidney cells responding to drops in renal perfusion pressure. According to the classical paradigm, renin then cleaves off a portion of the N-terminus of a circulating protein, angiotensinogen to generate the decapeptide angiotensin I. This inactive peptide is then hydrolyzed by angiotensin converting enzyme (ACE), an ecto-metallopeptidase on the surface of pulmonary vascular endothelial cells, to generate the angiotensin II, which binds to receptors in arteriolar smooth muscle to constrict vessels and acts on the adrenal gland and kidney to promote retention of sodium and water. This process is homeostatic, being designed to preserve blood pressure. Additional essentially protective roles may include vasoconstriction to reduce bleeding and edema during tissue injury. In this regard it is interesting to note the association between use of ACE inhibitors and more severe systemic reactions to insect stings 65. ACE and angiotensin II are unquestionably important in human health, as shown by the success of therapeutic agents directed against ACE or receptors of its product, angiotensin II. As discussed in more detail below, mast cells complicate the classical paradigm by providing the means of generating both angiotensin I and II outside of vessels, using pathways that neither involve ACE nor are blocked by ACE inhibitors. And because mast cells outside of the kidney can be a source of renin, they can initiate angiotensin production in a variety of tissues in response to events unconnected to regulation of blood pressure and volume. Recent research on angiotensins also suggests that angiotensin II is a trophic factor with chronic affects on growth and remodeling of many tissues, that angiotensin II-inactivating peptidases may be important in regulating angiotensin activity, and that there may be “good” and “bad” receptors for angiotensin II in the context, for example, of responses to lung injury 66, 67.
Generation of angiotensin II by chymases and cathepsin G
The idea that some mast peptidases generate bioactive angiotensin II is not new. Travis, Wintroub and others showed that human mast cell chymase and cathepsin G process angiotensin I into angiotensin II in vitro more than a quarter of a century ago 68, 69, not long after the enzymes were first purified. Later, convergence of work in different laboratories culminated in realization that a potent, non-ACE, angiotensin II-generating peptidase extractable from human heart tissue is, in fact, indistinguishable from human mast cell chymase 70–73. Although some evidence supports the possibility that this chymase is expressed in endothelial, vascular or cardiac muscle cells, it is clear that mast cells have far greater capacity to store chymase than any other cell type. Thus, it is probable that most chymase in extracts of human heart muscle, as in other tissues, arises from MCTC mast cells, which are easily detected in heart tissues. History was repeating itself, in the sense that a similar convergence occurred twice in the 1970’s in connection with the major mast cell chymotryptic protease of rat connective tissue, mast cell protease I, which was initially thought to be intrinsic to skeletal muscle 74, 75, and also in connection with an enzyme extracted from rat intestine thought to function to degrade intracellular pyridoxal phosphate-dependent enzymes—but found subsequently to be made and stored by an intestinal mast cell sub-population, then called “atypical” 76. These events in the history of mast cell peptidases are worth recounting if only because they remind us that the capacity of mast cells to store peptidases is prodigious—so much so that a minor cell in a tissue like skeletal muscle can produce the lion’s share of certain peptidase activities in tissue extracts.
Although human cathepsin G was shown long ago to be capable of selectively hydrolyzing angiotensin I to generate angiotensin II 68—and shown later to be an abundant product of MCTC mast cells 77, 78—rather little is known of its importance in generating angiotensin II in vivo, and even less known of its role in other animals. A preliminary comparison of the properties of mouse and human enzyme suggests that the former is more active and narrower in specificity, being more purely chymotryptic. In humans, cathepsin G is a major product of neutrophils (and to a lesser extent, macrophages), in addition to being a product of MCTC mast cells. Thus, it may contribute to the generation of angiotensin II in neutrophilic as well as mast cell-mediated pathologies. In this connection, it may be helpful that potent, dual inhibitors of human chymase and cathepsin G have been generated 79, 80. In humans, chymase can generate angiotensin II while bound to macroglobulin, which prolongs chymase’s during of action and escorts chymase away from site of generation, where it can be detected in blood 44. Chymase released into interstitial fluid or serum is much more likely to be trapped in this macroglobulin-bound but active form than to be inactivated irreversibly by serpin-class inhibitors, for which chymase has little affinity. Cathepsin G, on the other hand, is susceptible to α1-antichymotrypsin.
In mice, notwithstanding the apparent redundancy of chymase-like peptidases, it appears that mMCP-4 makes the major contribution, as suggested by studies in mMCP-4-null mice 81. However, mMCP-4 is not as selective as human chymase for the Phe8-His9 bond of angiotensin I, and is both activator and inactivator of angiotensin 82. Nonetheless, as established by the work of Husain and Dell’Italia and others, mMCP-4 and/or similar chymases appear to be responsible for ACE inhibitor-resistant generation of angiotensin II in mice—and for most extravascular generation of angiotensin II in heart muscle 83, as previously shown in dogs 84. Although, there is potential for production of mMCP-4 and other chymases by non-mast cells, the marked reduction of interstitial angiotensin II generation in mast cell-deficient KitW/KitW-v mice suggests that most of extravascular angiotensin II-generating capacity originates from mast cells 83. Presumably, the existence of this extravascular pathway serves a homeostatic purpose, like blood pressure control. However, many studies in this active area of research focus on potentially deleterious effects such as hypertension (as shown in transgenic mouse expressing rat vascular chymase 85), re-stenosis, fibrosis, worsening lung injury in ARDS, and cardiac arrhythmias 83, 86–88.
Mast cell renin
Studies by Mackins and colleagues 89, 90 establish that mast cells can store renin, which is released by mast cell activators like compound 48/80 and allergen and boosts local generation of angiotensin I, which is then processed to angiotensin II by ACE or chymase-like peptidases. Presumably, mast cell renin could serve homeostatic functions, although existing studies focus on potential pathological contributions. In perfused mouse hearts, mast cell deficiency is associated with less “spillover” of renin and fewer malignant ventricular arrhythmias after ischemia-reperfusion. Intriguingly, much of this work on cardiovascular implications of mast cell renin release has been conducted in guinea pigs 89, 90, which appear to lack angiotensin II-generating chymase 89. Whether mast cell-derived renin is important in myocardial ischemia and arrhythmias in humans needs to be established.
Mast cell proteases, de-worming and gut homeostasis
Perhaps the earliest in vivo evidence that a mast cell peptidase serves host defense was provided by Miller and colleagues, who developed a mouse deficient in the mucosal/intraepithelial mast cell chymase mMCP-1 91. This was the first mammal engineered to lack a mast cell serine peptidase. These Mcpt1-null mice have, difficulty expelling Trichinella spiralis 91, which is a parasitic roundworm that infects a variety of mammalian hosts and causes trichinellosis, a disease characterized most dramatically in humans by muscle inflammation from tissue deposition of larvae. MMCP-1-expressing mast cells increase dramatically in mice after worm infestation, and expulsion of Trichinella spiralis accompanies or precedes mMCP-1 release into intestinal mucosa and lumen. This presumably increases intestinal inflammation over the short term, but by expelling worms more quickly, restores gut health sooner. Curiously, mMCP-1’s de-worming function does not extend to all nematodes. For example, although mice infected with Nippostrongylus brasiliensis develop impressive jejunal mastocytosis and dramatic increases in gut content of mMCP-1, worm burden is unaffected by lack of mMCP-1 in Mcpt1-null mice 91–94. However, other mast cell proteases may be important. Indeed, recent studies suggest that the related β-chymase mMCP-4 plays a more general role in regulating gut barrier function, including permeability and epithelial migration, as indicated by the small intestine phenotype in Mcpt4-null mice and in mast cell-deficient mice with mast cell populations re-established by adoptive transfer of BMCMC originating from wild-type and Mcpt4 −/− mice 95. By activating PAR-2, mast cell tryptases may play similar roles in large intestine 96.
Chymases and control of allergic airway inflammation
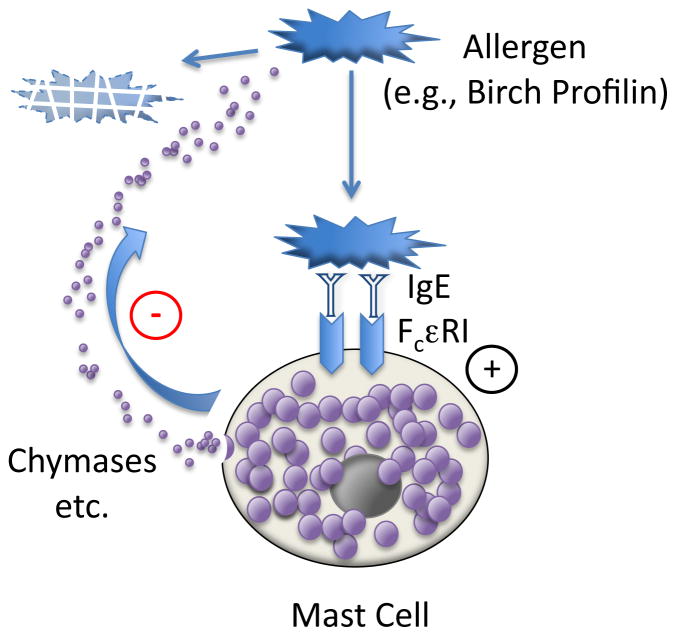
It is not yet clear whether the overall effect of mast cell chymases released during airway allergen challenge is to promote or oppose allergic inflammation (or both: e.g., pro-inflammatory in the early phase and anti-inflammatory in late or chronic phases). Although it is often assumed that release of chymase will augment inflammation, several experiments suggest mechanisms by which the actions of chymases could reduce or terminate inflammation. For example, chymases could disarm allergens by cleaving them, as occurs with canine chymase degrading birch profilin and destroying epitopes recognized by IgE 97 (see Figure 2). Furthermore, human mast cell chymase (and cathepsin G) destroy several cytokines associated with perpetuation of allergic inflammation 41. Indeed, the idea that human chymase opposes inflammation received further support from the observation that chymase-containing mast cells in the outer wall of small airways correlate with better lung function in asthmatics 98. In a model of asthma, anti-inflammatory activity was attributed to a specific chymase, mMCP4, based on the finding of increased airway responsiveness, inflammation, and smooth muscle volume in Mcpt4 −/− mice 99. A potential mechanism for increased smooth muscle volume is degradation of perimyocyte matrix and reduced proliferative responses to growth factors 100. Such actions may be related to pro-apoptotic effects of chymases on vascular smooth muscle 101, 102, which may increase susceptibility to aneurysm formation 103. On the other hand, inhibitors of human chymase and cathepsin G oppose development of airway hyper-responsiveness and early- and late-phase rises in airway resistance in Ascaris suum-sensitized sheep challenged with allergen, and, in mice exposed to tobacco smoke, reduce lung neutrophilia and production of neutrophil chemoattractant 79, 80. Explanations for observed differences in conclusions drawn from genetic versus pharmacological models likely relate in part to differences between mammals in the degree of chymase (and cathepsin G) redundancy—along with variations in enzymological properties, inhibitor susceptibilities, and cell-selective expression within and between mammals (reviewed in 4).
Figure 2. Self-termination of mast cell activation by allergen.
Multivalent protein allergens like birch profilin recognized by allergen-specific IgE bound to high-affinity IgE receptor on the surface of mast cells stimulates degranulation and release of epitope-destroying proteases, such as chymases. This is a negative feedback loop that reduces allergic inflammation by destroying allergen.
Tryptases and control of bacterial infection
This topic has been reviewed recently 104 and is briefly summarized and updated here. Among the most persuasive early evidence that mast cells control bacterial infection in vivo came from studies of responses of mast cell-deficient mice to enterobacteria 105, 106. In peritoneal infections, stimulation of mast cell release of TNFα early in infection is needed for timely recruitment of neutrophils to contain infection. Subsequent studies revealed that mast cell-derived IL-6 helps to contain peritoneal and lung infections with Klebsiella pneumoniae and other gut organisms 45, 107. The possibility that secreted mast cell proteases could also help to control gut infections was suggested by studies in mice showing that the mouse tryptase mMCP6 (but not the related protease mMCP7) attracts neutrophils when injected into the peritoneal cavity 108 and established that Mcpt6 −/− mice clear Klebsiella pneumoniae inefficiently and are more likely than +/+ mice to die following injection of Klebsiella pneumoniae into the peritoneal cavity 109. Other studies provide glimpses of some of the subtleties of mast cell protease contributions by establishing that reduced levels of active mast cell tryptase in Dppi −/− mice correlate with increased gut bacterial load following cecal ligation and puncture but with improved short-term survival, possibly related to higher levels of immunoprotective IL-6, which can be degraded by mMCP-6 45. Thus, although mouse studies clearly establish that some mast cell tryptases help to control and contain some types of bacteria, the impact on survival may depend on the nature and virulence of the organism—and in some cases tryptases may lower survival. In any case, several other mast cell factors, including TNFα and IL-6, are also important. Studies with human mast cell tryptases are more limited, although it has been shown that active human βI tryptase (but not α tryptase) attracts neutrophils when introduced to mouse airways 110. Whether mast cell tryptases are in fact major weapons in human anti-bacterial defenses is an unanswered question, the answer to which may determine whether pharmaceutical strategies involving systemic blockade of tryptase activity will block mast cell-mediated inflammation at the expense of creating serious immune deficits. In this regard, the failure to identify humans that are entirely bereft of active mast cell tryptases, despite the high frequency of deficiency alleles in most populations, is consistent with (although not proof of) key roles in host defense 111–113.
PRO-INFLAMMATORY ROLES of MAST CELL PEPTIDASES
Tryptases
Allergic inflammation of airway and skin
Tryptases are released with histamine from human skin mast cells in vivo in acute and chronic responses to allergen 114, 115. Levels of immunoreactive tryptases also increase in the airway in asthma 116, and transcripts encoding tryptases are among the most abundant and upregulated gene products in brush biopsies of asthmatic bronchial epithelium, consistent with intraepithelial mast cell migration or local proliferation 38, 117. It is not known if tryptases detected by immunoassays in airway fluids (or in blood, for that matter) is active. Overall, evidence from animal models as well as humans is compelling that tryptases released during allergic inflammation are not only markers of mast cell activation but contribute to resulting pathology. For example, small molecules designed to inhibit human tryptases markedly reduce airway eosinophilia and goblet cell hyperplasia in a mouse model of asthma 118. Similar findings are reported with nafamostat 119, which is a highly potent although not entirely selective inhibitor of tryptases 120. Several lines of evidence suggest that tryptase is a bona fide in vivo target of nafamostat. For example, nafamostat reduces scratching in mice induced by skin injection of tryptase or by mast cell-degranulator compound 48/80 17. Nafamostat’s effects on scratching are not seen in mast cell-deficient mice, and involve PAR-2, since the effects of tryptase and compound 48/80 on scratching are inhibited by PAR-2 antagonists. The simplest explanation of these findings is that tryptase from mast cells activates PAR-2 on nerves involved in itch pathways. Note that leeches make a potent and selective tryptase inhibitor 121, which is indirect evidence of a role for tryptase in signaling the presence of the leech, perhaps by activating neural itch and pain pathways or by spreading the degranulation signal.
Published studies of the effect of tryptase inhibition in humans are few. However, a topical (aerosolized) tryptase inhibitor reduced late-phase bronchoconstriction in a small study involving mild human atopic asthmatics 122 and decreased nasal symptoms and eosinophilia in humans with allergic rhinitis 123. Among several proposed pathways by which tryptases may promote asthmatic bronchoconstriction, it is not yet clear which are the most important. One potential mechanism proposed early on is tryptase-mediated destruction of vasoactive intestinal peptide 6, 25–27. A possibly distinct pathway involves augmentation of bronchoconstriction by histamine and other airway smooth muscle agonists, which is a phenomenon manifest in muscle bath preparations of bronchi from dogs and humans 19, 20, 22. Tryptase released from one mast cell under the influence of allergen also may promote degranulation of nearby mast cells, as suggested by mast cell-stabilizing actions of some tryptase inhibitors 124, 125 and by provocation of histamine release in sheep or guinea pig skin or airway by injected or inhaled human tryptase 23, 126–129. The early history of pharmaceutical interest in and development of tryptase inhibitors was thoroughly reviewed by Cairns 130 and will not be re-reviewed here. Notwithstanding the pharmaceutical interest in blocking more acute affects of tryptases on inflammation and smooth muscle constriction, chronic affects on airway remodeling (including growth of airway fibroblasts 131–134, smooth muscle 135 and vessels 136)—which may be responsible for bronchodilator-resistant airway obstruction—also provide rationales for therapeutic inhibition. The extent to which tryptase activation of PAR-2 is involved in allergic inflammatory, bronchoconstrictor and remodeling responses is not yet clear.
Arthritis and inflammatory bowel disease
A role for mast cell tryptases in arthritis is suggested by reduced inflammation in tryptase-deficient mice in two models or arthritis 137, 138. One of these models (methylated bovine serum albumin/IL-1β-induced) seems to require two tryptase gene products (namely, mMCP-6 and -7) for full expression of the inflammatory phenotype 137. These recent findings support prior speculation about the importance of mast cell products in arthritis based on studies in mast cell-deficient mice and the finding of tryptase-expressing mast cells in arthritic joints 139–142. The mechanisms by which mast cell tryptases contribute to various forms of experimental arthritis remain to be established—and of course tryptases and mast cells are not the sole factors contributing to the phenotype 141, 143. The importance of mast cells and tryptases to the pathogenesis of related human afflictions like rheumatoid arthritis also remains to be clarified. The picture is in some respects clearer in regard to inflammatory bowel disease, especially ulcerative colitis, which has been long associated with increases in mast cell numbers and activation in affected tissues 144, 145. At least two pharmacological lines of evidence suggest that tryptases released from mast cells contribute to the pathology of ulcerative colitis: 1) a human trial of a β-tryptase-selective inhibitor given systemically by subcutaneous injection appeared to reduce gastrointestinal symptoms in subjects with ulcerative colitis 146 and 2) treatment with the tryptase inhibitor nafamostat decreased the severity of pathological findings in a rat model of colitis caused by trinitrobenzene sulfonic acid 147.
Chymases and cathepsin G
Allergic inflammation in airway and skin
The ability of human chymase and cathepsin G to cleave angiotensin I selectively at Phe8 to generate angiotensin II, which can be a homeostatic process assisting support of blood pressure—but can also be pro-inflammatory—was summarized earlier. However, both enzymes have effects that are more classically inflammatory, especially by promoting tissue swelling. For example, dog chymase, injected into dog skin, increases the size of histamine-induced wheals without inducing wheals by itself 148. Furthermore, inhibition of chymase activity in vivo reduces size of wheals generated by mast cell-degranulating agents. The mechanism of these effects are not known, but could include breakdown and untethering of extracellular matrix so that fluid extravasated under the influence of histamine travels farther than it would do otherwise. Both chymase and cathepsin G are fairly omnivorous, and can separate the dermal-epidermal junction by degrading a variety of matrix proteins 149, as well as by activating MMPs 49. Possibly, these enzymes also destroy extracellular histaminases so that histamine levels are higher and more sustained. Chymase and cathepsin G also can stimulate gland secretion 150, 151. Indeed, in human airway, chymase-positive mast cells are a high fraction of mast cells lying within 20 μm of submucosal glands 152. The proteolytic activity of human chymase extends to albumin 153; however, this cleavage would not increase oncotic pressure because the nicked fragments remain joined by disulfide linkages. Acting subacutely or chronically, chymase-like peptidases also may promote inflammatory angiogenesis, as suggested by sponge granulomas in hamsters 154 and by mast cell-dependent angiogenesis in a model of skin carcinogenesis 49. The recently reported inhibition of several animal models of allergic and non-allergic inflammation by inhibitors of chymase and cathepsin G 80 further suggests that these enzymes are broadly capable of promoting inflammation. Possibly, they act synergistically when released from human mast cells, where they are usually found in the same granules, because their substrate preferences only partially overlap.
Ischemia-reperfusion injury, aneurysms and vascular stenosis
A mouse model of irreversible ischemia-reperfusion injury suggests that a specific mouse mast cell protease, mMCP-5, is responsible for irreversibly injuring skeletal muscle 155. This work establishes the principle that a mast cell protease can be cytotoxic in the context of ischemic inflammation, which is associated with mast cell activation. The mechanism by which muscle is damaged by mMCP-5, which is an elastolytic peptidase with no known functional equivalent in human mast cells, is not clear. However, the potential for chymase-related mast cell peptidases to damage and alter vessels themselves has gathered increased experimental support over the past few years. After initial studies suggested a role for mast cells in promoting arterial enlargement in a neutrophil elastase-induced mouse model of aortic aneurysm 156, subsequent studies showed at least partial dependence on mast cell expression of a particular chymase, mMCP-4 103. The mechanism by which this chymase promotes aneurysm formation in this model is hypothesized to include inflammatory activation of vessel wall-weakening MMPs and cathepsins and direct stimulation of aortic smooth muscle cell apoptosis. On the other hand, there is strong in vivo pharmacological evidence from studies in a variety of models of vascular injury that chymase-like enzymes promote cardiovascular remodeling, fibrosis and stenosis in response to injury 62, 88, 157–160.
Dipeptidyl peptidase I and other thiol cathepsins
Membrane-bound mast cell secretory granules harboring biogenic amines, proteases and proteoglycans are related to lysosomes and partly may serve lysosomal functions. Indeed they contain some lysosome-associated proteases. Cathepsin G is not a classic cathepsin because it is a serine (not thiol) peptidase with expression restricted to specialized granules of mast and myelomonocytic cells, especially neutrophils. Another granule peptidase, dipeptidyl peptidase I (DPPI; cathepsin C) also is atypical. Although it is expressed in many cells, it is much more abundant in mast cells, myelomonocytes and other specialized granulated cells, like cytotoxic T and natural killer cells. In uninflamed dog airway, mast cells are the dominant cell type staining positively for DPPI 161. Like other proteins of secretory granules, DPPI can be secreted 162 and it may cleave extracellular targets 161. However, it is not highly destructive because its activities are restricted compared to other thiol cathepsins by an “exclusion domain” ensuring preference for cleaving N-terminal dipeptides 163. These attributes suggest that DPPI is likely to serve an intragranular function not related to general protein degradation or typical lysosomal activity. At present, DPPI’s major identified role is to activate granule-associated immune cell peptidases related to chymases, cathepsin G, lymphocyte granzymes, and neutrophil elastase. It accomplishes this by removing the N-terminal pro-dipeptide that is a shared attribute of these enzymes 164–167. Given the effects of genetic inactivation of DPPI on activation of an impressive range of conserved immune serine peptidases, the phenotype might be expected to be more severe than it is. In fact, genetic deletion or inactivation of DPPI is not lethal to mice protected from infections, but DPPI-deficient mice have a variety of immune deficits and altered responses to sepsis 14, 168, including improved short term survival following cecal ligation and puncture 45. Humans with defects in DPPI have chronic periodontal infections 169. Mice lacking DPPI have little if any mast cell chymase activity, although at least one chymase (mMCP-4) is present in mast cell granules as an inactive pro-enzyme 166. The effect on mouse tryptase mMCP-6 is less dramatic, with activity being reduced but present 166. This is perhaps not surprising given that tryptases possess a much longer pro-peptide than chymases, granzymes and neutrophil elastase-like hydrolases 170, 171. Schwartz and colleagues suggest that the pro-peptide is removed from human β-tryptases by tandem cleavages initiated by autocatalysis to generate a remnant pro-dipeptide removed by DPPI 172. The extent to which human chymases and tryptases depend on DPPI for activation in vivo remains to be determined. Mast cells do express classical lysosomal thiol cathepsins, including cathepsin S, which can influence levels of chymase and/or carboxypeptidase independently of DPPI in mouse mast cells 173, 174. Thus, although DPPI has received more attention in studies of mast cell biology to date, other thiol cathepsins may be important to mast cell function.
Table I.
Comparison of some mast cell proteases in mice and humans.
| Protease class | Human | Mouse |
|---|---|---|
| SERINE | ||
| Tryptase-like | ||
| Active: | βI, βII, βIII, γ | mMCP-6, -7*, γ; mastin/mMCP-11** |
| Inactive: | α, δ, βIIIFS; mastin | mMCP-7* |
| Chymase-like | ||
| Active: | CMA-1/α; Cathepsin G | mMCP-1, -4, -5***; Cathepsin G |
| Inactive: | mMCP-2 | |
| Plasminogen activator | t-PA | ? |
| CYSTEINE/THIOL | ||
| Cathepsins | DPPI/C, ?others | DPPI/C, B, L, S |
| METALLO | ||
| Carboxypeptidase | CPA3 | CPA3 |
| Matrix metallo | MMP-9 | MMP-9 |
| ADAM, other | ? | ADAM17, neurolysin |
| ASPARTYL | Renin | Renin |
not expressed in some strains of laboratory mice
expressed primarily in basophils
elastolytic, not chymotryptic
References
- 1.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 2.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pejler G, Ronnberg E, Waern I, et al. Mast cell proteases-multifaceted regulators of inflammatory disease. Blood. 2010 doi: 10.1182/blood-2010-01-257287. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi NN, Caughey GH. Mast cell peptidases: Chameleons of innate immunity and host defense. Am J Respir Cell Mol Biol. 2010;42:257–267. doi: 10.1165/rcmb.2009-0324RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature. 1988;335:73–75. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- 6.Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol. 1990;3:27–32. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Walls AF, Brain SD, Desai A, et al. Human mast cell tryptase attenuates the vasodilator activity of calcitonin gene-related peptide. Biochem Pharmacol. 1992;43:1243–1248. doi: 10.1016/0006-2952(92)90498-8. [DOI] [PubMed] [Google Scholar]
- 8.Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 9.Corvera CU, Dery O, McConalogue K, et al. Mast cell tryptase regulates rat colonic myocytes through PAR-2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 11.Schmidlin F, Amadesi S, Vidil R, et al. Expression and function of PAR-2 in human bronchial smooth muscle. Am J Respir Crit Care Med. 2001;164:1276–1281. doi: 10.1164/ajrccm.164.7.2101157. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff M, Corvera CU, Thoma MS, et al. PAR-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 13.Compton SJ, Renaux B, Wijesuriya SJ, et al. Glycosylation and the activation of PAR-2 by human mast cell tryptase. Br J Pharmacol. 2001;134:705–718. doi: 10.1038/sj.bjp.0704303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottrell GS, Amadesi S, Pikios S, et al. PAR2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology. 2007;132:2422–2437. doi: 10.1053/j.gastro.2007.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stead RH, Dixon MF, Bramwell NH, et al. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff M, Neisius U, Ikoma A, et al. PrAR-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ui H, Andoh T, Lee JB, et al. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekizawa K, Caughey GH, Lazarus SC, et al. Mast cell tryptase causes airway smooth muscle hyperresponsiveness in dogs. J Clin Invest. 1989;83:175–179. doi: 10.1172/JCI113855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson PRA, Ammit AJ, Carlin SM, et al. Mast cell tryptase potentiates histamine-induced contraction in human sensitized bronchus. Eur Resp J. 1997;10:38–43. doi: 10.1183/09031936.97.10010038. [DOI] [PubMed] [Google Scholar]
- 21.Barrios VE, Middleton SC, Kashem MA, et al. Tryptase mediates hyperresponsiveness in isolated guinea pig bronchi. Life Sci. 1998;63:2295–2303. doi: 10.1016/s0024-3205(98)00518-9. [DOI] [PubMed] [Google Scholar]
- 22.Berger P, Compton SJ, Molimard M, et al. Mast cell tryptase as a mediator of hyperresponsiveness in human isolated bronchi. Clin Exp Allergy. 1999;29:804–812. doi: 10.1046/j.1365-2222.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 23.Molinari JF, Scuri M, Moore WR, et al. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. Am J Respir Crit Care Med. 1996;154:649–653. doi: 10.1164/ajrccm.154.3.8810600. [DOI] [PubMed] [Google Scholar]
- 24.Cocks TM, Fong B, Chow JM, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 25.Caughey GH, Leidig F, Viro NF, et al. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988;244:133–137. [PubMed] [Google Scholar]
- 26.Franconi GM, Graf PD, Lazarus SC, et al. Mast cell chymase and tryptase reverse airway smooth muscle relaxation induced by vasoactive intestinal peptide in the ferret. J Pharmacol Exp Ther. 1989;248:947–951. [PubMed] [Google Scholar]
- 27.Tam EK, Franconi GM, Nadel JA, et al. Protease inhibitors potentiate smooth muscle relaxation induced by vasoactive intestinal peptide in isolated human bronchi. Am J Respir Cell Mol Biol. 1990;2:449–452. doi: 10.1165/ajrcmb/2.5.449. [DOI] [PubMed] [Google Scholar]
- 28.Gruber BL, Marchese MJ, Suzuki K, et al. Synovial procollagenase activation by human mast cell tryptase. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M, Kume H, Oguma T, et al. Mast cell tryptase causes homologous desensitization of β-adrenoceptors by Ca2+ sensitization in tracheal smooth muscle. Clin Exp Allergy. 2008;38:135–144. doi: 10.1111/j.1365-2222.2007.02879.x. [DOI] [PubMed] [Google Scholar]
- 30.Piliponsky AM, Chen CC, Nishimura T, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14:392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 32.Schneider LA, Schlenner SM, Feyerabend TB, et al. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano A, Kishi F, Minami K, et al. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159:1987–1992. [PubMed] [Google Scholar]
- 34.Nagata N, Niwa Y, Nakaya Y. A novel 31-amino-acid-length endothelin, ET-1(1–31), can act as a biologically active peptide for vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;275:595–600. doi: 10.1006/bbrc.2000.3292. [DOI] [PubMed] [Google Scholar]
- 35.Nagase T, Kurihara H, Kurihara Y, et al. Airway hyperresponsiveness to methacholine in mutant mice deficient in endothelin-1. Am J Respir Crit Care Med. 1998;157:560–564. doi: 10.1164/ajrccm.157.2.9706009. [DOI] [PubMed] [Google Scholar]
- 36.Irani AM, Goldstein SM, Wintroub BU, et al. Human mast cell carboxypeptidase: Selective localization to MCTC cells. J Immunol. 1991;147:247–253. [PubMed] [Google Scholar]
- 37.Goldstein SM, Leong J, Schwartz LB, et al. Protease composition of exocytosed human skin mast cell protease-proteoglycan complexes: tryptase resides in a complex distinct from chymase and carboxypeptidase. J Immunol. 1992;148:2475–2482. [PubMed] [Google Scholar]
- 38.Dougherty RH, Sidhu SS, Raman K, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in Th2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiemann F, Brandt E, Gross R, et al. The cathelicidin LL-37 activates human mast cells and is degraded by mast cell tryptase: counter-regulation by CXCL4. J Immunol. 2009;183:2223–2231. doi: 10.4049/jimmunol.0803587. [DOI] [PubMed] [Google Scholar]
- 40.Schiemann F, Grimm TA, Hoch J, et al. Mast cells and neutrophils proteolytically activate chemokine precursor CTAP-III and are subject to counterregulation by PF-4 through inhibition of chymase and cathepsin G. Blood. 2006;107:2234–2242. doi: 10.1182/blood-2005-06-2424. [DOI] [PubMed] [Google Scholar]
- 41.Zhao W, Oskeritzian CA, Pozez AL, et al. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 42.Schechter NM, Sprows JL, Schoenberger OL, et al. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–21315. [PubMed] [Google Scholar]
- 43.Walter M, Sutton RM, Schechter NM. Highly efficient inhibition of human chymase by α2-macroglobulin. Arch Biochem Biophys. 1999;368:276–284. doi: 10.1006/abbi.1999.1309. [DOI] [PubMed] [Google Scholar]
- 44.Raymond WW, Su S, Makarova A, et al. α2-Macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. J Immunol. 2009;182:5770–5777. doi: 10.4049/jimmunol.0900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallen-St Clair J, Pham CT, Villalta SA, et al. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metz M, Piliponsky AM, Chen CC, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 47.Fang KC, Raymond WW, Lazarus SC, et al. Dog mastocytoma cells secrete a 92-kd gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang KC, Wolters PJ, Steinhoff M, et al. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-β. J Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- 49.Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells upregulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baram D, Vaday GG, Salamon P, et al. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNFα. J Immunol. 2001;167:4008–4016. doi: 10.4049/jimmunol.167.7.4008. [DOI] [PubMed] [Google Scholar]
- 51.Tchougounova E, Lundequist A, Fajardo I, et al. A key role for mast cell chymase in the activation of pro-MMP-9 and pro-MMP-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 52.McQuibban GA, Gong JH, Tam EM, et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 53.Greenlee KJ, Corry DB, Engler DA, et al. Proteomic identification of in vivo substrates for MMPs 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177:7312–7321. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baluk P, Raymond WW, Ator E, et al. Matrix metalloproteinase-2 and -9 expression increases in mycoplasma-infected airways but is not required for microvascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L307–317. doi: 10.1152/ajplung.00404.2003. [DOI] [PubMed] [Google Scholar]
- 56.Corry DB, Rishi K, Kanellis J, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corry DB, Kiss A, Song LZ, et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. Faseb J. 2004;18:995–997. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukkarinen H, Hogmalm A, Lappalainen U, et al. MMP-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2009;41:59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka A, Yamane Y, Matsuda H. Mast cell MMP-9 production enhanced by bacterial lipopolysaccharide. J Vet Med Sci. 2001;63:811–813. doi: 10.1292/jvms.63.811. [DOI] [PubMed] [Google Scholar]
- 60.Fang KC, Raymond WW, Blount JL, et al. Dog mast cell α-chymase activates progelatinase B by cleaving the Phe88-Phe89 and Phe91-Glu92 bonds of the catalytic domain. J Biol Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 61.Frank BT, Rossall JC, Caughey GH, et al. Mast cell tissue inhibitor of metalloproteinase-1 is cleaved and inactivated extracellularly by α-chymase. J Immunol. 2001;166:2783–2792. doi: 10.4049/jimmunol.166.4.2783. [DOI] [PubMed] [Google Scholar]
- 62.Kishi K, Muramatsu M, Jin D, et al. The effects of chymase on MMP-2 activation in neointimal hyperplasia after balloon injury in dogs. Hypertens Res. 2007;30:77–83. doi: 10.1291/hypres.30.77. [DOI] [PubMed] [Google Scholar]
- 63.Ishida K, Takai S, Murano M, et al. Role of chymase-dependent MMP-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2008;324:422–426. doi: 10.1124/jpet.107.131946. [DOI] [PubMed] [Google Scholar]
- 64.Inoue N, Muramatsu M, Jin D, et al. Effects of chymase inhibitor on angiotensin II-induced abdominal aortic aneurysm development in apolipoprotein E-deficient mice. Atherosclerosis. 2009;204:359–364. doi: 10.1016/j.atherosclerosis.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 65.Rueff F, Przybilla B, Bilo MB, et al. Predictors of severe systemic anaphylactic reactions in patients with hymenoptera venom allergy: importance of baseline serum tryptase. J Allergy Clin Immunol. 2009;124:1047–1054. doi: 10.1016/j.jaci.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 66.Marshall RP, Gohlke P, Chambers RC, et al. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 67.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reilly CF, Tewksbury DA, Schechter NB, et al. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem. 1982;257:8619–8622. [PubMed] [Google Scholar]
- 69.Wintroub BU, Schechter NB, Lazarus GS, et al. Angiotensin I conversion by human and rat chymotryptic proteinases. J Invest Derm. 1984;83:336–339. doi: 10.1111/1523-1747.ep12264144. [DOI] [PubMed] [Google Scholar]
- 70.Urata H, Kinoshita A, Misono KS, et al. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 71.Urata H, Kinoshita A, Perez DM, et al. Cloning of the gene and cDNA for human heart chymase. J Biol Chem. 1991;266:17173–17179. [PubMed] [Google Scholar]
- 72.Caughey GH, Zerweck EH, Vanderslice P. Structure, chromosomal assignment, and deduced amino acid sequence of a human gene for mast cell chymase. J Biol Chem. 1991;266:12956–12963. [PubMed] [Google Scholar]
- 73.Caughey GH, Schaumberg TH, Zerweck EH, et al. The human mast cell chymase gene (CMA1): mapping to the cathepsin G/granzyme gene cluster and lineage-restricted expression. Genomics. 1993;15:614–620. doi: 10.1006/geno.1993.1115. [DOI] [PubMed] [Google Scholar]
- 74.Woodbury RG, Everitt M, Sanada Y, et al. A major serine protease in rat skeletal muscle: Evidence for its mast cell origin. Proc Natl Acad Sci U S A. 1978;75:5311–5313. doi: 10.1073/pnas.75.11.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woodbury RG, Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980;114:189–195. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]
- 76.Woodbury RG, Gruzenski GM, Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978;75:2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schechter NM, Irani AM, Sprows JL, et al. Identification of a cathepsin G-like proteinase in the mctc type of human mast cell. J Immunol. 1990;145:2652–2661. [PubMed] [Google Scholar]
- 78.Benyon RC, Enciso JA, Befus AD. Analysis of human skin mast cell proteins by two-dimensional gel electrophoresis: identification of tryptase as a sialylated glycoprotein. J Immunol. 1993;151:2699–2706. [PubMed] [Google Scholar]
- 79.de Garavilla L, Greco MN, Sukumar N, et al. A novel, potent dual inhibitor of the leukocyte proteases cathepsin G and chymase: molecular mechanisms and anti-inflammatory activity in vivo. J Biol Chem. 2005;280:18001–18007. doi: 10.1074/jbc.M501302200. [DOI] [PubMed] [Google Scholar]
- 80.Maryanoff BE, de Garavilla L, Greco MN, et al. Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am J Respir Crit Care Med. 2010;181:247–253. doi: 10.1164/rccm.200904-0627OC. [DOI] [PubMed] [Google Scholar]
- 81.Lundequist A, Tchougounova E, Abrink M, et al. Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II. J Biol Chem. 2004;279:32339–32344. doi: 10.1074/jbc.M405576200. [DOI] [PubMed] [Google Scholar]
- 82.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell α- and β-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 83.Wei CC, Hase N, Inoue Y, et al. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010 doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei CC, Meng QC, Palmer R, et al. Evidence for angiotensin-converting enzyme- and chymase-mediated angiotensin ii formation in the interstitial fluid space of the dog heart in vivo. Circulation. 1999;99:2583–2589. doi: 10.1161/01.cir.99.19.2583. [DOI] [PubMed] [Google Scholar]
- 85.Ju H, Gros R, You X, et al. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice. Proc Natl Acad Sci U S A. 2001;98:7469–7474. doi: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiota N, Okunishi H, Takai S, et al. Tranilast suppresses vascular chymase expression and neointima formation in balloon-injured dog carotid artery. Circulation. 1999;99:1084–1090. doi: 10.1161/01.cir.99.8.1084. [DOI] [PubMed] [Google Scholar]
- 87.Sakaguchi M, Takai S, Jin D, et al. A specific chymase inhibitor, NK3201, suppresses bleomycin-induced pulmonary fibrosis in hamsters. Eur J Pharmacol. 2004;493:173–176. doi: 10.1016/j.ejphar.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 88.Takai S, Jin D, Muramatsu M, et al. Therapeutic applications of chymase inhibitors in cardiovascular diseases and fibrosis. Eur J Pharmacol. 2004;501:1–8. doi: 10.1016/j.ejphar.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 89.Mackins CJ, Kano S, Seyedi N, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kano S, Tyler E, Salazar-Rodriguez M, et al. Immediate hypersensitivity elicits renin release from cardiac mast cells. Int Arch Allergy Immunol. 2008;146:71–75. doi: 10.1159/000112505. [DOI] [PubMed] [Google Scholar]
- 91.Knight PA, Wright SH, Lawrence CE, et al. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wastling JM, Scudamore CL, Thornton EM, et al. Constitutive expression of mouse mast cell protease-1 in normal Balb/c mice and its up-regulation during intestinal nematode infection. Immunology. 1997;90:308–313. doi: 10.1046/j.1365-2567.1997.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight PA, Wright SH, Brown JK, et al. Enteric expression of the integrin αvβ6 is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol. 2002;161:771–779. doi: 10.1016/s0002-9440(10)64236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pemberton AD, Wright SH, Knight PA, et al. Anaphylactic release of mucosal mast cell granule proteases: role of serpins in the differential clearance of mouse mast cell proteases-1 and -2. J Immunol. 2006;176:899–904. doi: 10.4049/jimmunol.176.2.899. [DOI] [PubMed] [Google Scholar]
- 95.Groschwitz KR, Ahrens R, Osterfeld H, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of PAR-2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 97.Mellon MB, Frank BT, Fang KC. Mast cell α-chymase reduces ige recognition of birch pollen profilin by cleaving antibody-binding epitopes. J Immunol. 2002;168:290–297. doi: 10.4049/jimmunol.168.1.290. [DOI] [PubMed] [Google Scholar]
- 98.Balzar S, Chu HW, Strand M, et al. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am J Respir Crit Care Med. 2005;171:431–439. doi: 10.1164/rccm.200407-949OC. [DOI] [PubMed] [Google Scholar]
- 99.Waern I, Jonasson S, Hjoberg J, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 100.Lazaar AL, Plotnick MI, Kucich U, et al. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J Immunol. 2002;169:1014–1020. doi: 10.4049/jimmunol.169.2.1014. [DOI] [PubMed] [Google Scholar]
- 101.Leskinen M, Wang Y, Leszczynski D, et al. Mast cell chymase induces apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:516–522. doi: 10.1161/01.atv.21.4.516. [DOI] [PubMed] [Google Scholar]
- 102.Leskinen MJ, Heikkila HM, Speer MY, et al. Mast cell chymase induces smooth muscle cell apoptosis by disrupting nf-kappab-mediated survival signaling. Exp Cell Res. 2006;312:1289–1298. doi: 10.1016/j.yexcr.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 103.Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: Structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 105.Malaviya R, Ross E, Jakschik BA, et al. Mast cell degranulation induced by type 1 fimbriated Escherichia coli in mice. J Clin Invest. 1994;93:1645–1653. doi: 10.1172/JCI117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malaviya R, Ikeda T, Ross E, et al. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNFα. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 107.Sutherland RE, Olsen JS, McKinstry A, et al. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang C, Friend DS, Qiu WT, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- 109.Thakurdas SM, Melicoff E, Sansores-Garcia L, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 110.Huang C, De Sanctis GT, O’Brien PJ, et al. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 111.Soto D, Malmsten C, Blount JL, et al. Genetic deficiency of human mast cell α-tryptase. Clin Exp Allergy. 2002;32:1000–1006. doi: 10.1046/j.1365-2222.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 112.Trivedi NN, Raymond WW, Caughey GH. Chimerism, point mutation, and truncation dramatically transformed mast cell δ-tryptases during primate evolution. J Allergy Clin Immunol. 2008;121:1262–1268. doi: 10.1016/j.jaci.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 113.Trivedi NN, Tamraz B, Chu C, et al. Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J Allergy Clin Immunol. 2009;124:1099–1105. doi: 10.1016/j.jaci.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz LB, Atkins PC, Bradford TR, et al. Release of tryptase together with histamine during the immediate cutaneous response to allergen. J Allergy Clin Immunol. 1987;80:850–855. doi: 10.1016/s0091-6749(87)80276-2. [DOI] [PubMed] [Google Scholar]
- 115.Shalit M, Schwartz LB, Golzar N, et al. Release of histamine and tryptase in vivo after prolonged cutaneous challenge with allergen in humans. J Pharmacol Exp Ther. 1988;244:133–137. [PubMed] [Google Scholar]
- 116.Wenzel SE, Fowler A, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. Am Rev Respir Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 117.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oh S-W, Pae CI, Lee D-K, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 119.Chen CL, Wang SD, Zeng ZY, et al. Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma. J Allergy Clin Immunol. 2006;118:105–112. doi: 10.1016/j.jaci.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 120.Mori S, Itoh Y, Shinohata R, et al. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci. 2003;92:420–423. doi: 10.1254/jphs.92.420. [DOI] [PubMed] [Google Scholar]
- 121.Sommerhoff CP, Sollner C, Mentele R, et al. A kazal-type inhibitor of human mast cell tryptase: isolation from the medicinal leech Hirudo medicinalis, characterization, and sequence analysis. Biol Chem Hoppe-Seyler. 1994;375:685–694. doi: 10.1515/bchm3.1994.375.10.685. [DOI] [PubMed] [Google Scholar]
- 122.Krishna MT, Chauhan A, Little L, et al. Inhibition of mast cell tryptase by inhaled APC366 attenuates allergen-induced late-phase airway obstruction in asthma. J Allergy Clin Immunol. 2001;107:1039–1045. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- 123.Erin EM, Leaker BR, Zacharasiewicz A, et al. Effects of a reversible β-tryptase and trypsin inhibitor (RWJ-58643) on nasal allergic responses. Clin Exp Allergy. 2006;36:458–464. doi: 10.1111/j.1365-2222.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 124.He S, Aslam A, Gaca MD, et al. Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: effects of tryptase and other agonists of PAR-2 on histamine release. J Pharmacol Exp Ther. 2004;309:119–126. doi: 10.1124/jpet.103.061291. [DOI] [PubMed] [Google Scholar]
- 125.He S, Gaca MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 126.Molinari JF, Moore WR, Clark J, et al. Role of tryptase in immediate cutaneous responses in allergic sheep. J Appl Physiol. 1995;79:1966–1970. doi: 10.1152/jappl.1995.79.6.1966. [DOI] [PubMed] [Google Scholar]
- 127.Clark JM, Abraham WM, Fishman CE, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Resp Crit Care Med. 1995;152:2076–2083. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- 128.Wright CD, Havill AM, Middleton SC, et al. Inhibition of allergen-induced pulmonary responses by the selective tryptase inhibitor 1,5-bis-[4-[(3-carbamimidoyl-benzenesulfonylamino)-methyl]-phenoxy]-pen tane (amg-126737) Biochem Pharmacol. 1999;58:1989–1996. doi: 10.1016/s0006-2952(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 129.Rice KD, Wang VR, Gangloff AR, et al. Dibasic inhibitors of human mast cell tryptase. Part 2: Structure-activity relationships and requirements for potent activity. Bioorg Med Chem Lett. 2000;10:2361–2366. doi: 10.1016/s0960-894x(00)00485-6. [DOI] [PubMed] [Google Scholar]
- 130.Cairns JA. Inhibitors of mast cell tryptase β as therapeutics for the treatment of asthma and inflammatory disorders. Pulm Pharmacol Ther. 2005;18:55–66. doi: 10.1016/j.pupt.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 131.Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991;88:493–499. doi: 10.1172/JCI115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hartmann T, Ruoss SJ, Caughey GH. Modulation of thrombin and thrombin receptor peptide mitogenicity by human lung mast cell tryptase. Am J Physiol Lung Cell Mol Physiol. 1994;267:L113–L119. doi: 10.1152/ajplung.1994.267.2.L113. [DOI] [PubMed] [Google Scholar]
- 133.Gruber BL, Kew RR, Jelaska A, et al. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158:2310–2317. [PubMed] [Google Scholar]
- 134.Cairns JA, Walls AF. Mast cell tryptase stimulates synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brown JK, Tyler CL, Jones CA, et al. Tryptase, the dominant secretory granular protein in human mast cells, is a potent mitogen for cultured dog tracheal smooth muscle cells. Am J Resp Cell Mol Biol. 1995;13:227–236. doi: 10.1165/ajrcmb.13.2.7626290. [DOI] [PubMed] [Google Scholar]
- 136.Blair RJ, Meng H, Marchese MJ, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McNeil HP, Shin K, Campbell IK, et al. The mouse mast cell-restricted tetramer-forming tryptases mMCP6 and mMCP7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58:2338–2346. doi: 10.1002/art.23639. [DOI] [PubMed] [Google Scholar]
- 138.Shin K, Nigrovic PA, Crish J, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buckley MG, Walters C, Wong WM, et al. Mast cell activation in arthritis: detection of α- and β-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci (Colch) 1997;93:363–370. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- 140.Lee DM, Friend DS, Gurish MF, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 141.Nigrovic PA, Binstadt BA, Monach PA, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL–1. Proc Natl Acad Sci U S A. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 143.Zhou JS, Xing W, Friend DS, et al. Mast cell deficiency in KitW-sh mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Balazs M, Illyes G, Vadasz G. Mast cells in ulcerative colitis. Quantitative and ultrastructural studies. Virchows Arch. 1989;57:353–360. doi: 10.1007/BF02899101. [DOI] [PubMed] [Google Scholar]
- 145.Fox CC, Lazenby AJ, Moore WC, et al. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology. 1990;99:119–124. doi: 10.1016/0016-5085(90)91238-2. [DOI] [PubMed] [Google Scholar]
- 146.Tremaine WJ, Brzezinski A, Katz JA, et al. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC2059): an open-label pilot study. Aliment Pharmacol Ther. 2002;16:407–413. doi: 10.1046/j.1365-2036.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- 147.Isozaki Y, Yoshida N, Kuroda M, et al. Anti-tryptase treatment using nafamostat mesilate has a therapeutic effect on experimental colitis. Scand J Gastroenterol. 2006;41:944–953. doi: 10.1080/00365520500529470. [DOI] [PubMed] [Google Scholar]
- 148.Rubinstein I, Nadel JA, Graf PD, et al. Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J Clin Invest. 1990;86:555–559. doi: 10.1172/JCI114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Briggaman RA, Schechter NM, Fraki J, et al. Degradation of the epidermal-dermal junction by proteolytic enzymes from human skin and human polymorphonuclear leukocytes. J Exp Med. 1984;160:1027–1042. doi: 10.1084/jem.160.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sommerhoff CP, Caughey GH, Finkbeiner WE, et al. Mast cell chymase. A potent secretagogue for airway gland serous cells. J Immunol. 1989;142:2450–2456. [PubMed] [Google Scholar]
- 151.Sommerhoff CP, Nadel JA, Basbaum CB, et al. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990;85:682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matin R, Tam EK, Nadel JA, et al. Distribution of chymase-containing mast cells in human bronchi. J Histochem Cytochem. 1992;40:781–786. doi: 10.1177/40.6.1588024. [DOI] [PubMed] [Google Scholar]
- 153.Raymond WW, Waugh Ruggles S, Craik CS, et al. Albumin is a substrate of human chymase: prediction by combinatorial peptide screening and development of a selective inhibitor based on the albumin cleavage site. J Biol Chem. 2003;278:34517–34524. doi: 10.1074/jbc.M304087200. [DOI] [PubMed] [Google Scholar]
- 154.Muramatsu M, Katada J, Hattori M, et al. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur J Pharmacol. 2000;402:181–191. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 155.Abonia JP, Friend DS, Austen WG, Jr, et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol. 2005;174:7285–7291. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sun J, Sukhova GK, Yang M, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shiota N, Fukamizu A, Takai S, et al. Activation of angiotensin II-forming chymase in the cardiomyopathic hamster heart. J Hypertens. 1997;15:431–440. doi: 10.1097/00004872-199715040-00014. [DOI] [PubMed] [Google Scholar]
- 158.Takai S, Shiota N, Kobayashi S, et al. Induction of chymase that forms angiotensin II in the monkey atherosclerotic aorta. FEBS Lett. 1997;412:86–90. doi: 10.1016/s0014-5793(97)00752-7. [DOI] [PubMed] [Google Scholar]
- 159.Takai S, Yuda A, Jin D, et al. Inhibition of chymase reduces vascular proliferation in dog grafted veins. FEBS Lett. 2000;467:141–144. doi: 10.1016/s0014-5793(00)01125-x. [DOI] [PubMed] [Google Scholar]
- 160.Nishimoto M, Takai S, Kim S, et al. Significance of chymase-dependent angiotensin II-forming pathway in the development of vascular proliferation. Circulation. 2001;104:1274–1279. doi: 10.1161/hc3601.094304. [DOI] [PubMed] [Google Scholar]
- 161.Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- 162.Wolters PJ, Raymond WW, Blount JL, et al. Regulated expression, processing, and secretion of dog mast cell dipeptidyl peptidase I. J Biol Chem. 1998;273:15514–15520. doi: 10.1074/jbc.273.25.15514. [DOI] [PubMed] [Google Scholar]
- 163.Turk D, Janjic V, Stern I, et al. Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 2001;20:6570–6582. doi: 10.1093/emboj/20.23.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McGuire MJ, Lipsky PE, Thiele DL. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J Biol Chem. 1993;268:2458–2467. [PubMed] [Google Scholar]
- 165.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wolters PJ, Pham CT, Muilenburg DJ, et al. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- 167.Caughey GH. A pulmonary perspective on GASPIDs: Granule-associated serine peptidases of immune defense. Curr Resp Med Rev. 2006;2:263–277. doi: 10.2174/157339806778019024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Adkison AM, Raptis SZ, Kelley DG, et al. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pham CT, Ivanovich JL, Raptis SZ, et al. Papillon-Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173:7277–7281. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 170.Vanderslice P, Ballinger SM, Tam EK, et al. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci USA. 1990;87:3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vanderslice P, Craik CS, Nadel JA, et al. Molecular cloning of dog mast cell tryptase and a related protease: structural evidence of a unique mode of serine protease activation. Biochemistry. 1989;28:4148–4155. doi: 10.1021/bi00436a004. [DOI] [PubMed] [Google Scholar]
- 172.Sakai K, Ren S, Schwartz LB. A novel heparin-dependent processing pathway for human tryptase: autocatalysis followed by activation with dipeptidyl peptidase I. J Clin Invest. 1996;97:988–995. doi: 10.1172/JCI118523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Henningsson F, Yamamoto K, Saftig P, et al. A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci. 2005;118:2035–2042. doi: 10.1242/jcs.02333. [DOI] [PubMed] [Google Scholar]
- 174.Henningsson F, Wolters P, Chapman HA, et al. Mast cell cathepsins C and S control levels of carboxypeptidase A and the chymase, mouse mast cell protease 5. Biol Chem. 2003;384:1527–1531. doi: 10.1515/BC.2003.169. [DOI] [PubMed] [Google Scholar]