Abstract
So far, numerous genes have been found to associate with various strategies to resist and transform the toxic metalloid arsenic (here, we denote these genes as “arsenic-related genes”). However, our knowledge of the distribution, redundancies and organization of these genes in bacteria is still limited. In this study, we analyzed the 188 Burkholderiales genomes and found that 95% genomes harbored arsenic-related genes, with an average of 6.6 genes per genome. The results indicated: a) compared to a low frequency of distribution for aio (arsenite oxidase) (12 strains), arr (arsenate respiratory reductase) (1 strain) and arsM (arsenite methytransferase)-like genes (4 strains), the ars (arsenic resistance system)-like genes were identified in 174 strains including 1,051 genes; b) 2/3 ars-like genes were clustered as ars operon and displayed a high diversity of gene organizations (68 forms) which may suggest the rapid movement and evolution for ars-like genes in bacterial genomes; c) the arsenite efflux system was dominant with ACR3 form rather than ArsB in Burkholderiales; d) only a few numbers of arsM and arrAB are found indicating neither As III biomethylation nor AsV respiration is the primary mechanism in Burkholderiales members; (e) the aio-like gene is mostly flanked with ars-like genes and phosphate transport system, implying the close functional relatedness between arsenic and phosphorus metabolisms. On average, the number of arsenic-related genes per genome of strains isolated from arsenic-rich environments is more than four times higher than the strains from other environments. Compared with human, plant and animal pathogens, the environmental strains possess a larger average number of arsenic-related genes, which indicates that habitat is likely a key driver for bacterial arsenic resistance.
Introduction
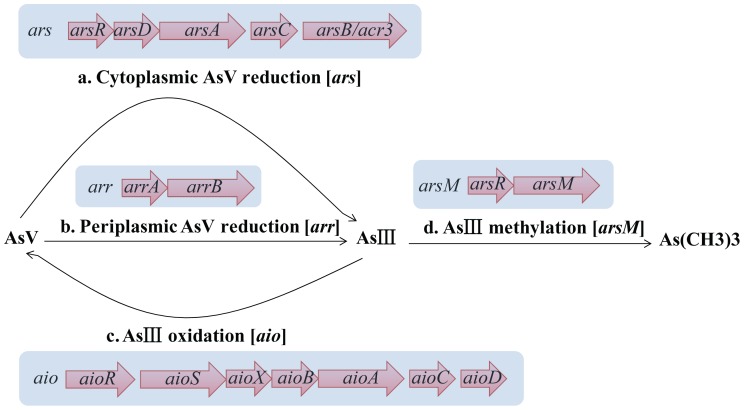
Arsenic (As) is considered one of the most toxic metalloids widely distributed on earth. Due to anthropogenic pollution and natural transformation, many countries have suffered from arsenic contamination and subsequent poisoning. Arsenic contamination, especially of soil and groundwater, has become a global environmental problem. Microbes play an important role in the global geochemical cycle of arsenic [1], [2]. To adapt to habitats contaminated with arsenic, microbes have developed multiple strategies for resistance to and transformation of arsenic. These strategies have primarily included the following: 1) cytoplasmic/periplasmic AsV reduction and As III extrusion; 2) As III oxidation and AsV extrusion; and 3) As III methylation and volatilization by way of the formation of a gas, also called biomethylation [2]–[6]. These strategies are summarized in Figure 1, and the genes associated with those processes are listed in Table 1.
Figure 1. Four major metabolic strategies for arsenic resistance and transformation were found in microbes.
a) cytoplasmic AsV reduction by ArsC and As III extrusion by ArsB or ACR3; b) periplasmic AsV reduction under anaerobic conditions by ArrAB; c) As III oxidation by AioAB and AsV extrusion through a phosphate transporter system; d) As III methylation to the gaseous compound As(CH)3 by ArsM. The gene organizations representative of these four processes are shown in the pale blue box, and the corresponding functions of the genes are listed in Table 1.
Table 1. Arsenic-related genes involved in bacterial arsenic resistance and transformation.
| Family | Gene | Product |
| ars | arsR | Arsenic transcriptional regulator |
| arsB | Arsenic efflux pump | |
| arsC | Arsenate reductase | |
| arsH | Putative flavoprotein | |
| acr3 | Arsenic efflux pump ACR3 family | |
| arsA | Arsenite active ATPase | |
| arsD | ars operon trans-acting repressor | |
| arsO | Monooxygenase | |
| glo | Glyoxalase/bleomycin resistance protein | |
| mfs | Major facilitator superfamily | |
| arr | arrA | Respiratory As(V) reductase large subunit |
| arrB | Respiratory As(V) reductase small subunit | |
| aio | aioA | Arsenite oxidase large subunit |
| aioB | Arsenite oxidase small subunit | |
| aioX | Phosphonate-binding periplasmic protein | |
| aioS | Periplasmic sensor, signal transduction, histidine kinase | |
| aioR | Two component, sigma54 specific, transcriptionalregulator, Fis family protein | |
| aioC | Cytochrome c, monoheme | |
| aioD | Molybdenum cofactor biosynthesis protein A | |
| arsM | arsM | Arsenite S-adenosylmethyltransferase |
Note: ars, cytoplasmic AsV reduction; arr, periplasmic AsV reduction; aio, arsenite oxidation; arsM, arsenite methylation.
In the past, arsenic-related genes have been reported to be widely distributed in bacterial genomes. The sequences of genes such as arsC, arrA, arsB/acr3, arsM, aioA and aioB displayed significant diversity, as determined through PCR-based approaches [1], [7]–[11] and high-throughput metagenomic approaches [12]–[14]. The PCR-based method is highly dependent on the coverage and specificity of the universal primers used to target the genes of interest. This method can underestimate the abundance of arsenic-related genes if multiple copies of the genes were present in the bacterial genome. As for the high-throughput metagenomic approach, certain false positives would occur due to very small read lengths (approximately 100 bp for an Illumina sequencer and 400–600 bp for a Roche 454 sequencer). Furthermore, this approach could not associate specific genes with the respective strains. Therefore, both approaches lack the complete and reliable information regarding the distribution of arsenic-related genes in individual bacteria. With the rapid development of high-throughput sequencing technology, a large number of microbial genomes have been sequenced in recent years. There is no doubt that genomic sequence of a strain contains nearly all of the information about its arsenic-related genes. Therefore, in this study, we used genomic information to investigate the distribution, abundance and organization of arsenic-related genes in bacteria.
We employed the genome sequences of strains in the Burkholderiales order as a case study to assess the evolution of arsenic related genes. We chose this order based on the numerous factors. 1) Strains in this order display phenotypic, metabolic and ecological diversity, which included bacteria from different niches and lifestyles [15]. 2) To date, a large number of genomes have been sequenced in Burkholderiales, and approximately 215 genomes are available in the National Center for Biotechnology Information (NCBI) database. These available strains include all five families: Burkholderiaceae, Oxalobacteraceae, Alcaligenaceae, Comamonadaceae and Sutterellaceae, as well as the unclassified family. 3) Many previously reported arsenic-resistant and arsenite-oxidizing strains belong to this order, and their genome sequences have been determined [16]–[20]. In the present study, we systematically re-annotated the arsenic-related genes based on protein-similarity, and we compared the relationship between the distribution of arsenic-related genes in strains and their habitats. With the results of this new analysis, we discuss the evolution of arsenic-related genes along the phylogeny of the Burkholderiales order.
Materials and Methods
Genome sequences and annotation
All available genomes of strains belonging to the Burkholderiales order were retrieved from bacterial genome database in NCBI, including 91 complete and 124 draft genomes (genomes available as of Jan 21, 2013). Among the 124 draft genomes, some genomes lacked annotation information. Therefore, we annotated these genomes with the RAST high-quality annotation system [21] using Glimmer 3.0 gene prediction software [22], and the annotation results are stored online (rast.nmpdr.org/; account: smark1984; password: 397310). In addition, the draft genomes with contig number greater than 1,000 were excluded from the analysis if their original genomic annotations were unavailable. In total, 188 genomes were used for the analysis presented in this study.
Phylogenetic analyses
The 16S rRNA gene-based tree was a fast and easy approach to reconstruct the phylogeny of the targeted strains. We first analyzed the phylogeny of these 188 strains using 16S rRNA genes. However, the 16S rRNA gene-based tree of these 188 strains could not clearly distinguish them. Thus, a phylogenetic analysis based on the conservation of proteins shared across the 188 genomes was performed. The conserved proteins of these 188 genomes were identified with blastP, using an “all vs. all” strategy. Based on the blastP analysis (threshold value: e-value = 1-e10; coverage > = 70%; identity > = 50%), the 188 genomes contained 10 conserved genes that had exactly one member per genome, and the lengths of each of the genes were nearly identical. Each set of the conserved proteins was aligned by clustalW [23], and all of the sets of the alignments were concatenated into a string of amino acids for each genome. Finally, the concatenated alignment data were used to infer phylogenetic relationships by PhyML with a maximum-likelihood (ML) algorithm [24]. One-thousand bootstrap repetitions were used to estimate tree reliability.
Arsenic-related gene annotation
Due to the extreme diversity in arsenic-related genes (such as arsR and arsC) [25], the annotated information of the genomes in NCBI or in the RAST system may include incorrect annotations for numerous genes. For example, some of the arsenic-related genes were annotated with other names. Thus, it is not appropriate to identify these genes simply by the names of their proteins. Therefore, we extracted the arsenic-related gene information according to our re-annotation strategy, as illustrated in Figure S1. First, we built a preliminary-screening database by gathering the arsenic-related sequences from the NCBI protein database. All of the predicted proteomics sets from these 188 genomes were searched against this “self-build arsenic database” using the blastP algorithm, and we used a custom Perl script to parse the blast results with conventional criteria (e-value = 1-e10; coverage> = 70%; identity > = 35%) to obtain the candidate genes. The candidate genes were filtered through protein functional classification, Clusters of Orthologous Groups (COG) [26] and ortholog clustering analyses by OrthoMCL, with an inflation value of 1.5 [27]. According to the results that we obtained, the relatively pure arsenic-related genes were divided to two groups (scattered genes and genes clustered together) by a manual analysis. Apparently, the genes clustered together were the actual arsenic-related genes. The scattered genes were searched against the genes that clustered together for further confirmation.
Heatmap analysis of the distribution of arsenic-related genes
To clearly display the distribution of the arsenic-related genes in these 188 strains, we made a matrix with 188 rows and 21 columns, in which the rows represented the 188 strains and the columns represented an individual arsenic-related gene or ars-like cluster in each strain. From top to bottom, the 188 strains were ordered according to the sequence of the strains in the core genes-based phylogenetic tree. This matrix was used to produce a heatmap with a custom script written in the R based language (http://www.r-project.org/).
Results
Overall information on the 188 Burkholderiales genomes
As of Jan. 21th, 2013, 215 strains in the Burkholderiales order have been sequenced, and most of these strains are involved in pathogenicity and other bio-applications (http://www.ncbi.nlm.nih.gov/genome/?term=Burkholderiales). To associate the distribution of arsenic-related genes with their phylogenetic affiliation, we first tried to determinate the phylogenetic structure among these strains. Our analysis was based on the core genomes of these strains rather than 16S genes because the 16S gene-based phylogenetic tree made it difficult to distinguish the actual relationships (Figure S2). To maintain a suitable size of core genes, 188 genomes were selected for phylogenetic interference and used for the subsequent analysis in this study (Table 2). Based on our analysis, 10 genes were shared among the 188 genomes, and these conserved proteins were used to construct a ML tree. As shown in Figure 2, the core gene-base tree could clearly group the strains into five families and one unclassified family, representing 35 genera and 70 species. The selected 188 strains were distributed among a diversity of ecological sites. According to the isolation sources [15], we could classify these strains into different groups (Table S1). These groups include the following: (i) human host (58 strains, denoted H in Table S1), (ii) plant pathogens (14 strains, P), (iii) animal host (11 strains, Z), (iv) rhizosphere and root nodules (27 strains, R), (v) soil (25 strains, S), (vi) sediment (7 strains, D), (vii) wastewater and sludge (23 strains, W), (viii) endosymbionts (3 strains, E) and (ix) miscellaneous sources (12 strains, U). In addition, the isolation sources of eight strains were unavailable (denoted NA in Table S1). Among these strains, Achromobacter arsenitoxydans SY8, Acidovorax sp. NO1, Alcaligenes faecalis subsp. faecalis NCIB 8687, Herminiimonas arsenicoxydans ULPAs1 and Thiomonas sp. 3As were the sequenced arsenite oxidizers isolated from niches contaminated with arsenic, in which, the mechanisms related to arsenic resistance and arsenite oxidation have been widespread investigated [16]–[19], [28]–[33].
Table 2. Phylogenetic information on the 188 Burkholderiales bacterial genomes.
| Family | Genus | Number | Total |
| Alcaligenaceae | Achromobacter | 6 | 29 |
| Advenella | 1 | ||
| Alcaligenes | 2 | ||
| Bordetella | 16 | ||
| Pusillimonas | 1 | ||
| Taylorella | 3 | ||
| Burkholderiaceae | Burkholderia | 78 | 101 |
| Cupriavidus | 7 | ||
| Pandoraea | 1 | ||
| Polynucleobacter | 2 | ||
| Ralstonia | 13 | ||
| Comamonadaceae | Acidovorax | 11 | 33 |
| Alicycliphilus | 3 | ||
| Comamonas | 4 | ||
| Delftia | 2 | ||
| Hydrogenophaga | 1 | ||
| Hylemonella | 1 | ||
| Limnohabitans | 1 | ||
| Polaromonas | 3 | ||
| Pseudacidovorax | 1 | ||
| Ramlibacter | 1 | ||
| Rhodoferax | 1 | ||
| Variovorax | 3 | ||
| Verminephrobacter | 1 | ||
| Oxalobacteraceae | Collimonas | 1 | 15 |
| Herbaspirillum | 9 | ||
| Herminiimonas | 1 | ||
| Janthinobacterium | 1 | ||
| Oxalobacter | 2 | ||
| Unclassified | 1 | ||
| Sutterellaceae | Parasutterella | 1 | 4 |
| Sutterella | 3 | ||
| Unclassified | Leptothrix | 1 | 6 |
| Methylibium | 1 | ||
| Rubrivivax | 2 | ||
| Thiomonas | 2 |
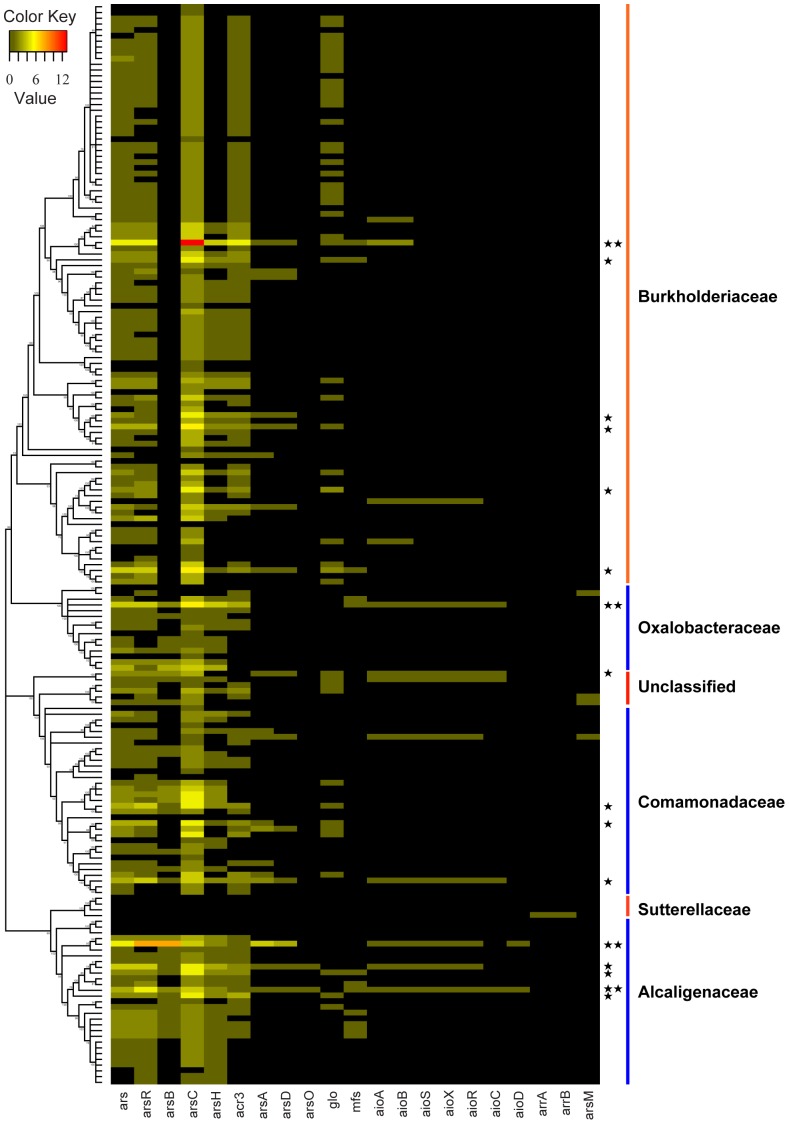
Figure 2. Distribution of arsenic-related genes in 188 Burkholderiales genomes.
From upstream to downstream in the 10 core genes-based tree, the 188 strains' names and their detailed distribution of the arsenic-related genes is listed in Table S3. The color of the bar indicates the gene numbers. One asterisk and double asterisks represent two times or four times as many as the average number of arsenic-related genes per genome, respectively.
Overall distribution of arsenic-related genes in Burkholderiales genomes
One-hundred and eighty eight genomes were investigated in detail to ascertain the distribution and organization of the arsenic-related genes based on our three-step re-annotation strategy (Figure S1). The number of arsenic-related genes detected in each genome was highly variable, and ranged from zero in the following ten strains [all three Sutterella strains (S. parvirubra YIT 11816, S. wadsworthensis 3_1_45B and S. wadsworthensis 2_1_59BFAA), all three Taylorella strains (T. asinigenitalis MCE3, T. equigenitalis ATCC 35865 and T. equigenitalis MCE9), Cupriavidus necator HPC(L), Oxalobacter formigenes HOxBLS, Polynucleobacter necessarius subsp. necessarius STIR1 and Verminephrobacter eiseniae EF01-2] to 35 in Burkholderia multivorans ATCC 17616 and 36 in A. faecalis subsp. faecalis NCIB8687 (Table S3). A total of 1,117 arsenic-related genes were identified in these genomes. Among these genes, 795 genes (71.2%) were grouped into an ars/aio cluster (at least two arsenic-related genes gather together at position). This result indicates that arsenic-related genes tended to group together. The distribution of arsenic-related genes is presented in Figure 2 and detailed in Table S2. According to the pathways of arsenic-resistance and transformation, there are 1,051 ars-like genes, 60 aio-like genes, two arr-like genes and four arsM genes. In our analysis, the ars-like genes are the predominant type of arsenic-related gene. In contrast, arr and arsM were identified only in a few genomes (Figure 2). A set of arrAB was only identified in Parasutterella excrementihominis YIT 11859, belonging to Sutterellaceae family. As for arsenite methylation, Oxalobacter formigenes OXCC13 in the Oxalobacteraceae family, Rhodoferax ferrireducens T118 and two Rubrivivaxstrains (R. benzoatilyticus JA2 and R. gelatinosus IL144) in the Comamonadaceae family were found to contain arsM genes. Twelve strains have genes encoding arsenite oxidase, and these strains were located in all of the families except Sutterellaceae. In addition, B. multivorans ATCC 17616 contained two sets of aioAB in its genome. The aio-like gene was found in the plasmid of Ralstonia solanacearum PSI07. Nearly 95% strains (178 out of 188) harbored arsenic-related genes in their genomes (Figure 2), which indicates that arsenic-related metabolism is widely present in Burkholderiales genomes.
The genome size of the 188 strains in Burkholderiales varied markedly, from 1.56 Mb (P. necessarius subsp. necessarius STIR1) to 11.29 (Burkholderia terrae BS001) Mb. Inevitably, genomes of a larger size had a greater number of genes. For example, some types of genes that are associated with resistance to antibiotics and toxic compounds, such as multidrug resistance (MDR) efflux pumps, have been reported in greater numbers if the strain has a larger genome [34]. However, unlike MDR efflux pumps, according to our statistical analysis, there was not a positive correlation between genomic size and the number of arsenic-related genes (r = 0.121; p>0.05).
The ars gene is highly abundant and has extreme diversity in its organization
The diversity of arsenic-related genes is reflected by of the ars-like genes, which made up 94.1% of the arsenic-related genes and were abundant in 174 strains. Overall, 5.6 ars-like genes per genome were observed in Burkholderiales strains (Table S2 and Figure 2). As shown in Figure 2, nearly every strain contained several copies of the arsC gene in their genomes. The arsC gene encodes arsenate reductase and is involved in the transformation of AsV to As III, which is then excreted by the arsenic efflux pump ArsB or ACR3. This mechanism benefits the bacteria itself, though it enhances the toxicity of the surrounding environment. The arsenite efflux pump could be classified into two types, ArsB and ACR3, based on different structures [35]. A total of 205 arsenite efflux pumps were identified in these genomes, including 151 copies of ACR3, which indicates that ACR3 is the primary form of arsenite efflux pump in Burkholderiales. Moreover, in the Burkholderiaceae family, the arsenite efflux pump was only present as the ACR3 type (Figure 2 and Table S3).
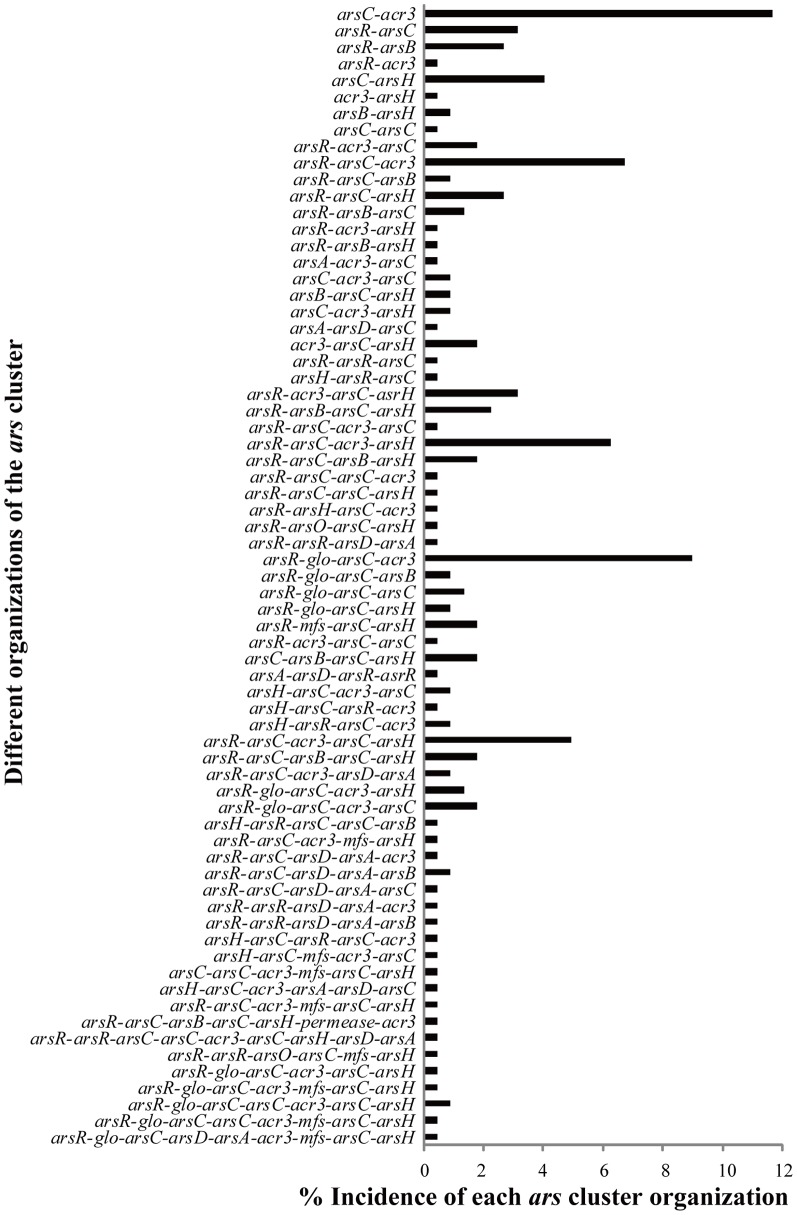
There are a total of 223 ars operons identified in 161 strains covering 2/3 ars-like genes (Figure 2). As shown in Table S3, 11 strains (A. arsenitoxydans SY8, Achromobacter piechaudii HLE, Acidovorax sp. JS42, Acidovorax sp. NO-1, A. faecalis subsp. faecalis NCIB 8687, B. multivorans ATCC 17616, Burkholderia phytofirmans PsJN, Delftia acidovorans SPH-1, Herbaspirillum sp. GW103, H. arsenicoxydans ULPAs1 and Ralstonia pickettii 12D) contained no less than three sets of ars operons in their genomes. According to their organizations, 223 ars operons contained 68 different forms (Figure 3).
Figure 3. Diversity of organizations of the arsenate-resistance operon (ars) cluster in the 161 Burkholderiales genomes.
Rare distribution of the arr-like gene in Burkholderiales genomes
Two-gene clusters (arrA and arrB) are involved in arsenate respiratory reduction, which is found in bacterial and archaea mainly isolated from aquifers and sediments. Of 188 strains, we found that only P. excrementihominis YIT 11859 contained one set of arrAB genes (Figure 2 and Table S2). The respiratory As(V) reductase large subunit ArrA and small subunit ArrB of P. excrementihominis YIT 11859 shared 46% and 42% identities, respectively, with those of Shewanella sp. ANA-3 [36]. In the Shewanella sp. ANA-3 genome, the arr cluster was flanked an ars-like cluster of arsD-arsA-arsB-arsC [36]. However, no ars-like genes were identified in P. excrementihominis YIT 11859.
Comparison analysis of the aio operon and flanking sequences
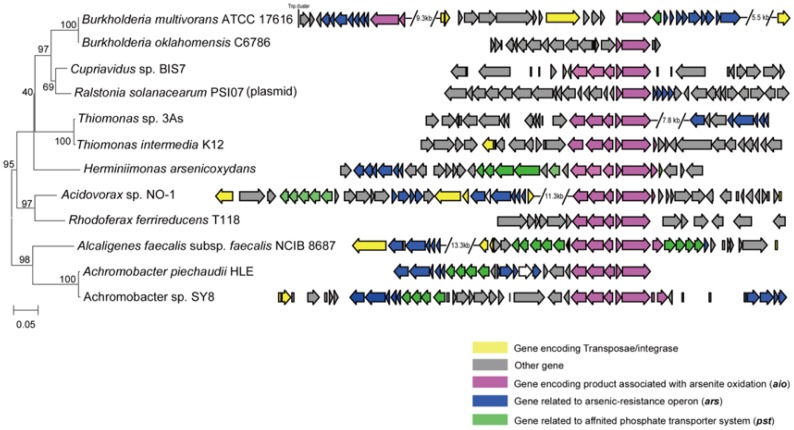
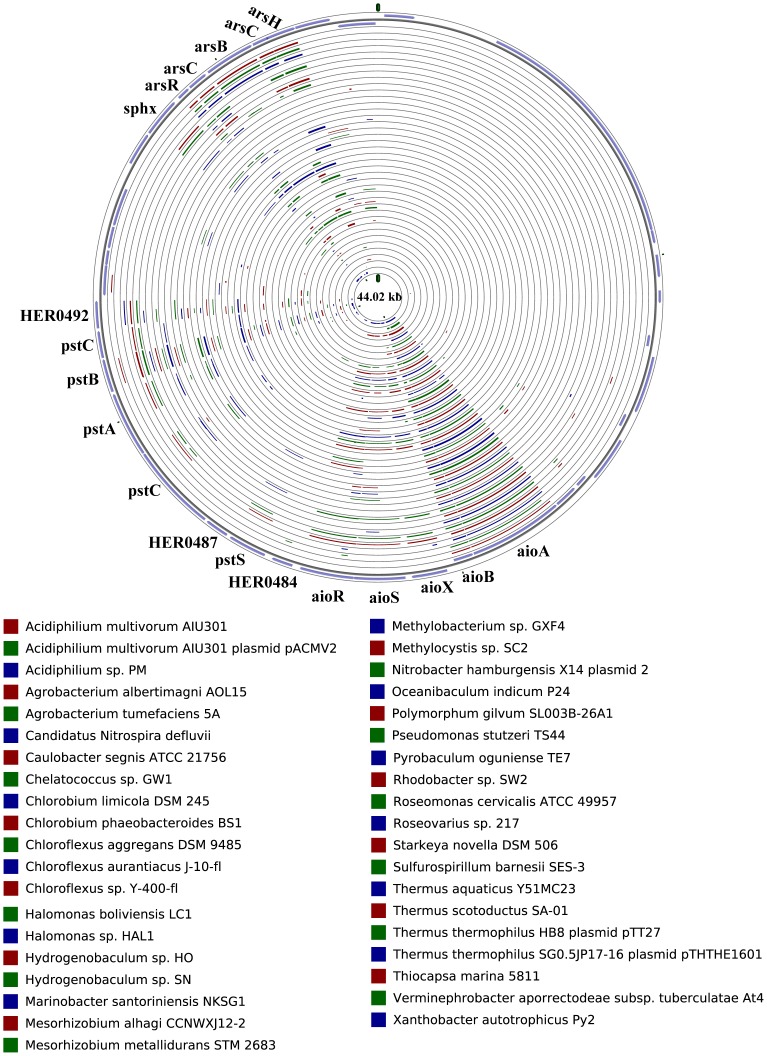
Bacterial arsenite oxidation transforms the more toxic As III to the less toxic AsV, which is considered an environmental detoxification pathway. Twelve strains were identified that carry aio operons in their genomes, among which only R. solanacearum PSI07 contained the aio operon in its plasmid. The organization of aio operons can be roughly grouped into two forms by the presence or absence of the three-component system AioX/AioS/AioR (Figure 4). The aio operon is frequently flanked with ars operons and genes encoding the high-affinity phosphate transport system pstSCAB, as is the case in the other 39 genomes identified in all of the sequenced genomes of bacteria and archaea from the NCBI database (Figure 5). However, comparison of the organization of these aio operons revealed a limited synteny of their flanking elements, which may indicate that the aio operon was obtained through horizontal gene transfer (HGT).
Figure 4. Multiple organizations of the aio gene cluster and flanking sequences were detected in arsenite-oxidizing bacteria in Burkholderiales.
Figure 5. Comparisons of the organization of the aio cluster and flanking sequence in 39 arsenite oxidizers genomes.
H. arsenicoxydans ULPAs1 is used as the reference genome. From outside to inside, first two rings donated ORF encoded from forward/reverse strand of the partial region of the H. arsenicoxydans ULPAs1 genome; rings 3 to 41 represent the 39 arsenite oxidizers at this order, which are shown under the cycle (from up to down and left to right).
The aio operon appeared to be randomly distributed in four families and the unclassified family of Burkholderiales, which is consistent with prediction described above (Figure 2). Although two types of aioAB were found throughout bacteria and archaea [37], the small number of strains carrying aio operons indicated that the capacity for arsenite oxidation by microbes is a relatively rare compared with that of the ars operon resistance system.
Distribution of arsM-like gene in Burkholderiales genomes
Microbial methylation of arsenite is mediated by arsM and has been found to be widespread in bacteria, archaea and eukarya [38]–[40]. The volatilization of As III in this process is thought to contribute to the global cycle of As. Based on a protein-similarity search, the arsM gene was identified in O. formigenes OXCC13 (Feature_id, 556269.7. peg.1267), R. ferrireducens T118 (Locus_tag, Rfer_1612), R. benzoatilyticus JA2 (RBXJA2T_04893) and R. gelatinosus IL144 (RGE_20810) (Figure 2 and Table S2). The arsM gene was mostly followed by arsR, which is believed to control the expression of arsM. As for our four arsM genes, we found one strain that did not contain arsR upstream to arsM (R. ferrireducens T118), which may suggest that arsM is constitutively expressed in R. ferrireducens T118.
Habitat influences the distribution of arsenic-related genes
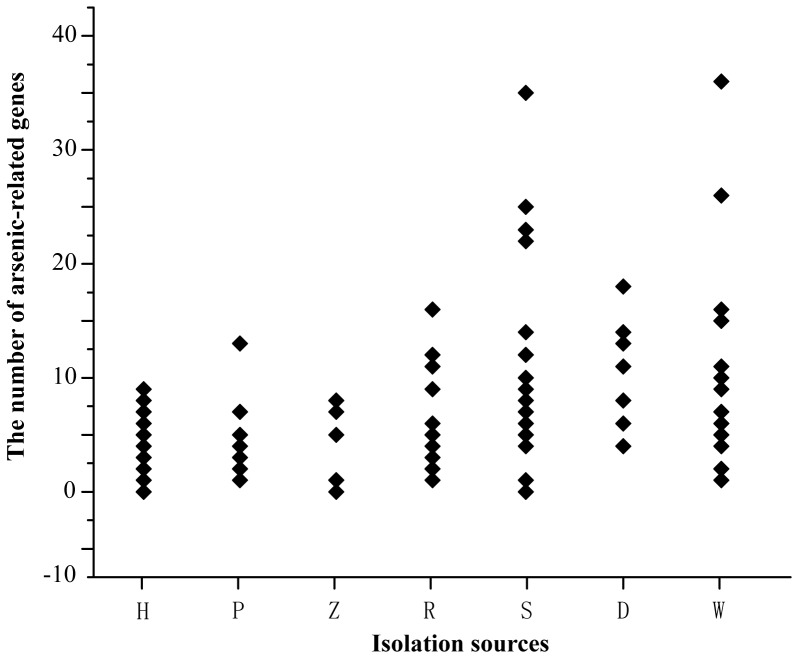
Compared among the abundance of arsenic-related genes of strains isolated from human, plant, animal, soil, sediment, wastewater or sludge and rhizosphere or root nodule, certain correlations were found: a) the number of the arsenic-related genes of strains isolated from soil (S) and wastewater or sludge (W) are larger than that of strains in the other environments (Figure 6); b) the six strains having more than 20 arsenic-related genes were recovered from S or W, and four of them are from arsenic-rich environment (Figure 6); c) the average number of arsenic-related genes per genome of human, plant and animal pathogens (H, P, Z) was less than that of strains isolated from S, W, sediment (D) and rhizosphere and root nodules (R) (Table S1, Table S3 and Figure 6), and d) the five isolates from the arsenic-rich niches (Table S1) contained more than four times average arsenic-related genes per genome compared to the other strains (25 vs 6 genes, Table S3 and Figure 2).
Figure 6. Habitat impacts the distribution of arsenic-related genes in Burkholderiales.
The scatter distribution of the number of arsenic-related genes per genome grouped by the isolation sources. The isolation sources included human (H), plant (P), animal (Z), rhizosphere or root nodules (R), soil (S), sediment (D) and wastewater or sludge (W) (Table S1).
Discussion
Previously, many studies have revealed the widespread distribution of arsenic-related genes in bacteria, and arsenic-related genes have been isolated from a large number of bacteria from different niches [1], [4], [8], [9], [11], [13], [41]. In light of these data, it has been assumed that arsenic-related genes were common in all bacteria, but clear evidence has been lacking. To date, numerous bacterial genomes (more than 10,000) have been sequenced. When looking through these genomes, nearly all of the genomes contain some arsenic-related genes despite the strains having been sampled from low-arsenic or arsenic-free habitats. This phenomenon puts us in mind to ensure the feasibility of using mass genomic information to detect the presence of arsenic-related genes in any bacteria. In this study, for the first time, we systematically analyzed the distribution and organization of arsenic-related genes using genome data from strains of Burkholderiales. Our studies provided the definitive evidence that nearly all Burkholderiales strains contained arsenic-related genes. This conclusion can most likely to be extended to all bacteria, despite the absence of direct evidence in this study. We could speculate that evolutionarily ancient microbes were exposed to “an arsenic surroundings” on ancient earth [42]. To overcome these selective pressures, microbes obtained numerous arsenic-related genes in their genomes for survival. Therefore, the arsenic-related gene may have very early origins, especially the ars-like gene. This speculation was supported in part by recent literatures showing that bacterial arsenic resistance and transformation was an acquired trait via HGT, driven by adaptation to habitats containing arsenic [17], [19], [43]–[45]. However, we found that the arsenic-related genes were absent in ten of the 188 examined strains, which suggest that some microbes may lose their arsenic-related genes during adaption to arsenic-free niches. In addition, the number of arsenic-related genes of strains isolated from arsenic-rich environments is much higher than the strains from other environments. Compared with human, plant and animal pathogens, the strains isolated from environmental sources possess a larger number of arsenic-related genes, which suggests that habitat likely plays an important role in influencing the distribution of arsenic-related genes [18], [45], [46].
The ars-like genes were highly abundant and displayed an extreme diversity in distribution. The ars-like genes were often found in the form of a cluster/operon, but they were also present as a scattered distribution, especially arsC. The diversity of organization of the ars-like cluster was very significant, and we observed up to 68 forms in 188 Burkholderiales strains (Figure 3). Previous research has demonstrated that the three-gene arsRB(/acr3)C and five-gene arsRDAB(/acr3)C are the typical organization structures of ars operons [35], [47]. Apart from these operons, there are a few other operons derived from these main structures. In the Burkholderiales order, the number of operon structures was exceeded our expectation because these strains descended from a recent common ancestor. This result indicates that the ars operon has a high diversity of organization. Considering the recent common ancestor for these strains, multiple forms of the ars-like operon within Burkholderiales may emerge through the HGT or by gene rearrangement. In any case, this result hints at the potentially efficient movement of ars-like genes. However, one should keep in mind that the number of different arrangements of ars-like clusters may not be very accurate because some genomes are in draft status, which may split an ars-like cluster into more than one cluster or lead an ars-like cluster to separate the different genes. However, in genomic analyses, such errors occur at a very low probability. There are five main forms (>4.5%) of the ars-like cluster: arsC-acr3, arsR-arsC-acr3, arsR-arsC-acr3-arsH, arsR-glo-arsC-acr3 and arsR-arsC-acr3-arsC-arsH. The arsC and acr3 genes are shared among these five organizations, which supports a key role for these two genes in resistance to arsenic. This prediction was agreement with the opinion that arsB/acr3 contribute to the basic resistance to arsenic in bacteria [7], [35]. Currently, several genes have been reported to be involved in the arsenic resistance system and are defined as ars-like genes: arsR, arsA, arsD,arsB, acr3,arsC,arsH [48], arsO [49] and arsP (putative membrane permease) [50]. In this study, the glo gene, encoding the glyoxalase/bleomycin resistance-related product, was found to be located in the ars-like cluster (Figure 3) in numerous Burkholderiales genomes. This result suggests that this gene contributes to arsenic-resistance, as functionally related genes tend to cluster together.
Arsenate-respiring bacteria reduce AsV to As III and affect the speciation and mobilization of arsenic in various locales worldwide, especially in anaerobic conditions. In these 188 genomes, the AsV respiratory reductase gene arrAB was only found in P. excrementihominis YIT 11859. This strain is a strictly anaerobic bacterium that was isolated from the human gut [51]. In Shewanella sp. ANA-3, expression of arrAB was silent under aerobic conditions, and these two genes were predicted to be obtained through HGT [36]. Therefore, the fact that arrAB genes were not identified in most of the 188 strains may be explained by the requirement for anaerobic conditions for AsV respiratory reductase to function [11], as most strains came from aerobic niches (Table S1).
As for the aio-like gene, multiple lines of evidence have demonstrated that HGT plays an important role in spreading aio-like genes among bacteria [45]. The aio-like genes identified in the R. solanacearum PSI07 plasmid also supported the above conclusion. In this study, numerous genomes have been found to contain arsenite oxidation and phosphate-related genes (such as the pst transport system and pho regulatory element) together. A previous study showed that the phosphate transport system (Pst) flanking the aioAB genes could bind phosphate selectively over arsenate (at least 103-fold excess), even in arsenate-rich conditions [52], which seems to weaken the relationship between arsenic and phosphorus metabolism. However, recently, it was reported that the expression of aioAB was under the control of the phosphate regulators phoBR in A. tumefaciens 5A [53] . In addition, we found that in Agrobacterium tumefaciens GW4 [54], the Pst1 located near the AioAB could bind both phosphate and arsenate (Wang et al., submitted to Environmental Microbiology) which suggests significant relatedness between arsenic and phosphorus metabolism.
The arsenite S-adenosylmethyltransferase encoding gene (arsM) was only identified in few Burkholderiales genomes, which may indicate a low frequency of occurrence in the Burkholderiales order. The arsM gene was widely found in bacteria, archaea and eukarya (excluding plants) and displayed a high diversity of sequence [1]. However, a small number of ArsM are currently available in the NCBI proteins database compared with ars-like genes. One possible reason for the low number of Burkholderiales strains harboring arsM may be that As III biomethylation is not a primary pathway for bacterial arsenic detoxification. Bacteria have two mechanisms to deal with As III in vivo, As III biomethylation and As III oxidation. These two mechanisms share the common substrate of As III. In Burkholderiales, we found that the four potential As III biomethylation strains did not contain the aioAB genes in their genomes. However, the arsM gene was identified in some of the 39 arsenite-oxidizer genomes, such as Candidatus Nitrospira defluvii (Locus_tag, NIDE3709) and Thiocapsa marina 5811 (Locus_tag, ThimaDRAFT_0102), which suggests that the pathways of As III biomethylation and As III oxidation could coexist in one strain.
Supporting Information
The flowchart displaying the process used to determine the arsenic-related genes in Burkholderiales genomes.
(TIF)
The 16S rRNA genes based phylogenetic tree of 184 Burkholderiales strains. Four strains (Acidovorax avenae subsp. avenae RS-1, Bordetella holmesii 44057, Burkholderia ambifaria IOP40-10 and Burkholderia ambifaria MEX-5) are not involved in this phylogenetic analysis due to the 16S rRNA genes not identified in their genomes.
(DOCX)
The isolation sources of 188 Burkholderiales strains obtained from literature in order to be classified in nine groups according to their original habitats.
(XLSX)
The detail distribution of arsenic-related genes in 188 Burkholderiales genomes.
(XLSX)
The names of 188 strains to construct a phylogenetic tree based on 10 core genes from their genomes ( Figure 2 , left) and the original data shown the presence or absence of arsenic-related genes ( Figure 2 , right).
(XLSX)
Funding Statement
This work was supported by the National Natural Science Foundation of China 31010103903 and 31170106. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jia Y, Huang H, Zhong M, Wang FH, Zhang LM, et al. (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47: 3141–3148. [DOI] [PubMed] [Google Scholar]
- 2. Dhuldhaj UP, Yadav IC, Singh S, Sharma NK (2013) Microbial interactions in the arsenic cycle: adoptive strategies and applications in environmental management. Rev Environ Contam Toxicol 224: 1–38. [DOI] [PubMed] [Google Scholar]
- 3. Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26: 311–325. [DOI] [PubMed] [Google Scholar]
- 4. Cavalca L, Corsini A, Zaccheo P, Andreoni V, Muyzer G (2013) Microbial transformations of arsenic: perspectives for biological removal of arsenic from water. Future Microbiol 8: 753–768. [DOI] [PubMed] [Google Scholar]
- 5. Kruger MC, Bertin PN, Heipieper HJ, Arsene-Ploetze F (2013) Bacterial metabolism of environmental arsenic—mechanisms and biotechnological applications. Appl Microbiol Biotechnol 97: 3827–3841. [DOI] [PubMed] [Google Scholar]
- 6. Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300: 939–944. [DOI] [PubMed] [Google Scholar]
- 7. Shen Z, Han J, Wang Y, Sahin O, Zhang Q (2013) The contribution of ArsB to arsenic resistance in Campylobacter jejuni . PLoS One 8: e58894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158: 128–137. [DOI] [PubMed] [Google Scholar]
- 9. Heinrich-Salmeron A, Cordi A, Brochier-Armanet C, Halter D, Pagnout C, et al. (2011) Unsuspected diversity of arsenite-oxidizing bacteria as revealed by widespread distribution of the aoxB gene in prokaryotes. Appl Environ Microbiol 77: 4685–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quemeneur M, Heinrich-Salmeron A, Muller D, Lievremont D, Jauzein M, et al. (2008) Diversity surveys and evolutionary relationships of aoxB genes in aerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74: 4567–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malasarn D, Saltikov CW, Campbell KM, Santini JM, Hering JG, et al. (2004) arrA is a reliable marker for As(V) respiration. Science 306: 455. [DOI] [PubMed] [Google Scholar]
- 12. Bertin PN, Heinrich-Salmeron A, Pelletier E, Goulhen-Chollet F, Arsene-Ploetze F, et al. (2011) Metabolic diversity among main microorganisms inside an arsenic-rich ecosystem revealed by meta- and proteo-genomics. ISME J 5: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai L, Yu K, Yang Y, Chen BW, Li XD, et al. (2013) Metagenomic exploration reveals high levels of microbial arsenic metabolism genes in activated sludge and coastal sediments. Appl Microbiol Biotechnol 97: 9579–9588. [DOI] [PubMed] [Google Scholar]
- 14. Plewniak F, Koechler S, Navet B, Dugat-Bony E, Bouchez O, et al. (2013) Metagenomic insights into microbial metabolism affecting arsenic dispersion in Mediterranean marine sediments. Mol Ecol 22: 4870–4883. [DOI] [PubMed] [Google Scholar]
- 15. Perez-Pantoja D, Donoso R, Agullo L, Cordova M, Seeger M, et al. (2012) Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales . Environ Microbiol 14: 1091–1117. [DOI] [PubMed] [Google Scholar]
- 16. Arsene-Ploetze F, Koechler S, Marchal M, Coppee JY, Chandler M, et al. (2010) Structure, function, and evolution of the Thiomonas spp. genome. PLoS Genet 6: e1000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Hu Y, Gong J, Lin Y, Johnstone L, et al. (2012) Genome sequence of the highly efficient arsenite-oxidizing bacterium Achromobacter arsenitoxydans SY8. J Bacteriol 194: 1243–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muller D, Medigue C, Koechler S, Barbe V, Barakat M, et al. (2007) A tale of two oxidation states: bacterial colonization of arsenic-rich environments. PLoS Genet 3: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang Y, Li H, Rensing C, Zhao K, Johnstone L, et al. (2012) Genome sequence of the facultative anaerobic arsenite-oxidizing and nitrate-reducing bacterium Acidovorax sp. strain NO1. J Bacteriol 194: 1635–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trimble WL, Phung le T, Meyer F, Silver S, Gilbert JA (2012) Draft genome sequence of Achromobacter piechaudii strain HLE. J Bacteriol 194: 6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salzberg SL, Delcher AL, Kasif S, White O (1998) Microbial gene identification using interpolated Markov models. Nucleic Acids Res 26: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guindon S, Delsuc F, Dufayard JF, Gascuel O (2009) Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol 537: 113–137. [DOI] [PubMed] [Google Scholar]
- 25. Jackson CR, Dugas SL (2003) Phylogenetic analysis of bacterial and archaeal arsC gene sequences suggests an ancient, common origin for arsenate reductase. BMC Evol Biol 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tatusov RL, Koonin EV, Lipman DJ (1997) A genomic perspective on protein families. Science 278: 631–637. [DOI] [PubMed] [Google Scholar]
- 27. Li L, Stoeckert CJ Jr, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13: 2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiss S, Carapito C, Cleiss J, Koechler S, Turlin E, et al. (2009) Enhanced structural and functional genome elucidation of the arsenite-oxidizing strain Herminiimonas arsenicoxydans by proteomics data. Biochimie 91: 192–203. [DOI] [PubMed] [Google Scholar]
- 29. Cleiss-Arnold J, Koechler S, Proux C, Fardeau ML, Dillies MA, et al. (2010) Temporal transcriptomic response during arsenic stress in Herminiimonas arsenicoxydans . BMC Genomics 11: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muller D, Lievremont D, Simeonova DD, Hubert JC, Lett MC (2003) Arsenite oxidase aox genes from a metal-resistant beta-proteobacterium. J Bacteriol 185: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koechler S, Cleiss-Arnold J, Proux C, Sismeiro O, Dillies MA, et al. (2010) Multiple controls affect arsenite oxidase gene expression in Herminiimonas arsenicoxydans . BMC Microbiol 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phung le T, Trimble WL, Meyer F, Gilbert JA, Silver S (2012) Draft genome sequence of Alcaligenes faecalis subsp. faecalis NCIB 8687 (CCUG 2071). J Bacteriol 194: 5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson GL, Williams J, Hille R (1992) The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J Biol Chem 267: 23674–23682. [PubMed] [Google Scholar]
- 34. Piddock LJ (2006) Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19: 382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosen BP (1999) Families of arsenic transporters. Trends Microbiol 7: 207–212. [DOI] [PubMed] [Google Scholar]
- 36. Saltikov CW, Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci U S A 100: 10983–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zargar K, Conrad A, Bernick DL, Lowe TM, Stolc V, et al. (2012) ArxA, a new clade of arsenite oxidase within the DMSO reductase family of molybdenum oxidoreductases. Environ Microbiol 14: 1635–1645. [DOI] [PubMed] [Google Scholar]
- 38. Yin XX, Chen J, Qin J, Sun GX, Rosen BP, et al. (2011) Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol 156: 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin J, Rosen BP, Zhang Y, Wang G, Franke S, et al. (2006) Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci U S A 103: 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin S, Shi Q, Nix FB, Styblo M, Beck MA, et al. (2002) A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem 277: 10795–10803. [DOI] [PubMed] [Google Scholar]
- 41. Escudero LV, Casamayor EO, Chong G, Pedros-Alio C, Demergasso C (2013) Distribution of microbial arsenic reduction, oxidation and extrusion genes along a wide range of environmental arsenic concentrations. PLoS One 8: e78890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oremland RS, Saltikov CW, Wolfe-Simon F, Stolz JF (2009) Arsenic in the evolution of earth and extraterrestrial ecosystems. Geomicrobiol J 26: 522–536. [Google Scholar]
- 43. Villegas-Torres MF, Bedoya-Reina OC, Salazar C, Vives-Florez MJ, Dussan J (2011) Horizontal arsC gene transfer among microorganisms isolated from arsenic polluted soil. Int Biodeter Biodegr 65: 147–152. [Google Scholar]
- 44. Li X, Gong J, Hu Y, Cai L, Johnstone L, et al. (2012) Genome sequence of the moderately halotolerant, arsenite-oxidizing bacterium Pseudomonas stutzeri TS44. J Bacteriol 194: 4473–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai L, Liu G, Rensing C, Wang G (2009) Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Hu Y, Gong J, Zhang L, Wang G (2013) Comparative genome characterization of Achromobacter members reveals potential genetic determinants facilitating the adaptation to a pathogenic lifestyle. Appl Microbiol Biotechnol 97: 6413–6425. [DOI] [PubMed] [Google Scholar]
- 47. Carlin A, Shi W, Dey S, Rosen BP (1995) The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol 177: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H (2005) Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti . J Bacteriol 187: 6991–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang L, Chen S, Xiao X, Huang X, You D, et al. (2006) arsRBOCT arsenic resistance system encoded by linear plasmid pHZ227 in Streptomyces sp. strain FR-008. Appl Environ Microbiol 72: 3738–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang L, Jeon B, Sahin O, Zhang Q (2009) Identification of an arsenic resistance and arsenic-sensing system in Campylobacter jejuni . Appl Environ Microbiol 75: 5064–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagai F, Morotomi M, Sakon H, Tanaka R (2009) Parasutterella excrementihominis gen. nov., sp. nov., a member of the family Alcaligenaceae isolated from human faeces. Int J Syst Evol Microbiol 59: 1793–1797. [DOI] [PubMed] [Google Scholar]
- 52. Elias M, Wellner A, Goldin-Azulay K, Chabriere E, Vorholt JA, et al. (2012) The molecular basis of phosphate discrimination in arsenate-rich environments. Nature 491: 134–137. [DOI] [PubMed] [Google Scholar]
- 53. Kang YS, Heinemann J, Bothner B, Rensing C, McDermott TR (2012) Integrated co-regulation of bacterial arsenic and phosphorus metabolisms. Environ Microbiol 14: 3097–3109. [DOI] [PubMed] [Google Scholar]
- 54. Fan H, Su C, Wang Y, Yao J, Zhao K, et al. (2008) Sedimentary arsenite-oxidizing and arsenate-reducing bacteria associated with high arsenic groundwater from Shanyin, Northwestern China. J Appl Microbiol 105: 529–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flowchart displaying the process used to determine the arsenic-related genes in Burkholderiales genomes.
(TIF)
The 16S rRNA genes based phylogenetic tree of 184 Burkholderiales strains. Four strains (Acidovorax avenae subsp. avenae RS-1, Bordetella holmesii 44057, Burkholderia ambifaria IOP40-10 and Burkholderia ambifaria MEX-5) are not involved in this phylogenetic analysis due to the 16S rRNA genes not identified in their genomes.
(DOCX)
The isolation sources of 188 Burkholderiales strains obtained from literature in order to be classified in nine groups according to their original habitats.
(XLSX)
The detail distribution of arsenic-related genes in 188 Burkholderiales genomes.
(XLSX)
The names of 188 strains to construct a phylogenetic tree based on 10 core genes from their genomes ( Figure 2 , left) and the original data shown the presence or absence of arsenic-related genes ( Figure 2 , right).
(XLSX)