Abstract
Rationale
Although non-medical use of oxycodone continues to be a growing problem in the United States, there are no animal studies examining the effects of long term oxycodone self administration (SA).
Objectives
The current study was designed to examine chronic oxycodone SA by mice (14 days), in novel extended (4 hours) SA sessions and its effect on selective striatal neurotransmitter receptor mRNA expression.
Methods
Adult male C57/BL6J mice were either allowed to self administer oxycodone (0.25 mg/kg/infusion, FR1) or served as yoked-saline controls in an extended access paradigm. Mice self administered oxycodone for 4 hours/day for 14 consecutive days. Comparison groups with 14-days exposure to 1-hour SA sessions were also studied. Within one hour of the last extended SA session, mice were sacrificed, dorsal striatum was isolated and selective neurotransmitter receptor mRNA levels were examined.
Results
The oxycodone groups poked the active hole significantly more times than the yoked controls. The number of nose pokes at the active hole rose over the 14 days in the oxycodone group with extended access. The expression of thirteen neurotransmitter receptor mRNAs was significantly altered in the dorsal striatum, including the GABA A receptor beta 2 subunit (Gabrb2) showing experiment-wise significant decrease, as a result of extended oxycodone SA.
Conclusion
C57BL/6J mice escalated the amount of oxycodone self administered across 14 consecutive daily extended sessions, but not 1-hour sessions. Decreases in Gabrb2 mRNA levels may underlie escalation of oxycodone intake in the extended access SA sessions.
Keywords: Extended self-administration sessions, Mouse, Oxycodone, dorsal striatum, neurotransmitter receptor mRNA
Introduction
Over the past decade there has been a dramatic increase in the non-medical use of prescription opioids. Oxycodone is one of the most commonly abused prescription opioids and accounts for a substantial percentage of opioid related deaths in the United States (CDC, 2011). Despite the fact that illicit use of oxycodone has increased significantly in recent years, there is limited information about oxycodone’s impact on behavioral and neurobiological alterations in animal models.
Prescription opioids activate mu opioid receptors (MOP-r) located on the GABAergic interneurons in the ventral tegmental area (VTA) and the substantia nigra (SN), disinhibiting dopamine neurons and resulting in increases in dopamine release in the dopaminergic terminal regions in the striatum (Johnson and North 1992). The increases of dopamine levels subsequently alter gene expression in the striatum (e.g., (Angulo and McEwen 1994) which may be associated with changes in the behavioral effects of opioids in rodents (Chen et al. 2006; Kruzich et al. 2003, Seip-Cammack et al. 2012, Lenoir et al. 2013, Zhang et al. 2009, Picetti et al. 2012). Although numerous studies were conducted to examine the behavioral and neurobiological effects of MOP-r agonists such as morphine or heroin in rats (e.g., Lenoir et al. 2013, Picetti et al. 2012), there have been few animal studies investigating the effects of oxycodone using a paradigm that mimics oxycodone self administration (SA) in humans. SA is a reliable paradigm used to study voluntary consumption of drugs of abuse in laboratory settings. SA studies in mice are crucial since many transgenic animal models, which may offer insight into specific neurobiological mechanisms, exist only in the mouse. The majority of these mouse models have the C57BL/6 background. Studies have shown that extended access self-administration paradigms lead to an escalation of drug intake that is not seen in shorter paradigms in rat (e.g., Ahmed and Koob 1998; Mantsch et al. 2004; Picetti et al. 2012). However, there have been no reports using extended access self administration in mouse. We hypothesized that the mouse would escalate the amount of drug self-administered when given extended access sessions and such escalation of drug intake would result in neurobiological changes in brain regions related to reward and habitual learning. The dorsal striatum has been involved in neuronal adaptations to drug SA, locomotor regulation and habitual learning (e.g., Ito et al. 2002; Porrino et al. 2007; Belin and Everitt 2008). Thus, dorsal striatum was prioritized herein for initial brain analysis.
In the present study, extended access (4-hrs) SA sessions were used to examine the reward-associated behavioral and neurobiological alterations induced by oxycodone in C57BL/6J mice. Specifically, this study was carried out to determine how relatively long SA sessions for 14 consecutive days affect oxycodone SA behavior in mouse. Additionally, we examined how such extended access SA of oxycodone by the mouse affects transcription profiles of neurotransmitter receptors in the dorsal striatum since multiple neurotransmitter systems may be involved in the behavioral profile resulting from oxycodone SA.
We found that extended access oxycodone self administration led to escalation of oxycodone intake over the course of 14 days; such escalation was not found in the short access (1-hr) self administration sessions. The expression of several striatal neurotransmitter receptor genes was altered following extended access oxycodone self administration, giving initial insight into the neurobiological adaptations after self exposure to this widely abused prescription opioid.
Materials and Methods
Subjects
Adult Male C57BL/6J mice (11 weeks old) on arrival from Jackson Laboratory (Bar Harbor, ME) were housed in groups of four with free access to food and water in a light-(12:12 hour light/dark cycle, light on at 7:00 pm and off at 7:00 am) and temperature- (25 °C) controlled room. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996). The experimental protocols used were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Self-Administration Procedure
Catheter Implantation and Operant Conditioning Chambers
Following acclimation for 7 days, the mice were anesthetized with a combination of xylazine (8.0 mg/kg i.p.) and ketamine (80 mg/kg i.p.). For detail surgical procedure and description of operant conditioning chambers, see Zhang et al., 2009.
Oxycodone Self-administration
1. A 4-hour SA session was conducted once a day for 14 consecutive days. Each day, mice were weighed and heparinized saline (0.01 ml of 30 IU/ml solution) was used to flush the catheter to maintain patency. During SA sessions, mice were placed in the operant conditioning chambers and in the oxycodone groups, a nose-poke through the active hole led to 0.25 mg/kg infusion of oxycodone (Sigma, St. Louis, MO) under an FR1 schedule. Of a total 31 mice that started in the studies, 22 mice finished the 14 days SA sessions and passed the catheter patency test. Of these 22 mice, the brain tissues of 12 (6 were from each group) mice were randomly chosen to examine changes in mRNA expression. 2. In separate groups of mice, a 1-hour SA session was also conducted once a day for 14 consecutive days. The procedures were exactly the same as the 4-hour SA sessions except that every session lasted for only 1hour. Of a total 16 mice that started in the studies including oxycodone and yoked saline control, 13 finished the 14 days SA sessions and passed the patency test.
RNA Extraction
Mice were sacrificed within 1 hr after the last SA session (Day 14); the dorsal striatum from each mouse was dissected from the brain and homogenized in Qiazol (Qiagen, Valencia, CA). Total RNA was isolated from homogenates using the miRNeasy Kit (Qiagen, Valencia, CA). The quality and quantity of RNA from each sample was determined using Agilent 2100 bioanalyzer. Genomic DNA was removed from the isolated total RNA using RT2 HT First Strand Kit (Qiagen, Valencia, CA). Complementary DNA was then synthesized from 500 ng of total RNA with the same kit.
Mouse Neurotransmitter Receptors RT2-Profiler™ PCR Array
The mouse neurotransmitter receptors RT2 Profiler™ PCR Array (PAMM-060Z, SABioscience Version 3.0 profiles the expression of 84 genes involved in modulating biological processes through neurotransmitter receptors and five housekeeping genes (β-actin, Actb; glyceraldehyde 3-phosphate dehydrogenase, Gapdh; hypoxanthine guanine phosphoribosyl transferase 1, Hprt-1; Heat shock protein 90 alpha (cytosolic), class B member 1, Hsp90ab1 and β-glucuronidase, Gusb) by real-time PCR using the SYBR Green detection method. Total RNA (500 ng) was reverse transcribed using RT2 HT First Strand Kit (Qiagen, Valencia, CA) following manufacturer’s instructions. The generated cDNA was diluted with an appropriate volume of instrument-specific reagent (2x SuperArray RT2 Real-Time™ SYBR Green PCR Master Mix (PA-012) and ultra pure water) and 10 μl of this reaction mix was added to each well of the PCR array. The real-time PCR reaction was performed in an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA), applying the following program: 2 minutes at 50°C, 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The ABI Prism 7900 HT Sequence Detection System was used to calculate the Ct value for each well. Data were normalized to the mean of the five housekeeping genes and analyzed by the comparative Ct-method (2−ΔCT). The complete list of genes assayed on the array is shown in Table 2 and can also be found at the manufacturer’s website.
Table 2.
The symbol and description of the 84 neurotransmitter receptor genes in the array used in the current study.
| Position | Symbol | Description |
|---|---|---|
| A01 | Ache | Acetylcholinesterase |
| A02 | Anxa9 | Annexin A9 |
| A03 | Brs3 | Bombesin-like receptor 3 |
| A04 | Cckar | Cholecystokinin A receptor |
| A05 | Cckbr | Cholecystokinin B receptor |
| A06 | Chat | Choline acetyltransferase |
| A07 | Chrm1 | Cholinergic receptor, muscarinic 1, CNS |
| A08 | Chrm2 | Cholinergic receptor, muscarinic 2, cardiac |
| A09 | Chrm3 | Cholinergic receptor, muscarinic 3, cardiac |
| A10 | Chrm4 | Cholinergic receptor, muscarinic 4 |
| A11 | Chrm5 | Cholinergic receptor, muscarinic 5 |
| A12 | Chrna1 | Cholinergic receptor, nicotinic, alpha polypeptide 1 (muscle) |
| B01 | Chrna2 | Cholinergic receptor, nicotinic, alpha polypeptide 2 (neuronal) |
| B02 | Chrna3 | Cholinergic receptor, nicotinic, alpha polypeptide 3 |
| B03 | Chrna4 | Cholinergic receptor, nicotinic, alpha polypeptide 4 |
| B04 | Chrna5 | Cholinergic receptor, nicotinic, alpha polypeptide 5 |
| B05 | Chrna6 | Cholinergic receptor, nicotinic, alpha polypeptide 6 |
| B06 | Chrna7 | Cholinergic receptor, nicotinic, alpha polypeptide 7 |
| B07 | Chrnb1 | Cholinergic receptor, nicotinic, beta polypeptide 1 (muscle) |
| B08 | Chrnb2 | Cholinergic receptor, nicotinic, beta polypeptide 2 (neuronal) |
| B09 | Chrnb3 | Cholinergic receptor, nicotinic, beta polypeptide 3 |
| B10 | Chrnb4 | Cholinergic receptor, nicotinic, beta polypeptide 4 |
| B11 | Chrnd | Cholinergic receptor, nicotinic, delta polypeptide |
| B12 | Chrne | Cholinergic receptor, nicotinic, epsilon polypeptide |
| C01 | Chrng | Cholinergic receptor, nicotinic, gamma polypeptide |
| C02 | Comt | Catechol-O-methyltransferase |
| C03 | Drd1a | Dopamine receptor D1A |
| C04 | Drd2 | Dopamine receptor D2 |
| C05 | Drd3 | Dopamine receptor D3 |
| C06 | Drd4 | Dopamine receptor D4 |
| C07 | Drd5 | Dopamine receptor D5 |
| C08 | Gabra1 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 1 |
| C09 | Gabra2 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 2 |
| C10 | Gabra3 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 3 |
| C11 | Gabra4 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 4 |
| C12 | Gabra5 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 5 |
| D01 | Gabra6 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 6 |
| D02 | Gabrb2 | Gamma-aminobutyric acid (GABA) A receptor, subunit beta 2 |
| D03 | Gabrb3 | Gamma-aminobutyric acid (GABA) A receptor, subunit beta 3 |
| D04 | Gabrd | Gamma-aminobutyric acid (GABA) A receptor, subunit delta |
| D05 | Gabrg1 | Gamma-aminobutyric acid (GABA) A receptor, subunit gamma 1 |
| D06 | Gabrg2 | Gamma-aminobutyric acid (GABA) A receptor, subunit gamma 2 |
| D07 | Gabrp | Gamma-aminobutyric acid (GABA) A receptor, pi |
| D08 | Gabrq | Gamma-aminobutyric acid (GABA) A receptor, subunit theta |
| D09 | Gabrr1 | Gamma-aminobutyric acid (GABA) C receptor, subunit rho 1 |
| D10 | Gabrr2 | Gamma-aminobutyric acid (GABA) C receptor, subunit rho 2 |
| D11 | Gad1 | Glutamic acid decarboxylase 1 |
| D12 | Galr1 | Galanin receptor 1 |
| E01 | Galr2 | Galanin receptor 2 |
| E02 | Galr3 | Galanin receptor 3 |
| E03 | Glra1 | Glycine receptor, alpha 1 subunit |
| E04 | Glra2 | Glycine receptor, alpha 2 subunit |
| E05 | Glra3 | Glycine receptor, alpha 3 subunit |
| E06 | Glra4 | Glycine receptor, alpha 4 subunit |
| E07 | Glrb | Glycine receptor, beta subunit |
| E08 | Qrfpr | Pyroglutamylated RFamide peptide receptor |
| E09 | Npffr1 | Neuropeptide FF receptor 1 |
| E10 | Prokr1 | Prokineticin receptor 1 |
| E11 | Prokr2 | Prokineticin receptor 2 |
| E12 | Npffr2 | Neuropeptide FF receptor 2 |
| F01 | Gpr83 | G protein-coupled receptor 83 |
| F02 | Grpr | Gastrin releasing peptide receptor |
| F03 | Htr3a | 5-hydroxytryptamine (serotonin) receptor 3A |
| F04 | Maoa | Monoamine oxidase A |
| F05 | Mc2r | Melanocortin 2 receptor |
| F06 | Nmur1 | Neuromedin U receptor 1 |
| F07 | Nmur2 | Neuromedin U receptor 2 |
| F08 | Npy1r | Neuropeptide Y receptor Y1 |
| F09 | Npy2r | Neuropeptide Y receptor Y2 |
| F10 | Npy5r | Neuropeptide Y receptor Y5 |
| F11 | Npy6r | Neuropeptide Y receptor Y6 |
| F12 | Ntsr1 | Neurotensin receptor 1 |
| G01 | Pgr15l | G protein-coupled receptor 15-like |
| G02 | Ppyr1 | Pancreatic polypeptide receptor 1 |
| G03 | Prlhr | Prolactin releasing hormone receptor |
| G04 | Slc5a7 | Solute carrier family 5 (choline transporter), member 7 |
| G05 | Sstr1 | Somatostatin receptor 1 |
| G06 | Sstr2 | Somatostatin receptor 2 |
| G07 | Sstr3 | Somatostatin receptor 3 |
| G08 | Sstr4 | Somatostatin receptor 4 |
| G09 | Sstr5 | Somatostatin receptor 5 |
| G10 | Tacr1 | Tachykinin receptor 1 |
| G11 | Tacr2 | Tachykinin receptor 2 |
| G12 | Tacr3 | Tachykinin receptor 3 |
Confirmation of Changes in Gene Expression Using Real Time PCR
We reexamined all the genes found to be significantly altered in the array study following 14-day extended access oxycodone SA. For detail PCR procedures, see Zhang et al., 2013.
Statistical Analysis
Differences in SA measured as numbers of nose pokes in the active hole in each SA session across the 14 sessions were assessed by a two-way analysis of variance (ANOVA), Drug Condition × Session (repeated measure). Differences in expression of genes between the two groups were analyzed by t-tests and allowance for testing multiple genes was achieved by the Bonferroni correction. To evaluate whether a group of small p-values occurred more often than expected by chance, the partition test was applied to the t-tests (Ott et al. 2012). For a given threshold, r, ranging between 0 and a suitable upper limit like 0.10, the observed number of p-values smaller than r are contrasted by a chi-square statistic with the expected number, nr, where n = number tests carried out. The largest chi-square statistic over the range of r represents the test statistic whose associated (experiment-wise) significance level is obtained in randomized samples (labels case and controls are randomly permuted).
Results
Oxycodone self administration in extended access (4-hour) sessions
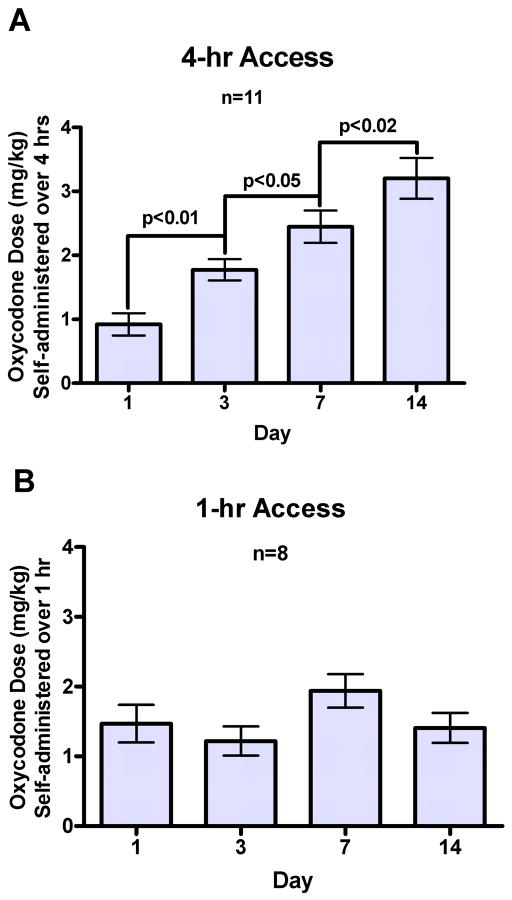
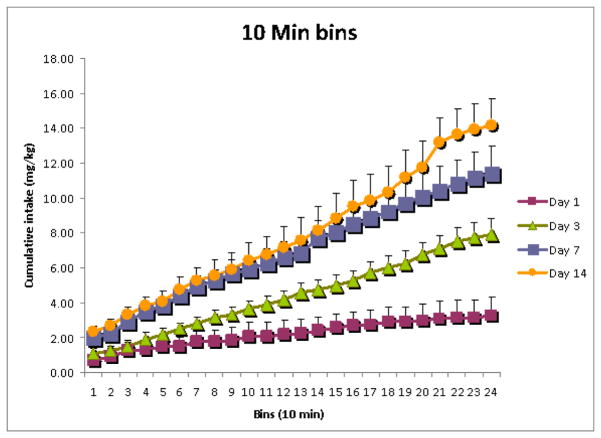
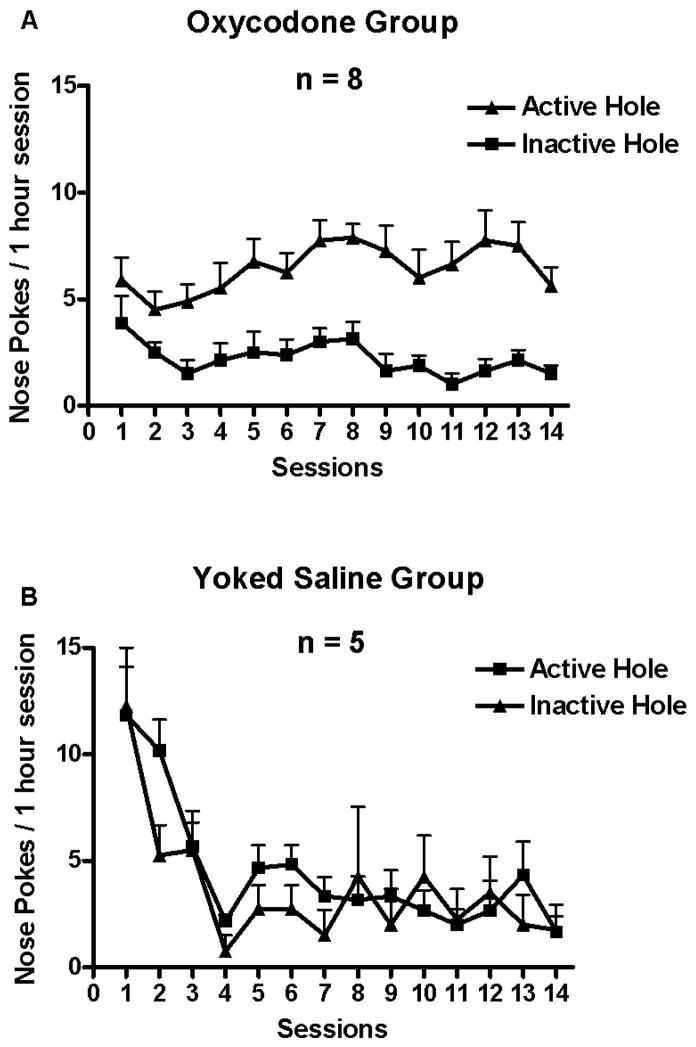
The behavior of oxycodone SA by adult mice in 4-hour session across 14 days is shown in Fig. 1A. Mice in the oxycodone group nose poked significantly more at the active hole than at the inactive hole (Fig. 1A). In contrast, the yoked saline control group did not differ in nose poking between the “active” and inactive hole, as shown in the Fig. 1B. One-way ANOVA showed that there was a significant main effect of the amount of oxycodone SA across days 1, 3, 7 and 14, F (3, 30) = 23.32, p < 0.000001 (Fig. 3A). Newman-Keuls post hoc tests showed that the amount of oxycodone SA on day 3 was greater than on day 1, p < 0.01; the amount of oxycodone SA on day 7 was greater than on day 3, p < 0.05; the amount of oxycodone SA on day 14 was greater than on day 7, p < 0.02. The cumulative mg/kg oxycodone intake measured in 10-min time bins on day 1, 3, 7 and 14 of SA sessions is shown in Fig. 4. There was a significant main effect of Days, F (3, 15) = 27.23, p < 0.000005.
Figure 1.
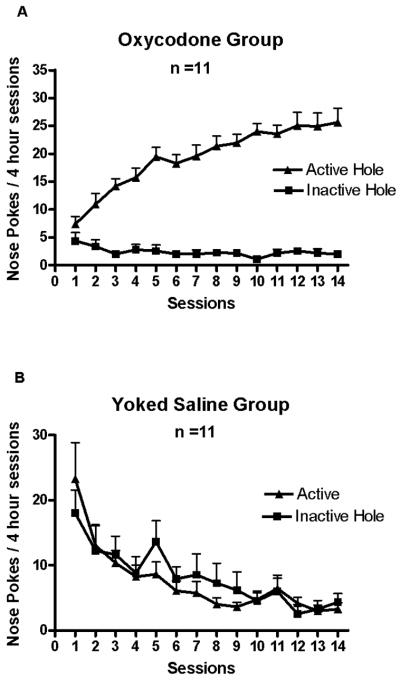
Oxycodone self administration in 4-hour sessions across the 14 days is shown. Data represent mean daily number of nose pokes (+SEM). Mice in the oxycodone group nose poked significantly more at the active hole than at the inactive hole (1A). Mice in the yoked saline group did not differ in nose poking between the “active” and inactive hole (1B).
Figure 3.
A. There was a significant increase in the amount of oxycodone self-administered across days 1, 3, 7 and 14 in 4-hour sessions. B. There was no significant increase in the amount of oxycodone self-administered across days 1, 3, 7 and 14 in 1-hour sessions.
Figure 4.
The cumulative mg/kg oxycodone intake (+SEM) measured in 10-min time bins on day 1, 3, 7 and 14 of the extended self administration sessions is presented.
Oxycodone self administration in short access (1-hour) sessions
The behavior of oxycodone SA by adult C57BL/6J mice in 1-hour sessions across 14 days is shown in Fig. 2. Mice in the oxycodone group nose poked significantly more at the active hole than at the inactive hole (Fig. 2A). In contrast, mice in the yoked saline control group did not differ in nose poking between the “active” and inactive hole (Fig. 2B). One-way ANOVA found that there was no significant main effect of the amount of oxycodone SA across days 1, 3, 7 and 14, F (3, 21) = 2.31, p = 0.105 (Fig. 3B).
Figure 2.
Oxycodone self administration in 1-hour sessions across the 14 days is shown. Data represent mean daily number of nose pokes (+SEM). Mice in the oxycodone group nose poked significantly more at the active hole than at the inactive hole (2A). Mice in the yoked saline group did not differ in nose poking between the “active” and inactive hole (2B).
Effects of oxycodone SA in extended access sessions on neurotransmitter receptor gene expression in the dorsal striatum
The effect of extended access oxycodone SA on the expression of 84 neurotransmitter receptor genes was examined. Of the 84 genes, 16 (Brs3, Cckar, Chrna1, Chrna6, Chrnb3, Chrnd, Chrng, Drd4, Gabra6, Gabrp, Galr3, Prokr1, Mc2r, Npy6r, Ppyr1, Tacr2) were not analyzed because of low abundance (CT values greater than 32, a threshold set for this assay).
Of the 68 genes analyzed by t-test, Gamma-aminobutyric acid (GABA) A receptor, subunit beta 2 (Gabrb2) exhibited an experiment-wise significant result, pB = 0.0085 (Bonferroni-corrected for 68 tests) (see Table 1). Eleven of the remaining 68 genes showed point-wise statistical significance (p ≤ 0.05, Table 1).
Table 1.
Genes differing between mice that had self administered oxycodone in 4 hrs session for 14 days and mice that served as yoked-saline controls in the dorsal striatum
| Gene symbol | Protein | P value | Fold Change | Change Direction |
|---|---|---|---|---|
| Gabrb2 | Gamma-aminobutyric acid (GABA) A receptor, subunit beta 2 | 0.00013 | 0.77 | ↓ |
| Slc5a7 | Solute carrier family 5 (choline transporter), member 7 | 0.00570 | 1.12 | ↑ |
| Gpr83 | G protein-coupled receptor 83 | 0.02004 | 1.23 | ↑ |
| Htr3a | 5-hydroxytryptamine (serotonin) receptor 3A | 0.02196 | 1.48 | ↑ |
| Tacr3 | Tachykinin receptor 3 | 0.02594 | 1.09 | ↑ |
| Chrnb4 | Cholinergic receptor, nicotinic, beta polypeptide 4 | 0.02689 | 0.56 | ↓ |
| Chrna7 | Cholinergic receptor, nicotinic, alpha polypeptide 7 | 0.03008 | 0.81 | ↓ |
| Chrm5 | Cholinergic receptor, muscarinic 5 | 0.03402 | 1.65 | ↑ |
| Gabra1 | Gamma-aminobutyric acid (GABA) A receptor, subunit alpha 1 | 0.03645 | 0.85 | ↓ |
| Glra4 | Glycine receptor, alpha 4 subunit | 0.03731 | 1.32 | ↑ |
| Npy5r | Neuropeptide Y receptor Y5 | 0.04055 | 1.14 | ↑ |
| Gabrr1 | Gamma-aminobutyric acid (GABA) C receptor, subunit rho 1 | 0.04979 | 1.47 | ↑ |
| Gabrr2 | Gamma-aminobutyric acid (GABA) C receptor, subunit rho 2 | 0.05466 | 1.62 | ↑ |
For further analysis, the partition test was performed (Ott et al. 2012). The strongest discrimination between observed and expected small p-values occurred with a threshold of 0.05467; p-values for 13 genes were observed below this level, whereas only 3.72 (0.05467 × 68) genes were expected. The associated experiment-wise significance level is pE = 0.0271, estimated in 50,000 randomization samples. Thus, we can confidently say that 13 genes showed different expression levels between oxycodone and yoked saline controls (Table 1).
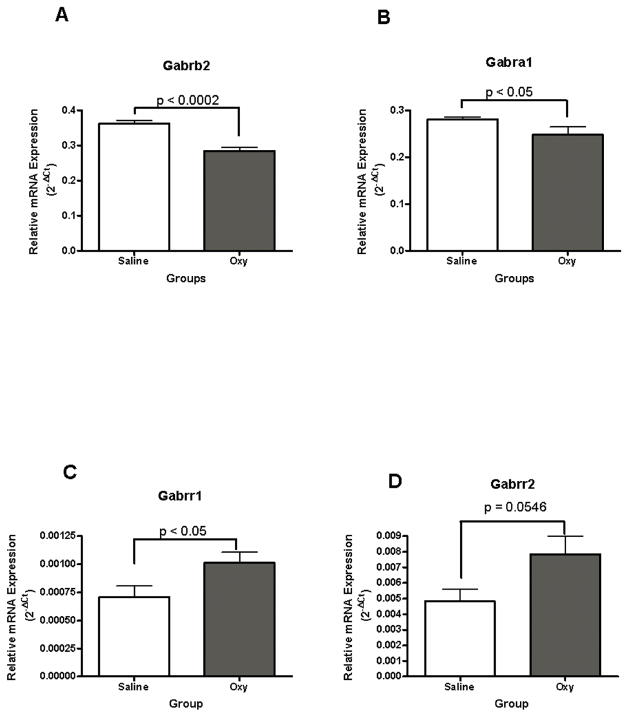
Gamma-aminobutyric acid (GABA) A receptor, subunit beta 2 (Gabrb2), subunit alpha 1 (Gabra1), subunit rho 1(Gabrr1) and subunit rho 2 (Gabrr2) mRNA levels were significantly different between the oxycodone and yoked saline groups, t (10) = −6.03, p < 0.0002; t (10) = −2.41, p < 0.05; t (10) = 2.65 p < 0.02; t (10) = 2.17, p < 0.05, respectively (Fig. 5A, 5B, 5C, 5D). Extended access oxycodone self administration significantly decreased Gabra1 and Gabrb2 mRNA levels, but increased Gabrr1 and Gabrr2 mRNA levels in the dorsal striatum compared to yoked saline controls.
Figure 5.
Oxycodone self administration in 4-hour sessions significantly affected GABA A subunits Gabra1, Gabrb2 and GABA C subunits Gabrr1 and Gabrr2 mRNA levels in the dorsal striatum. Gabrb2 (5A) and Gabra1 (5B) mRNA levels decreased whereas Gabrr1(5C) and Garbrr2 (5D) mRNA levels increased in the oxycodone group compared with yoked saline controls.
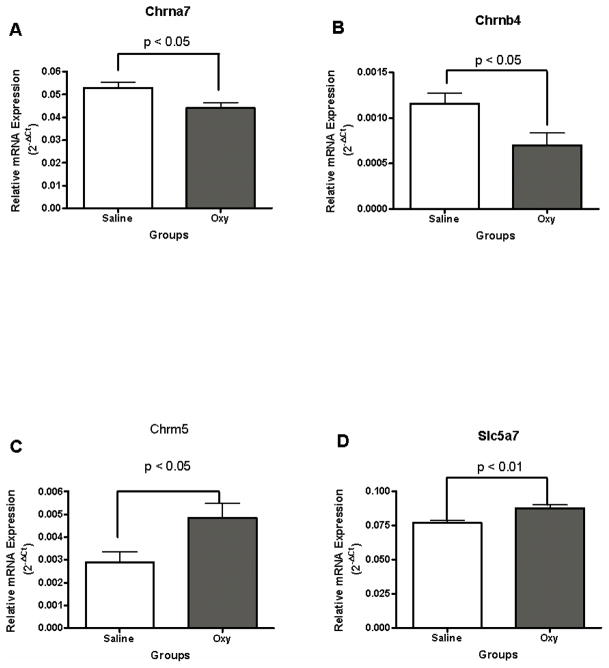
Cholinergic receptor, nicotinic, alpha polypeptide 7 (Chrna7) and cholinergic receptor, nicotinic, beta polypeptide 4 (Chranb4) mRNA profiles were significantly altered in mice that self administered oxycodone for 14 consecutive days, t (10) = −2.53, p < 0.05; t (10) = −2.59, p < 0.05, respectively (Fig. 6A and 6B). Oxycodone self administration decreased Chrna7 and Chranb4 mRNA levels.
Figure 6.
Oxycodone self administration in 4-hour sessions decreased cholinergic receptor, nicotinic, alpha subunit 7 Chrna7 (6A) and beta subunit 4 Chranb4 (6B) mRNA levels compared with yoked saline controls. In contrast, oxycodone self administration significantly increased cholinergic receptor, muscarinic 5 receptor (Chrm5) (6C) and choline transporter, member 7 (Slc5a7) (6D) mRNA levels in the dorsal striatum.
Cholinergic receptor, muscarinic 5 (Chrm5) mRNA level in the dorsal striatum was also changed by oxycodone self administration, t (10) = 2.45, p < 0.05 (Fig. 6C). Oxycodone self administration significantly increased Chrm5 mRNA levels.
Solute carrier family 5 (choline transporter), member 7 (Slc5a7) mRNA levels showed a difference between oxycodone SA and yoked saline control groups, t (10) = 3.50, p < 0.01 (Fig. 6D). Oxycodone self administration increased Slc5a7 mRNA levels.
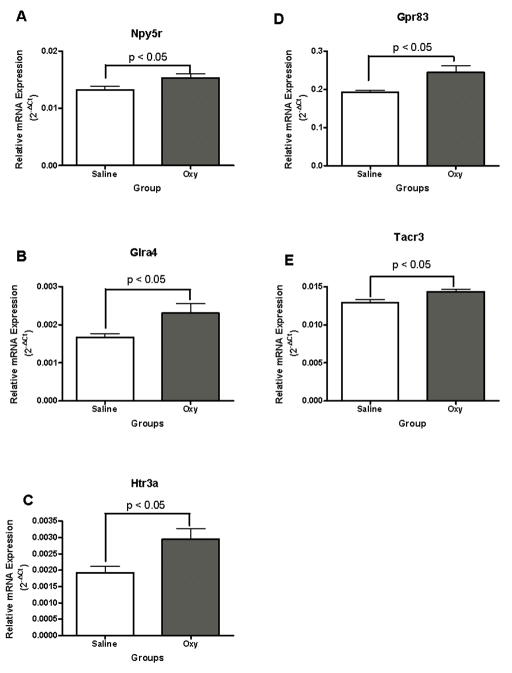
Neuropeptide Y receptor Y5 (Npy5r), Glycine receptor, alpha 4 subunit (Glra4) and 5-hydroxytryptamine (serotonin) receptor 3A (Htr3a) mRNA levels in the dorsal striatum showed alterations in response to oxycodone SA compared to controls, t (10) = 2.35, p < 0.05; t (10) = 2.40, p < 0.05; t (10) = 2.71, p < 0.05, respectively (Fig. 7A, 7B, 7C). Oxycodone SA significantly increased Npy5r, Glra4 and Htr3a mRNA levels.
Figure 7.
Oxycodone self administration in 4-hour sessions significantly increased Neuropeptide Y receptor Y5 (Npy5r) (6A), Glycine receptor, alpha 4 subunit (Glra4) (7B), 5-hydroxytryptamine (serotonin) receptor 3A (Htr3a) mRNA levels (7C), G protein-coupled receptor 83 (Gpr83) (7D) and Tachykinin receptor (Tacr3) (7E) in the dorsal striatum.
Similarly, G protein-coupled receptor 83 (Gpr83) and Tachykinin receptor (Tacr3) mRNA levels in the dorsal striatum also showed point-wise significant differences between mice that had self administered oxycodone and yoked saline controls t (10) = 2.76, p < 0.02 and t (10) = 2.61, p < 0.05, respectively (Fig. 7D, 7E).
Differences in Chrna7, Chrnb4, Grbrb2, Glra4, Htr3a mRNA levels between the oxycodone and yoked saline mice confirmed by real time PCR
Five out of 13 genes in Table 1 were confirmed to be significantly changed using real time PCR. T-tests found that there were significant differences between the oxycodone and yoked saline control for Chrna7 mRNA, t (10) = 2.30, p < 0.05; Chrnb4 mRNA, t (10) = 2.32, p < 0.05; Grbrb2 mRNA, t (10) = 2.23, p < 0.05; Glra4 mRNA, t (10) = 3.85, p < 0.01; Htr3a mRNA, t (10) = 5.90, p < 0.001, respectively.
Discussion
In the current study in mice, we used 14 consecutive days of self administration sessions of longer than typical duration, to examine oxycodone self-administration behavior and its neurobiological effect on striatal neurotransmitter receptor gene expression. To our knowledge, this is one of the first studies using 4-hour and 14-day self administration sessions to examine chronic oxycodone or any MOP-r agonist self exposure in mice. We found that adult C57BL/6J mice showed an escalation in oxycodone intake, which was accompanied by specific changes of neurotransmitter receptor mRNA levels in the dorsal striatum.
Conventionally, mouse self-administration studies involve sessions that do not last more than 3 hours (e.g., Deroche et al. 1997; Caine et al. 2007; Szumlinski et al. 2004; Berrendero et al 2013.; Szumlinski et al. 2004; Brown et al. 2012). In this study, 4 hour sessions, were used, in a chronic 14-day paradigm. Consistent with what was found in earlier studies on other drugs of abuse in rats (e.g., Ahmed and Koob, 1998; Mantsch et al. 2004; Picetti et al. 2012), the longer sessions used in the current study led to escalation of oxycodone intake over the sessions, which was not found in the short access self-administration paradigm. Specifically, the amount of oxycodone self administered rose significantly from days 1–3, 3–7, and 7–14. The significant difference in the total number of nose pokes (active and inactive) between the oxycodone and yoked saline mice in the early self-administration sessions (sessions 1–2) indicated that initial oxycodone intake exerted an inhibitory effect on nose poking behavior in mice. Increases in the number of nose pokes at the active hole and decreases in the number of nose pokes at the inactive hole observed over the 14-day sessions indicated chronic oxycodone intakes produced a rewarding effect on the mice in the oxycodone group.
Processes of escalation in drug intake have been discussed in the past, usually focused on rat models (e.g., Zernig et al., 2007). An increase in hedonic set point may be a potential mechanism for increased drug intake (Ahmed and Koob, 1998). It has been suggested that experimental animals and human addictive disease patients increase drug intake not only because they are tolerant to the rewarding effects of drugs of abuse, but because they are attempting to reach and maintain a higher hedonic state. Interestingly, results from a new study showed that intracranial self-stimulation (ICSS) thresholds increased following extended access to methamphetamine self-administration in rats. Such increases in ICSS thresholds were correlated with the increase of drug intake (Jang et al., 2013). However, it can not be completely ruled out that tolerance may have developed during the course of long-term self-exposure to oxycodone. Furthermore, the escalation in oxycodone intake indicated development of neuronal adaptation to the rewarding effect of oxycodone.
To explore neurobiological mechanisms underlying escalation of oxycodone intake, we examined transcription profiles of neurotransmitter receptors in the dorsal striatum, a brain region that is closely involved in reward, locomotor regulation and habitual learning (e.g., Ito et al. 2002; Porrino et al. 2007; Belin and Everitt 2008). Interestingly, a group of genes belonging to the Cys-loop receptor family including GABA type-A (GABAAR) receptors, glycine receptors (GlyRs) (Betz and Laube 2006), nicotinic acetylcholine receptors (nAChR) and serotonin receptor (5-HT3) changed in response to extended access oxycodone self administration in the current analysis. Both GABA and glycine play important roles in inhibitory neurotransmission. Genes encoding two subunits of GABAA receptor (Gabra1 and Gabrb2) showed significant differences in expression between the oxycodone self administration group and yoked saline controls. Association of the expression of several GABA-A subunits with morphine self administration was reported earlier in rats (Rodriguez Parkitna et al. 2004). Morphine withdrawal increased expression of GABAA receptor epsilon subunit mRNA in the locus coeruleus neurons (Heikkila et al. 2001). GABAA alpha1 subunit mRNAs were decreased in the shell of nucleus accumbens but increased in the core following chronic morphine administration whereas the GABAA delta subunit was unregulated in the shell of nucleus accumbens (Hemby 2004). Self-administration of morphine for 40 sessions changed expression of the gamma1 subunit of GABAA receptor in the rat amygdala (Rodriguez Parkitna et al. 2004). In the present study, 14-day oxycodone self administration led to decreases in both alpha1 and beta2 subunits of the GABAA receptor compared to the yoked saline controls. Such a finding is consistent with earlier reports showing that opioid self administration altered GABAA subunit expression in the central nervous system. Of interest the mRNA level of only one subunit, Gabrb2, showed an experiment-wise significant decrease in mice that had self administered oxycodone. However, such an alteration in one of the subunits may affect GABAA receptor function more broadly, since GABAA receptors are composed of five subunits that belong to different subunit classes. Thus, GABAA receptors exhibit distinct pharmacological and electrophysiological properties based on their subunit composition (e.g., Sieghart 1999, Scato-Jackson and Sieghart, 2008). Although the proportion of the beta 2 subunit needed to assemble the pentameric receptor in the dorsal striatum is not clear, these data lead to the hypothesis that decrease in the Gabrb2 subunit may cause decrease in GABAA receptor in the medium spiny neurons of this brain region. The resulting change in GABAA function may in part contribute to increase in oxycodone self administered over the 14-day sessions, as dorsal striatum is known to be involved in mediating habitual learning and compulsive-like behaviors that occur after more prolonged drug self-exposure (e.g., Ito et al. 2002; Porrino et al. 2007; Belin and Everitt 2008). This hypothesis could be tested in future studies, to determine whether protein levels of the beta 2 subunit and total GABAA receptor are decreased under such conditions. We also found that glycine, alpha 4 subunit mRNA levels were significantly increased in mice that had self administered oxycodone compared to saline controls. To our knowledge, this is the first study showing GABAA and glycine receptor subunits to be changed as a result of oxycodone self administration.
The genes encoding two subunits of nicotinic cholinergic receptor alpha7 and beta4 (Chrna7 and Chrnb4) nAChRs showed significant differences in expression between the oxycodone self-administration group and yoked saline controls. Neuronal nAChR subunits form pentameric receptors, closely resembling the nAChR found at the neuromuscular junction (Leonard and Bertrand, 2001). An earlier study reported that blocking alpha4beta2 nicotinic acetylcholine receptors inhibited the reinstatement of morphine-induced conditioned place preference by morphine priming in mice (Feng et al. 2008). Further, deletion of α4, α6 or β2 subunits abolished intravenous nicotine self-administration, which was restored by re-expression of these subunits in the VTA (Pons et al. 2008). In contrast, deletions of either Chrna7 or Chrnb4 subunits in mice did not affect dopamine release in striatal synaptosomes (Salminen et al. 2004). Thus, the decrease in both nAChRs alpha7 and beta4 subunits found in the current study may not play a key role in mediating oxycodone self-administration behavior.
Additionally, mRNA for a cholinergic receptor, the muscarinic 5 receptor was increased in the oxycodone self-administration group, compared to the controls. Thus subunits of nicotinic and muscarinic cholinergic receptors are altered as a result of oxycodone self administration. Earlier studies found that all five muscarinic cholinergic receptors (mAChRs) are expressed in the striatum, and muscarinic 5 receptors are the only mAChR subtype expressed in dopaminergic neurons (Weiner et al. 1990), where they function to facilitate dopamine release (Forster et al. 2002; Bendor et al. 2010; Steidl et al.2011). Thus, increases in muscarinic 5 receptor mRNA levels in the dorsal striatum may be involved in enhanced dopamine release in the mice that had self administered oxycodone. Furthermore, mRNA levels of the choline transporter also increased in mice that had self administered oxycodone compared to the saline control group, adding evidence that the cholinergic systems in the dorsal striatum were altered as a result of oxycodone self administration and may participate in mediating chronic oxycodone self-administration behaviour.
Several other genes also showed point-wise significant difference in expression between the oxycodone and saline control in the array study. Little is known about the involvement of Gpr83, Npy5r, Glra4, Htr3a, Gabrr1 and Gabrr2 in drug addiction models, and these would be valuable targets for follow-up. However, an earlier study found that administration of tachykinin 3 receptor agonists attenuated the intake of alcohol and tachykinin 3 receptor can also mediate the acute and chronic behavioral effects of cocaine (Foroud et al. 2008).
Interestingly, human studies have linked polymorphisms in some of the genes mentioned above, such as serotonin receptor 3A (altered by oxycodone self administration) with heroin addiction in humans (e.g., Levran et al. 2008; Levran et al. 2009). This indicates some translational value in the current study and needs to be followed up in future studies.
There are several other important neurotransmitter and neuropeptide receptor genes including glutamatergic, noradrenergic and opioid receptors genes which are not included in the array used in the current study. Future studies are needed to examine changes in these receptors following extended oxycodone self administration.
In summary, mice escalated oxycodone intake in a relatively long access, chronic self-administration paradigm. Escalation of oxycodone intake for 14 days resulted in identification of alterations in specific neurotransmitter receptor gene expression that may underlie oxycodone self-administration behavior in mice. The genes identified in this study support findings from earlier studies, and may constitute promising candidates for further studies to determine the mechanistic impact of these targets and their relationship to self-exposure to the widely abused prescription opioid oxycodone.
Supplementary Material
Acknowledgments
We thank Drs. Brian Reed, Connie Zhao, Joel Correa da Rosa, Orna Levran and Vadim Yuferov for their help in preparing the manuscript. This work was supported by NIH-NIDA 1R01DA029147-01A1 (YZ) and NIH-NIDA DA05030 (MJK).
Footnotes
Disclosure/Conflict of Interest
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bendor J, Lizardi-Ortiz JE, Westphalen RI, Brandstetter M, Hemmings HC, Jr, Sulzer D, Flajolet M, Greengard P. AGAP1/AP-3-dependent endocytic recycling of M5 muscarinic receptors promotes dopamine release. EMBO J. 29:2813–26. doi: 10.1038/emboj.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, Maldonado R. Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology. 2013;37:2332–44. doi: 10.1038/npp.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–10. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Brown RM, Stagnitti MR, Duncan JR, Lawrence AJ. The mGlu5 receptor antagonist MTEP attenuates opiate self-administration and cue-induced opiate-seeking behaviour in mice. Drug Alcohol Depend. 123:264–8. doi: 10.1016/j.drugalcdep.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–50. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6 x SJL and BALB/cByJ mice. Pharmacol Biochem Behav. 1997;57:429–40. doi: 10.1016/s0091-3057(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Feng B, Xing JH, Jia D, Liu SB, Guo HJ, Li XQ, He XS, Zhao MG. Blocking alpha4beta2 and alpha7 nicotinic acetylcholine receptors inhibits the reinstatement of morphine-induced CPP by drug priming in mice. Behav Brain Res. 220:100–5. doi: 10.1016/j.bbr.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila AT, Echenko O, Uusi-Oukari M, Sinkkonen ST, Korpi ER. Morphine withdrawal increases expression of GABA(A) receptor epsilon subunit mRNA in locus coeruleus neurons. Neuroreport. 2001;12:2981–5. doi: 10.1097/00001756-200109170-00045. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126:689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–53. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Chen AC, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPgammaS binding. Synapse. 2003;47:243–9. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7:720–9. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Randesi M, Rotrosen J, Casadonte P, Linzy S, Ott J, Adelson M, Kreek MJ. Heroin addiction in African Americans: a hypothesis-driven association study. Genes Brain Behav. 2009;8:531–40. doi: 10.1111/j.1601-183X.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Ott J, Liu Z, Shen Y. Challenging False Discovery Rate: A Partition Test Based on p Values in Human Case-Control Association Studies. Hum Hered. 2012;74:45–50. doi: 10.1159/000343752. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 220:163–72. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Parkitna JM, Bilecki W, Mierzejewski P, Stefanski R, Ligeza A, Bargiela A, Ziolkowska B, Kostowski W, Przewlocki R. Effects of morphine on gene expression in the rat amygdala. J Neurochem. 2004;91:38–48. doi: 10.1111/j.1471-4159.2004.02697.x. [DOI] [PubMed] [Google Scholar]
- Seip-Cammack KM, Reed B, Zhang Y, Ho A, Kreek MJ. Tolerance and sensitization to chronic escalating dose heroin following extended withdrawal in Fischer rats: possible role of mu-opioid receptors. Psychopharmacology (Berl) 225:127–40. doi: 10.1007/s00213-012-2801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Miller AD, Blaha CD, Yeomans JS. M(5) muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PLoS One. 6:e27538. doi: 10.1371/journal.pone.0027538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–13. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–4. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–22. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Svenningsson P, Picetti R, Schlussman SD, Nairn AC, Ho A, Greengard P, Kreek MJ. Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32. J Neurosci. 2006;26:2645–51. doi: 10.1523/JNEUROSCI.3923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ. Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology. 2013;67:259–66. doi: 10.1016/j.neuropharm.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.