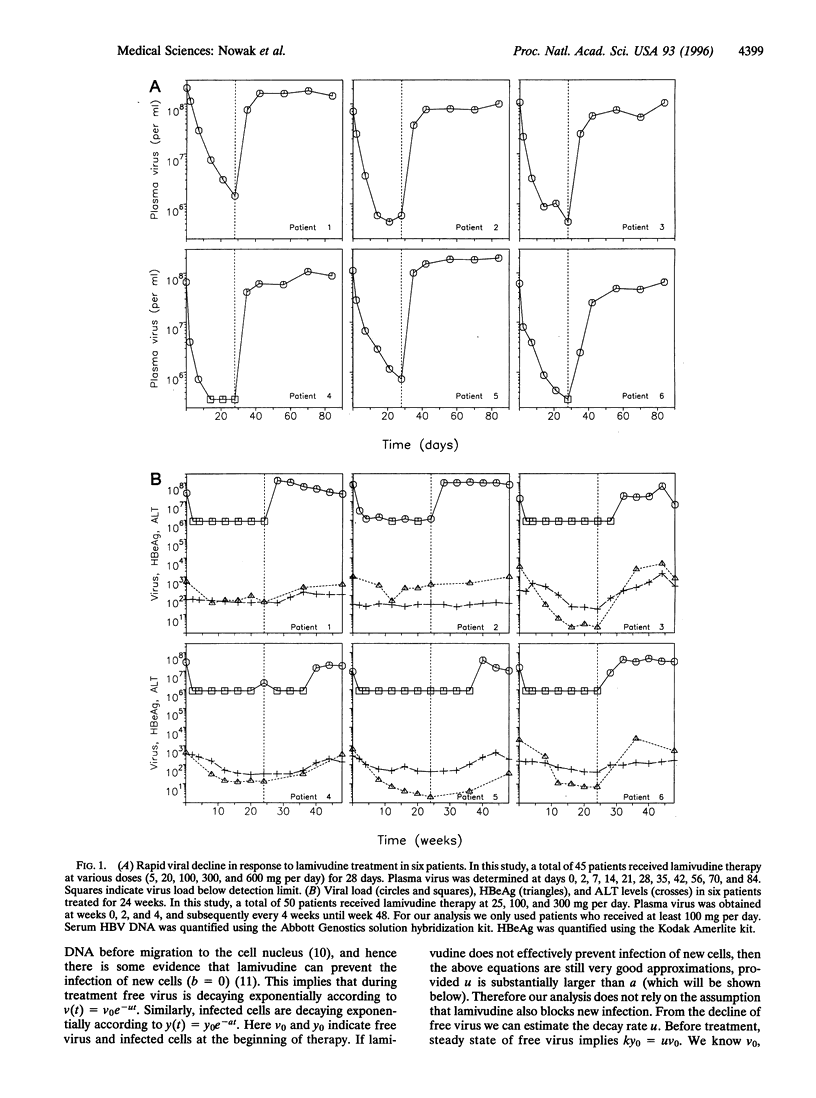
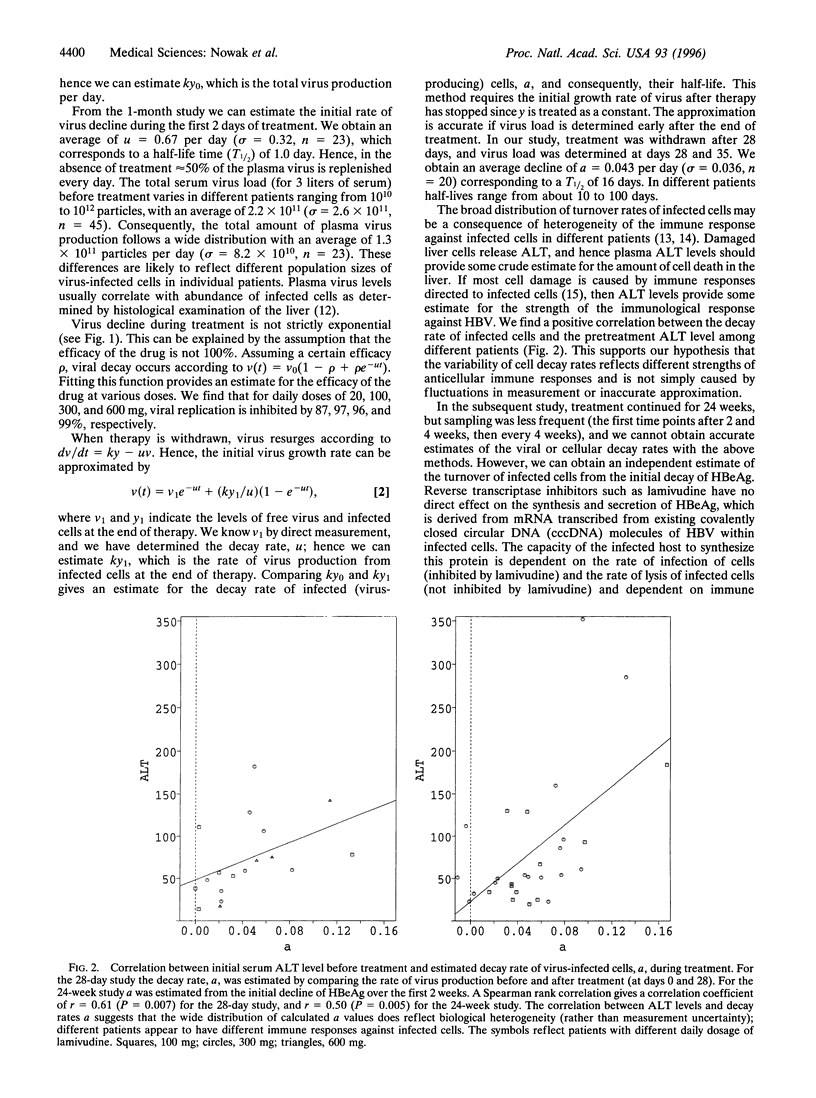
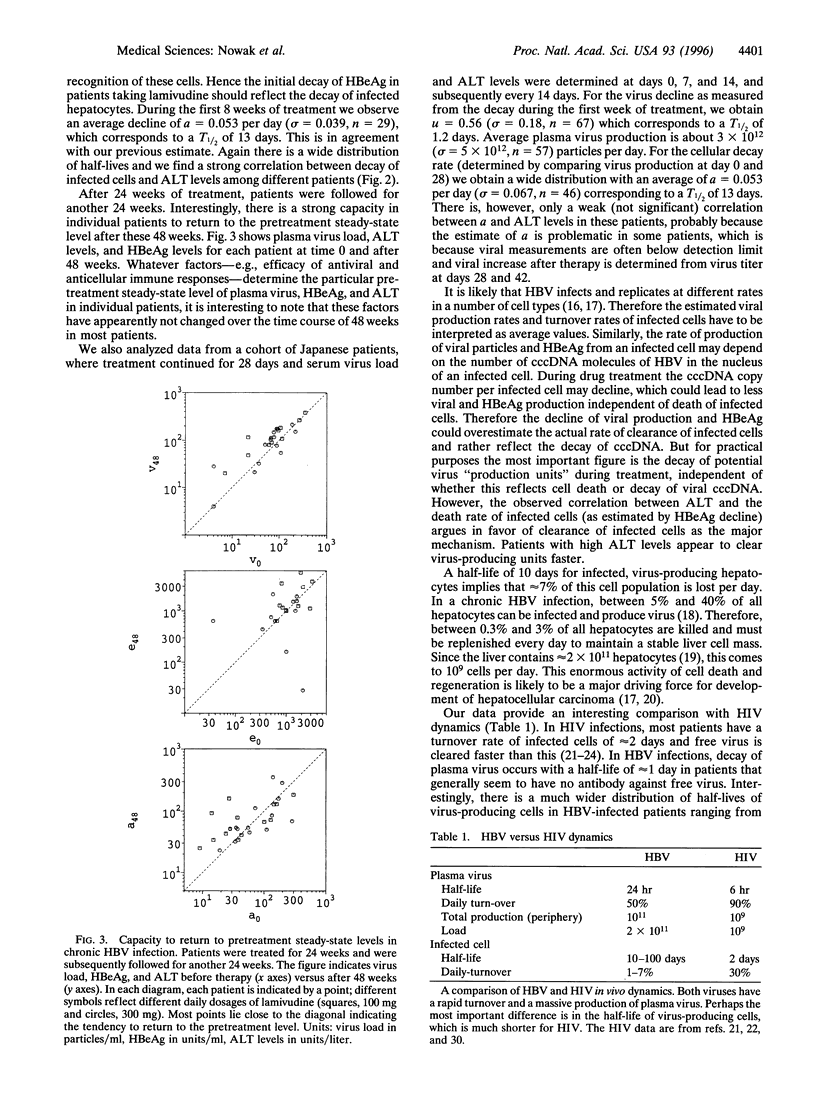
Abstract
Treatment of chronic hepatitis B virus (HBV) infections with the reverse transcriptase inhibitor lamivudine leads to a rapid decline in plasma viremia and provides estimates for crucial kinetic constants of HBV replication. We find that in persistently infected patients, HBV particles are cleared from the plasma with a half-life of approximately 1.0 day, which implies a 50% daily turnover of the free virus population. Total viral release into the periphery is approximately 10(11) virus particles per day. Although we have no direct measurement of the infected cell mass, we can estimate the turnover rate of these cells in two ways: (i) by comparing the rate of viral production before and after therapy or (ii) from the decline of hepatitis B antigen during treatment. These two independent methods give equivalent results: we find a wide distribution of half-lives for virus-producing cells, ranging from 10 to 100 days in different patients, which may reflect differences in rates of lysis of infected cells by immune responses. Our analysis provides a quantitative understanding of HBV replication dynamics in vivo and has implications for the optimal timing of drug treatment and immunotherapy in chronic HBV infection. This study also represents a comparison for recent findings on the dynamics of human immunodeficiency virus (HIV) infection. The total daily production of plasma virus is, on average, higher in chronic HBV carriers than in HIV-infected patients, but the half-life of virus-producing cells is much shorter in HIV. Most strikingly, there is no indication of drug resistance in HBV-infected patients treated for up to 24 weeks.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Stevens C. E., Wang K. Y., Sun T. S., Hsieh F. J., Szmuness W. Hepatitis B immune globulin (HBIG) efficacy in the interruption of perinatal transmission of hepatitis B virus carrier state. Initial report of a randomised double-blind placebo-controlled trial. Lancet. 1981 Aug 22;2(8243):388–393. doi: 10.1016/s0140-6736(81)90832-1. [DOI] [PubMed] [Google Scholar]
- Bertoletti A., Sette A., Chisari F. V., Penna A., Levrero M., De Carli M., Fiaccadori F., Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994 Jun 2;369(6479):407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Skalski V., Zhou J. H., Cheng Y. C. Biochemical pharmacology of (+)- and (-)-2',3'-dideoxy-3'-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992 Nov 5;267(31):22414–22420. [PubMed] [Google Scholar]
- Coffin J. M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995 Jan 27;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Perrillo R. P., Schiff E. R., Bartholomew M., Vicary C., Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995 Dec 21;333(25):1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- Doong S. L., Tsai C. H., Schinazi R. F., Liotta D. C., Cheng Y. C. Inhibition of the replication of hepatitis B virus in vitro by 2',3'-dideoxy-3'-thiacytidine and related analogues. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C., Penna A., Bertoletti A., Valli A., Antoni A. D., Giuberti T., Cavalli A., Petit M. A., Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990 Nov 15;145(10):3442–3449. [PubMed] [Google Scholar]
- Ganem D., Pollack J. R., Tavis J. Hepatitis B virus reverse transcriptase and its many roles in hepadnaviral genomic replication. Infect Agents Dis. 1994 Apr-Jun;3(2-3):85–93. [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Cullen J., Saputelli J., Wu T. T., Liu C., London W. T., Lustbader E., Schaffer P., O'Connell A. P., Fourel I. Characterization of the antiviral effects of 2' carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994 Feb;19(2):398–411. [PubMed] [Google Scholar]
- Mason W. S. The problem of antiviral therapy for chronic hepadnavirus infections. J Hepatol. 1993;17 (Suppl 3):S137–S142. doi: 10.1016/s0168-8278(05)80439-8. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Nowak M. A., Bonhoeffer S., Loveday C., Balfe P., Semple M., Kaye S., Tenant-Flowers M., Tedder R. HIV results in the frame. Results confirmed. Nature. 1995 May 18;375(6528):193–193. doi: 10.1038/375193a0. [DOI] [PubMed] [Google Scholar]
- Payne R. J., Nowak M. A., Blumberg B. S. A cellular model to explain the pathogenesis of infection by the hepatitis B virus. Math Biosci. 1994 Sep;123(1):25–58. doi: 10.1016/0025-5564(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Perelson A. S., Neumann A. U., Markowitz M., Leonard J. M., Ho D. D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996 Mar 15;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- Schuurman R., Nijhuis M., van Leeuwen R., Schipper P., de Jong D., Collis P., Danner S. A., Mulder J., Loveday C., Christopherson C. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J Infect Dis. 1995 Jun;171(6):1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- Severini A., Liu X. Y., Wilson J. S., Tyrrell D. L. Mechanism of inhibition of duck hepatitis B virus polymerase by (-)-beta-L-2',3'-dideoxy-3'-thiacytidine. Antimicrob Agents Chemother. 1995 Jul;39(7):1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Weissberg J. I., Andres L. L., Smith C. I., Weick S., Nichols J. E., Garcia G., Robinson W. S., Merigan T. C., Gregory P. B. Survival in chronic hepatitis B. An analysis of 379 patients. Ann Intern Med. 1984 Nov;101(5):613–616. doi: 10.7326/0003-4819-101-5-613. [DOI] [PubMed] [Google Scholar]



