Abstract
Rationale
Carrageenan-induced hyperalgesia is a widely used pain model in rodents. However, characteristics of carrageenan-induced hyperalgesia and effects of analgesic drugs under these conditions are unknown in nonhuman primates.
Objective
The aims of this study were to develop carrageenan-induced hyperalgesia in rhesus monkeys and determine the efficacy and potency of agonists selective for the four opioid receptor subtypes in this model versus acute pain, as compared to NSAIDs.
Results
Tail-injection of carrageenan produced long-lasting thermal hyperalgesia in monkeys. Systemically administered agonists selective for opioid receptor subtypes i.e. fentanyl (mu/MOP), U-50488H (kappa/KOP), SNC80 (delta/DOP) and Ro 64-6198 (nociceptin/orphanin FQ/NOP) dose-dependently attenuated carrageenan-induced thermal hyperalgesia with different potencies. In absence of carrageenan, these agonists, except SNC80, blocked acute thermal nociception. Opioid-related ligands, especially Ro 64-6198, were much more potent for their antihyperalgesic than antinociceptive effects. Both effects were mediated by the corresponding receptor mechanisms. Only fentanyl produced scratching at antihyperalgesic and antinociceptive doses consistent with its pruritic effects in humans, illustrating a translational profile of MOP agonists in nonhuman primates. Similar to SNC80, systemically administered NSAIDs ketorolac and naproxen dose-dependently attenuated carrageenan-induced hyperalgesia but not acute nociception.
Conclusion
Using two different pain modalities in nonhuman primates, effectiveness of clinically available analgesics like fentanyl, ketorolac and naproxen was distinguished and their efficacies and potencies were compared with the selective KOP, DOP, and NOP agonists. The opioid-related ligands displayed differential pharmacological properties in regulating hyperalgesia and acute nociception in the same subjects. Such preclinical primate models can be used to investigate novel analgesic agents.
Keywords: opioids, opioid receptors, NSAIDs, monkey pain model, carrageenan, nociceptin/orphanin FQ, NOP receptors, hyperalgesia
Introduction
Safe and effective treatment of pain poses a major challenge in pain research, owing to inadequate pain relief and treatment related side effects. In order to understand the pathophysiology and pharmacology of pain conditions, a number of experimental animal models have been developed, mostly in rodents. These models adapt the key behavioral characteristics of pain such as allodynia and hyperalgesia and are critical for making advances in preclinical pain research. However, there are examples where compounds that demonstrate efficacy and tolerability in rodent models have failed to show sufficient efficacy or safety in humans e.g. neurokinin-1 receptor antagonists (Hill 2000), selective sodium channel blockers (Wallace et al. 2002a) and glycine site NMDA antagonist (Wallace et al. 2002b). Efforts to develop ligands for promising analgesic targets like transient receptor potential ankyrin-1 and bradykinin B1 receptors are hindered due to differential pharmacology and receptor distribution among humans and rodents (Bianchi et al. 2012; Hawkinson et al. 2007). It is therefore crucial to develop behavioral assays in nonhuman primates, which are more closely homologous to humans and validate the pharmacological findings from multiple species.
In rhesus monkeys, warm water tail withdrawal procedure has been widely used to assess acute nociception (Hu et al. 2010; Ko et al. 1998). When algogenic substances such as capsaicin (Butelman et al. 2003), bradykinin (Butelman et al. 1995) and prostaglandin E2 (Negus et al. 1993) are applied or injected in the tail, it is sensitized to non-noxious thermal stimuli and allodynia/hyperalgesia are manifested as a decreased or faster tail withdrawal latency. These chemical-induced thermal hypersensitivities only last for 30 to 90 min. It is worth developing a longer duration of hyperalgesia in order to evaluate to what extent different analgesics display their effectiveness across different pain modalities and durations. Moreover, the longer duration of thermal allodynia and hyperalgesia may recruit mechanisms more similar to those involved in human pain syndromes. Such models will facilitate the evaluation of a ligand’s analgesic potential and allow direct comparisons of efficacy and potency between clinically used drugs and newly developed compounds.
One of the well-characterized rodent pain models is carrageenan-induced inflammatory hyperalgesia, which lasts up to 72 hours (Joris et al. 1987). Carrageenan causes inflammation from prostaglandin release via cyclooxygenase (COX) enzymes (Dirig et al. 1998). Activity of COX enzymes and inflammatory hyperalgesia is blocked by non-steroidal anti-inflammatory drugs (NSAIDs). Selective mu-opioid receptor (MOP) agonists are also reported to block carrageenan-induced hyperalgesia. This is likely due to increased expression of these receptors in the peripheral and central nervous system (Ji et al. 1995; Stanfa et al. 1992). In addition, peripherally acting MOP or kappa opioid receptor (KOP) agonists can block carrageenan-induced hyperalgesia in rodents (Barber et al. 1994; Whiteside et al. 2005). Nevertheless, characteristics of carrageenan-induced hyperalgesia as well as effects of opioids and NSAIDs in nonhuman primates are currently unknown.
MOP agonists and NSAIDs are primary treatment options for pain management. However, both types of analgesics possess distinct troublesome side effect profiles (Castellsague et al. 2012; Warner 2012). Given that the functions of other opioid receptor subtypes in primates, i.e., delta (DOP), KOP, and nociceptin/orphanin FQ (NOP), in regulating hyperalgesia are largely unknown, it is important to investigate effects of agonists selective for DOP, KOP, and NOP as compared to MOP agonists and NSAIDs. Therefore, the aims of the present study were to develop a nonhuman primate model of carrageenan-induced hyperalgesia, determine the efficacy and potency of agonists selective for each opioid receptor subtype between acute and inflammatory pain, and compare their effects with NSAIDs under these conditions.
Materials and Methods
Subjects
Eight adult (4 male and 4 female) rhesus monkeys (Macaca mulatta) ranging in body weight (7.4 – 12.1 kg) were used. The monkeys were individually housed and their daily diet consisted of approximately 25–30 biscuits (Purina Monkey Chow; Ralston Purina Co., St. Louis, MO), fresh fruit, and free access to water. All monkeys used were previously trained in the warm water tail-withdrawal assay and acclimated to being video-recorded in-cage. For one month prior to the study, the monkeys did not have exposure to any opioid compound. The monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the principles of laboratory animal care of the University Committee on the Use and Care of Animals in the University of Michigan (Ann Arbor, MI) and Wake Forest University (Winston-Salem, NC), and the Guide for the Care and Use of Laboratory Animals (publication number: 85–23, revised 1985) as adopted and promulgated by the U.S. National Institutes of Health (Bethesda, MD).
Procedures
Nociceptive Responses
Acute thermal nociception
The warm water tail-withdrawal assay was used to measure nociceptive responses to thermal stimuli and antinociceptive effects of test compounds (Hu et al. 2010). Previous studies have reported that monkeys and humans have similar thresholds to detect thermal stimuli. Monkeys escape the 51°C stimulus, which is also perceived as painful by humans. Similarly, monkeys do not withdraw from stimuli at temperatures between 43°C and 47°C that humans perceive as warm but not painful (Kupers et al. 1997). Therefore, the temperature range selected in the present studies is relevant to humans. Monkeys were seated in primate-restraining chairs, which allowed access to their shaved backs and exposure of their shaved tails (approximately 15 cm) to thermal flasks containing water maintained at 42, 46, or 50 °C. 42 and 46 °C water were used as normally non-noxious stimuli whereas 50°C water was used as an acute noxious stimulus. If monkeys did not remove their tails within 20 sec, the flask was removed and a maximum time of 20 sec was recorded. Test session began with control determinations at each temperature. Then, tail-withdrawal latencies were determined at multiple time points after administration of the test compound. A minimum of 1 week separated tests using the acute nociceptive assay.
Carrageenan-induced hyperalgesia
The tail-withdrawal latency in 46 °C water following administration of carrageenan was measured to evaluate antihyperalgesic effects of test compounds (Hawkinson et al. 2007). The 46 °C water was the thermal threshold of these subjects for expressing thermal hypersensitivity following administration of chemical irritants or pronociceptive agents (Butelman et al. 2003; Ko and Naughton 2009). To produce thermal hypersensitivity, carrageenan (0.3–2 mg) was subcutaneously administered into the terminal 3–5 cm of the tail in 0.1 mL. Carrageenan caused hyperalgesia or allodynia like hypersensitivity which was indicated by reduced tail-withdrawal latency from a maximum value of 20 sec to approximately 2–3 seconds in 46 °C water. Based on observations of the potency and duration of carrageenan (see Results), the antihyperalgesic effect of the test compound was evaluated 2 hours after administration of 2 mg of carrageenan. A minimum of 3–4 weeks separated tests using carrageenan-induced hyperalgesia. The hyperalgesic responses were scored by individuals who were blinded to the experimental conditions.
Itch/Scratching Responses
Monkeys were recorded in-cage for scratching behavior, which has been previously associated to an itch sensation (Ko et al. 2004). Recording was done in 15-minute intervals and scored by individuals who were blinded to experimental conditions. A scratch was counted and defined as a short (≤ 1 sec) episode of a scraping motion of the monkey’s front or hind paw against its body.
Experimental Design
The first part of the study was to determine the potency and duration of carrageenan-induced thermal hypersensitivity. Different doses (0.3–2 mg/tail) of carrageenan were administered by using a single dosing procedure. Tail-withdrawal latencies were measured at 15-min intervals during the first hour, then at 30-min intervals for the remaining 4-hour time course. The tail-withdrawal latencies at 42 and 46°C following carrageenan administration were used to detect potential hyperalgesic/pronociceptive effects.
The second part of the study was to determine and compare the potency of opioid-related ligands in the same group (n=4) of subjects receiving either acute nociceptive stimulus 50 °C water or carrageenan. Over a wide dose range, agonists selective for each receptor subtype, i.e., fentanyl (MOP), U-50488H (KOP), SNC80 (DOP), and Ro 64-6198 (NOP), were studied by using a cumulative dosing procedure. Drugs were administered at 30-min intervals. For each injection-test interval, a dose was given at time 0, and then the tail-withdrawal latency was measured between 20–30 min after each injection. For active antihyperalgesic/antinociceptive doses, additional test session was conducted separately in the same group of subjects in their home cages to record and quantify the itch scratching effects of these opioid-related ligands. Furthermore, antagonist studies were conducted to validate the receptor mechanisms underlying antihyperalgesic and antinociceptive effects. A single, MOP-selective dose of naltrexone (0.01 mg/kg), an antagonist with relative MOP selectivity as well as a single dose of KOP antagonist (nor-binaltorphimine, 3 mg/kg), DOP antagonist (naltrindole, 1 mg/kg), or NOP antagonist (J-113397, 0.03 mg/kg) was used. Previous studies have demonstrated that these dosing regimens produced selective antagonism for each receptor subtype. Naltrexone, naltrindole and J-113397 were administered 15 min prior to their selective agonists whereas nor-binaltorphimine was administered as a 24 h pretreatment to the KOP agonist. (Brandt et al. 2001; Butelman et al. 1993; Ko et al. 1998; Ko and Naughton 2009). The dose-response curves of opioid-related ligands were redetermined after pretreatment with corresponding antagonists.
The third part of the study was to determine and compare the potency of NSAIDs in the same group (n=4) of subjects receiving either carrageenan or acute nociceptive stimulus 50 °C water. Both ketorolac (0.3–6 mg/kg) and naproxen (3–20 mg/kg) were studied by using a cumulative dosing procedure with a 30-min inter-injection interval. In addition, antagonist studies were conducted to clarify the involvement of opioid receptors underlying antihyperalgesic effects. A single dose of the MOP antagonist (naltrexone, 0.01 or 0.3 mg/kg) was selected based on previous studies, demonstrating that these dosing regimens produced selective MOP or overall opioid receptor antagonism (Ko et al. 1998). The dose-response curve of ketorolac was redetermined after pretreatment with naltrexone.
Data Analysis
Mean values (mean ± S.E.M.) were calculated from individual values for all behavioral endpoints. Comparisons were made for the same subjects across all test sessions in the same experiment. The time course of carrageenan-induced hyperalgesia was analyzed by using two-way analysis of variance followed by the Newman-Keuls test for multiple comparisons. The scratching-eliciting effects of opioid-related ligands were analyzed by using one-way analysis of variance followed by the Dunnett test for multiple comparisons. The criterion for significance for all tests was set at p<0.05. For analyzing the dose-response curve for antinociceptive effects, individual tail-withdrawal latencies were converted to the percentage of maximum possible effect. The formula of the % maximum possible effect is defined as [(test latency – control latency)/(cutoff latency, 20 sec – control latency)]×100. For analyzing the dose-response curve for antihyperalgesic effects, individual tail-withdrawal latencies were converted to the percentage of maximum possible effect. The formula of the % maximum possible effect is defined as [(test latency – control latency determined 90 min after carrageenan)/(cutoff latency, 20 sec – control latency determined 90 min after carrageenan)]×100. ED50 values were calculated by least-squares regression with the portion of the dose-response curves spanning the 50% maximum possible effect and the 95% confidence limits were determined accordingly. Mean ED50 values were considered to be significantly different when their 95% confidence limits did not overlap; therefore, they provided the dose ratios (i.e., degree of rightward shift) of the dose-response curves with and without antagonists.
Drugs
Fentanyl HCl, naltrexone HCl, naltrindole HCl (National Institute on Drug Abuse, Bethesda, MD), nor-binaltorphimine dihydrochloride, naproxen sodium (Tocris Bioscience, Ellisville, MO), U-50488H methanesulfonate salt, and lambda carrageenan (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile water. SNC80 free base (NIDA, Bethesda, MD) was dissolved in 3% lactic acid in sterile water. J-113397 (Tocris Bioscience, Ellisville, MO) and Ro 64-6198 (Amgen, Thousand Oaks, CA) was dissolved in a solution of dimethyl sulfoxide/Tween 80/sterile water in a ratio of 1:1:8. Ketorolac tromethamine was purchased as an injectable solution (Hospira, Lake Forest, IL). Doses are presented in the compound forms listed above. All test compounds, i.e., opioid-related ligands, NSAIDs, and antagonists, were administered subcutaneously around the scapular region of the back at a volume of 0.1 mL/kg.
Results
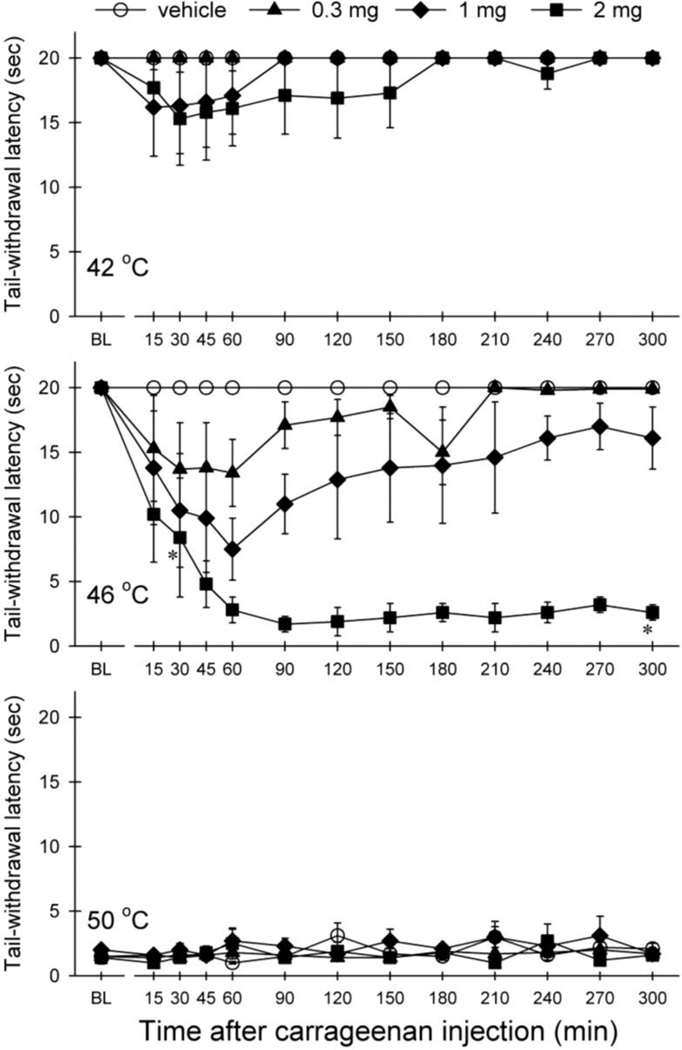
Figure 1 illustrates the time course and potency of carrageenan-induced thermal hypersensitivity in nonhuman primates. Carrageenan produced thermal hyperalgesia in 46 °C water in both dose-[F(3,9)=42.0; p<0.05] and time-dependent [F(12,36)=4.2; p<0.05] manners. In particular, 2 mg of carrageenan produced thermal hyperalgesia at 30 min after administration; then the thermal hyperalgesia peaked at 60 min and sustained through the 5-hour time point. Signs of tissue damage were not observed after injection with carrageenan. As noted, the subjects’ nociceptive threshold recovered at 24 hour after administration, i.e., displayed 20 sec in the presence of 46 °C water (data not shown).
Fig 1.
Thermal hyperalgesia induced by tail injection of carrageenan. Results are expressed as effect of carrageenan on tail withdrawal latencies at 42 °C (top), 46 °C (middle) and 50 °C (bottom) observed at different time points. Symbols represent different doses of carrageenan. Each value represents mean ± S.E.M (n=4). * significant difference from the vehicle between 30- and 300-min time points (p<0.05).
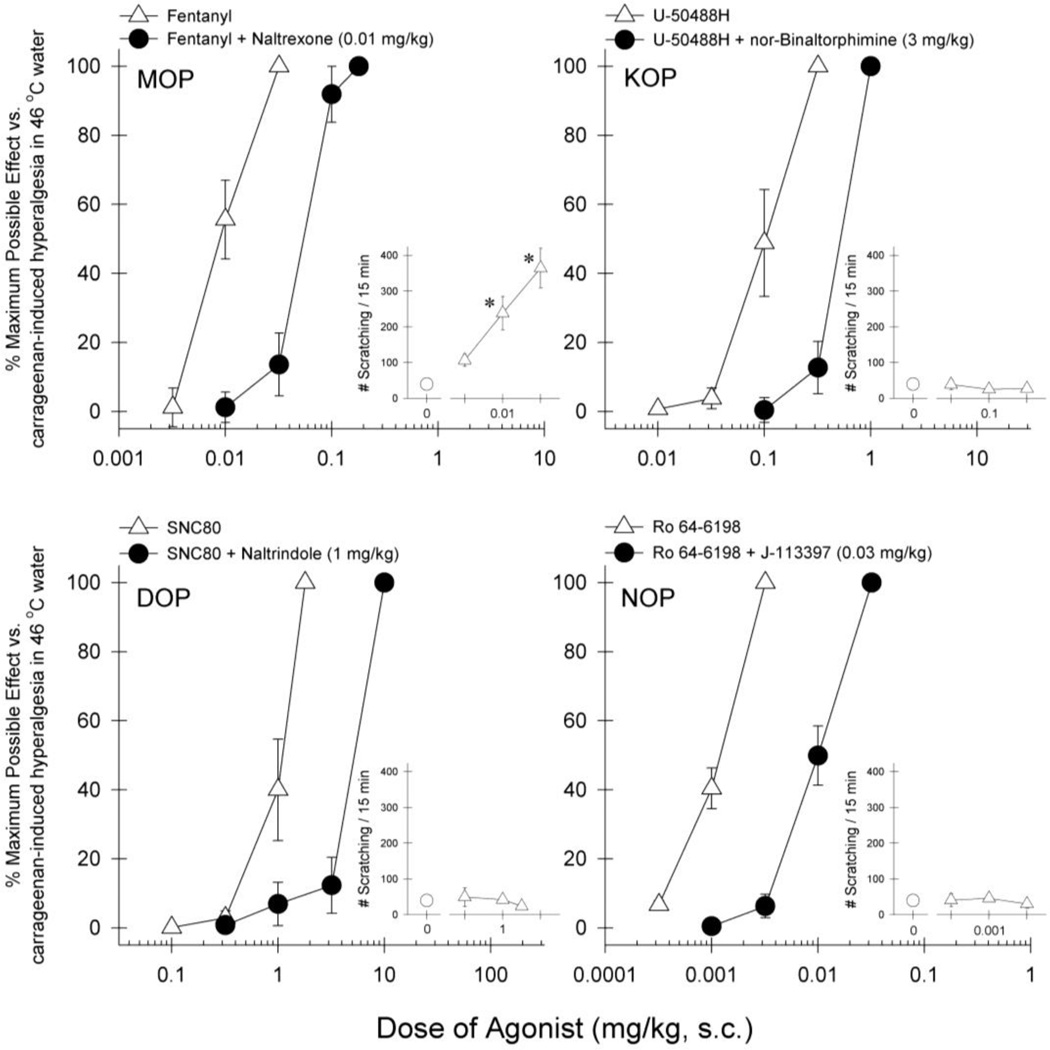
Figure 2 illustrates the effects of opioid-related ligands on carrageenan-induced thermal hyperalgesia. Agonists selective for each opioid receptor subtype were effective in attenuating carrageenan-induced hyperalgesia with different potencies, i.e., fentanyl [ED50 (95% confidence limits), 0.0089 (0.0055–0.014) mg/kg], U-50488H [0.10 (0.058–0.19) mg/kg], SNC80 [0.99 (0.64–1.53) mg/kg], and Ro 64-6198 [0.0012 (0.0009–0.0015) mg/kg]. In a separate test session examining itch-eliciting effects of opioid-related ligands, only fentanyl significantly evoked scratching responses at antihyperalgesic doses [F(3,12)=14.7; p<0.05]. In addition, antagonist pretreatment revealed that corresponding receptor antagonists produced 5–8 fold rightward shifts (i.e., 5–8 fold dose ratios determined by ED50 values) of the dose-response curves, i.e., fentanyl + naltrexone [0.054 (0.039–0.074) mg/kg], U-50488H + nor-binaltorphimine [0.51 (0.43–0.61) mg/kg], SNC80 + naltrindole [5.12 (4.25–6.17) mg/kg], and Ro 64–6198 + J-113397 [0.0098 (0.0065–0.0148) mg/kg].
Fig 2.
Dose response curves for the antihyperalgesic effects of opioid-related ligands against carrageenan-induced thermal hyperalgesia and effect on scratching behavior. Results are expressed as % maximum possible effect for each agonist against carrageenan-induced hyperalgesia at 46 °C (large panels) and number of scratches observed (enclosed panels). Open symbols represent the treatment with agonist alone. Closed symbols represent pretreatment with single dose of naltrexone 0.01 mg/kg, nor-binaltorphimine 3 mg/kg, naltrindole 1 mg/kg or J-113397 0.03 mg/kg for corresponding agonists. Each value represents mean ± S.E.M (n=4). *significant difference from baseline scratching responses (p<0.05)
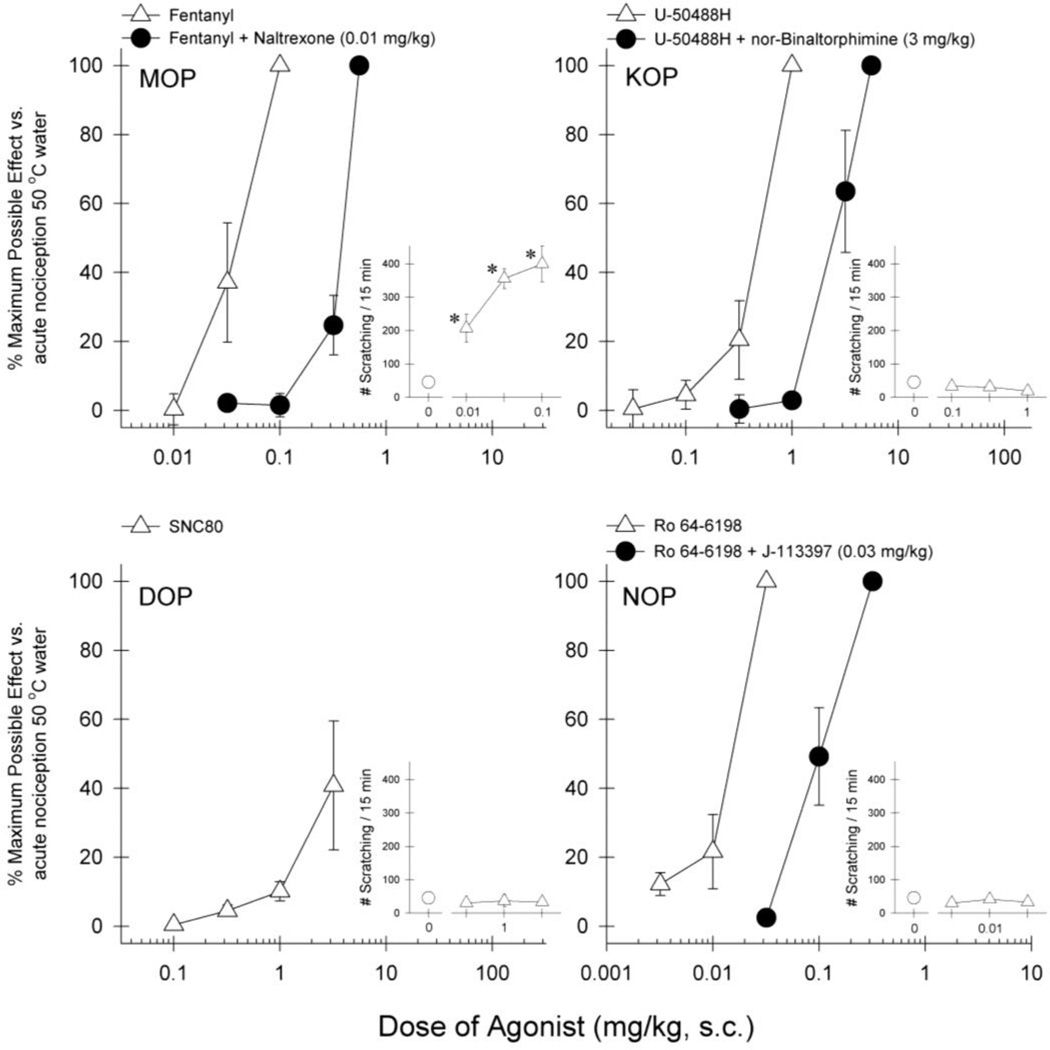
Figure 3 illustrates the effects of opioid-related ligands on acute nociceptive stimulus 50 °C water. Agonists selective for MOP, KOP, or NOP produced antinociceptive effects with different potencies, i.e., fentanyl [ED50 (95% confidence limits), 0.037 (0.019–0.069) mg/kg], U-50488H [0.46 (0.33–0.64) mg/kg], and Ro 64-6198 [0.014 (0.010–0.020) mg/kg]. The DOP agonist SNC80 did not produce full antinociceptive effects. After receiving the dose of 3 mg/kg of SNC80, two subjects displayed proconvulsive activities; therefore the test session was terminated due to the safety of the subjects. In a separate test session examining itch-eliciting effects of opioid-related ligands, only fentanyl significantly evoked scratching responses at antinociceptive doses [F(3,12)=14.6; p<0.05]. In addition, antagonist pretreatment revealed that corresponding receptor antagonists produced 5–10 fold rightward shifts of the dose-response curves, i.e., fentanyl + naltrexone [0.38 (0.33–0.43) mg/kg], U-50488H + nor-binaltorphimine [2.60 (1.57–4.32) mg/kg], and Ro 64-6198 + J-113397 [0.10 (0.060–0.17) mg/kg].
Fig 3.
Dose response curves for the antinociceptive effects of opioid-related ligands against acute thermal nociception and effect on scratching behavior. Results are expressed as % maximum possible effect for each agonist against acute thermal stimulus at 50 °C (large panels) and number of scratches observed (enclosed panels). Open symbols represent the treatment with agonist alone. Closed symbols represent pretreatment with single dose of naltrexone 0.01 mg/kg, nor-binaltorphimine 3 mg/kg or J-113397 0.03 mg/kg for corresponding agonists. Each value represents mean ± S.E.M (n=4). *significant difference from baseline scratching responses (p<0.05)
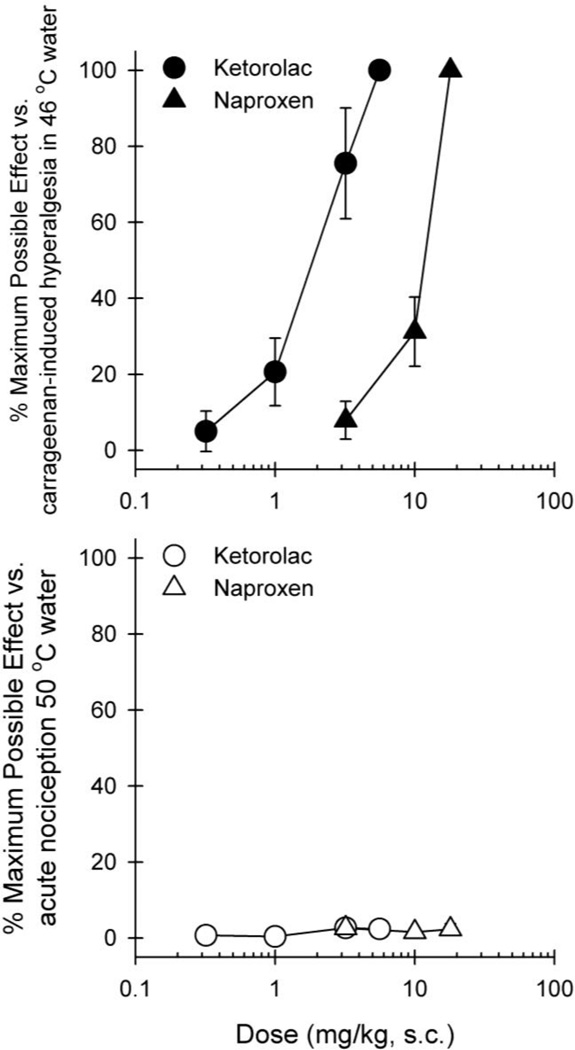
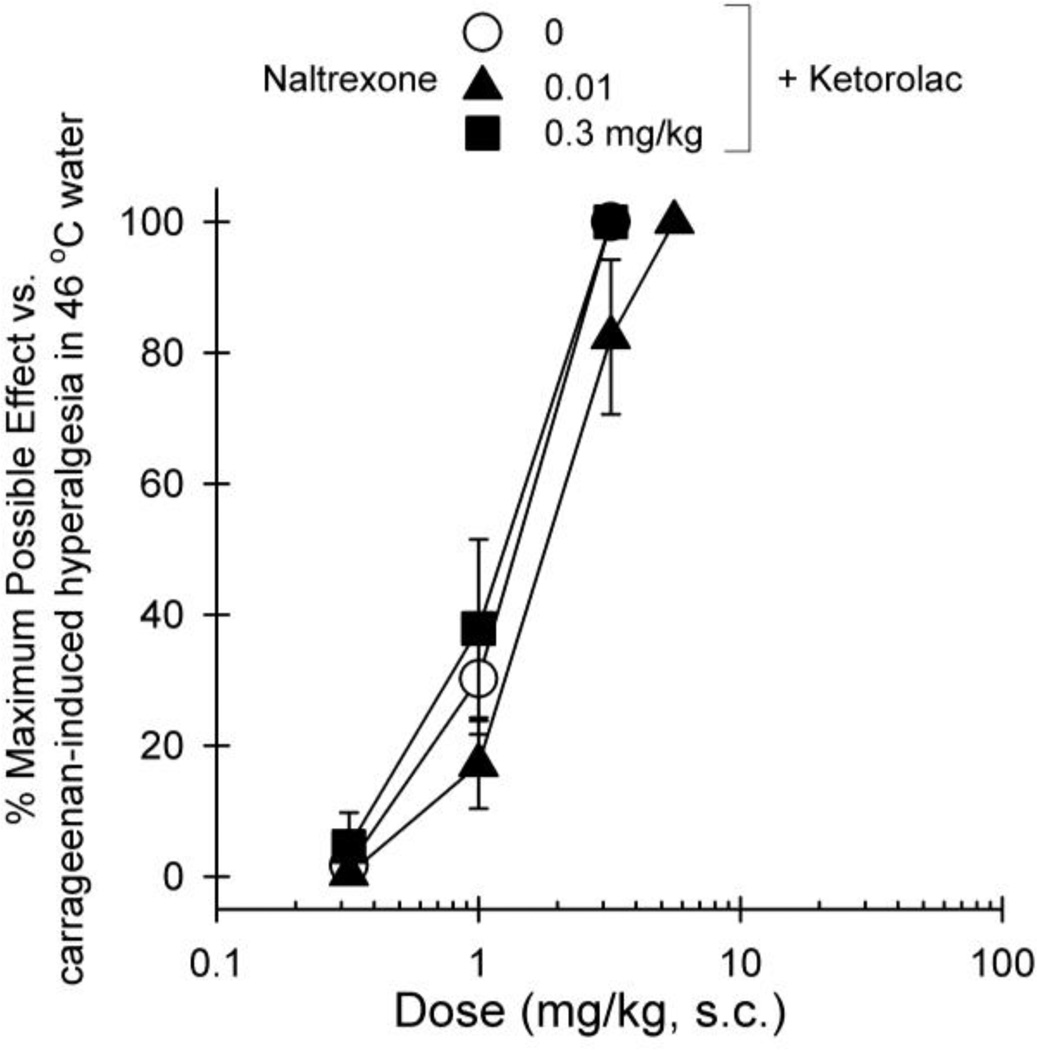
Figure 4 compares the effectiveness and potency of NSAIDs in the same group of subjects receiving either carrageenan or acute nociceptive stimulus 50 °C water. Both ketorolac and naproxen were effective in attenuating carrageenan-induced hyperalgesia, i.e., ketorolac [2.0 (1.0–3.8) mg/kg] and naproxen [11.5 (9.8–13.4) mg/kg]. However, both drugs did not produce antinociceptive effects against acute nociceptive stimulus 50 °C water. Pretreatment with different doses of naltrexone did not produce a rightward shift of the dose-response of ketorolac (Figure 5), i.e., vehicle + ketorolac [1.3 (0.9–1.8) mg/kg], naltrexone 0.01 mg/kg + ketorolac [1.9 (1.1–3.1) mg/kg], and naltrexone 0.3 mg/kg + ketorolac [1.2 (0.6–2.1) mg/kg].
Fig 4.
Dose response curves for the antihyperalgesic and antinociceptive effects of ketorolac (circles) and naproxen (triangles). Results are expressed as % maximum possible effect for ketorolac and naproxen against carrageenan-induced thermal hyperalgesia (top) and acute thermal stimulus (bottom). Each value represents mean ± S.E.M (n=4)
Fig 5.
Effect of naltrexone pretreatment on the dose response curve for the antihyperalgesic effects of ketorolac. Results are expressed as % maximum possible effect for ketorolac alone (open symbols) or with the pretreatment with naltrexone (closed symbols) against carrageenan-induced hyperalgesia. Each value represents mean ± S.E.M (n=4)
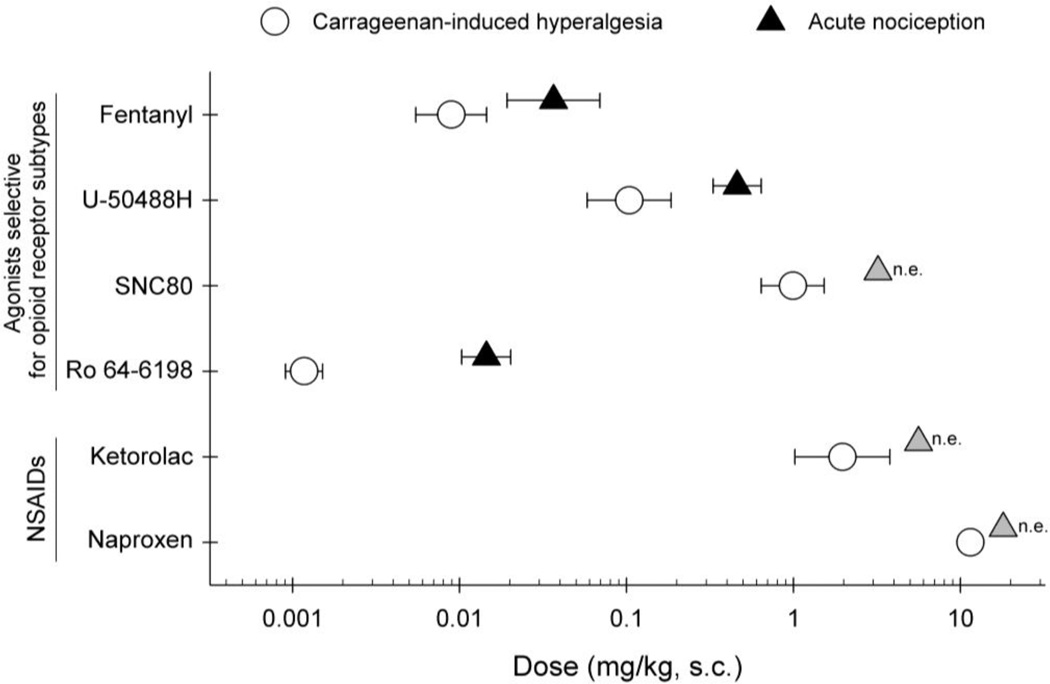
Figure 6 compares the antihyperalgesic and antinociceptive potencies of systemically administered opioid-related ligands and NSAIDs. Fentanyl, U-50488H, and Ro 64-6198 were more potent in producing antihyperalgesic effects. In particular, Ro 64-6198 was approximately 10-fold more potent in producing such effects. Effects of SNC80 were similar to those of NSAIDS, i.e., not effective against acute nociception, but effective in ameliorating carrageenan-induced hyperalgesia.
Fig 6.
Comparison of the antihyperalgesic and antinociceptive potencies of opioid-related ligands and NSAIDs. Results are presented as ED50 values for each drug with 95% confidence limit. Open symbols represent mean ED50 values of antihyperalgesic effects against carrageenan-induced hyperalgesia. Closed symbols represent mean ED50 values of antinociceptive effects against acute thermal nociception. Grey symbols indicate that the drug was not effective (n.e.) in blocking acute thermal nociception. Each value represents mean ± S.E.M (n=4)
Discussion
The present studies provide the first evidence of carrageenan-induced hyperalgesia in rhesus monkeys and compare the potency and effectiveness of the two primary classes of analgesics - opioids and NSAIDs – under these conditions. The first part of the study established the basic characteristics of carrageenan-induced hyperalgesia in monkeys. Tail injection of carrageenan induced thermal hypersensitivity in monkeys in a dose-dependent manner and caused hyperalgesia-like behaviors in response to water at non-noxious temperature. These behaviors began within an hour after the injection and lasted more than 5 hours, which are different from other chemical-induced allodynia/hyperalgesia with quick onset and short duration (Butelman et al. 2003; Butelman et al. 1995; Negus et al. 1993). The long duration of this model therefore allows for the drug to be administered after hyperalgesia is established which is more closely related to the clinical settings. Hence, carrageenan-induced long lasting hyperalgesia provides a novel experimental context to investigate a wide range of analgesic drugs in nonhuman primates.
The second part of the study determined effects of opioid-related ligands against carrageenan-induced thermal hyperalgesia as compared to acute thermal nociception (figures 2 and 3). Fentanyl (MOP), U-50488H (KOP), SNC80 (DOP) and Ro 64-6198 (NOP) attenuated carrageenan-induced thermal hyperalgesia in dose dependent manners. MOP antagonist naltrexone produced an approximate 5-fold parallel rightward shift in fentanyl dose response curve indicating that the antihyperalgesic effects of fentanyl were mediated by MOP. Similarly, KOP antagonist nor-binaltorphimine, DOP antagonist naltrindole and NOP antagonist J-113397 produced rightward shifts in the dose response curves of U-50488H, SNC80 and Ro 64-6198, respectively. This demonstrates the role of KOP, DOP and NOP receptors in driving the antihyperalgesic effects of their selective agonists. When noxious thermal stimulus was tested in absence of carrageenan, only fentanyl, U-50488H and Ro 64-6198 blocked acute thermal nociception. Together, MOP, KOP and NOP agonists were efficacious against both carrageenan-induced hyperalegsia and acute thermal nociception whereas DOP agonist was only efficacious against carrageenan-induced hyperalgesia (figures 2 and 3). The same antagonist produced an equal degree of rightward shifts of the agonist’s dose-response curves under both acute nociception and carrageenan-induced hyperalgesia. These findings indicate that fentanyl, U-50488H, and Ro 64-6198 attenuated carrageenan-induced hyperalgesia and produced acute antinociception both through the corresponding receptor mechanisms. There could be minimal contributions of endogenous opioids under both pain modalities as the dose ratios after the antagonist pretreatment are similar. Selective DOP agonists are reported to cause dose-dependent convulsions in rodents (Comer et al. 1993). In the present study, SNC80 was tested up to the highest dose that has elicited proconvulsive activities in monkeys but failed to block acute thermal nociception. This indicates that activation of DOP receptors at these doses is not sufficient to block nociception from acute noxious stimulus. Together these findings provide information on how different opioid receptor subtypes modulate antinociception in primates across different types of pain states such as in the presence or absence of inflammation.
Monkeys were also observed for the drug-induced behaviors such as scratching. Only fentanyl produced scratching at antihyperalgesic and antinociceptive doses. This observation parallels with the clinical profile of fentanyl where systemically administered fentanyl produces itch as a side effect at analgesic doses in humans (Ellis et al. 1990; Reich and Szepietowski 2010). In monkeys, scratching evoked by intrathecal administration of MOP agonist morphine was blocked by low doses of KOP agonists like nalfurafine (Ko and Husbands 2009), although high doses of KOP agonists produced sedation (Butelman et al. 2001). Similarly, in humans nalfurafine effectively attenuated pruritus in hemodialysis patients (Kumagai et al. 2010). This illustrates a translational aspect of opioid receptor functions in nonhuman primates. It is speculated that itch may be evoked by inhibition of pain (Ikoma et al. 2006). However, the present studies indicate that itch neurotransmission induced by pain inhibition may only be interpreted by MOP, but not KOP and NOP agonists. Together, the data provide direct comparison between the opioid-related ligands for their antinociceptive, antihyperalgesic and pruritic effects.
MOP, KOP and NOP agonists were more potent in blocking carrageenan-induced hyperalgesia than acute thermal nociception (Figure 6). These observations are similar to the findings in rodents indicating that the antinociceptive potency of opioids is enhanced under the state of inflammation as compared to the acute noxious stimulus (Stein and Lang 2009). There are several mechanisms for the increased potency of opioids in blocking inflammation-induced pain, which include peripheral and central effects. Activation of peripheral MOP, KOP, DOP and NOP receptors by local administration of opioids is shown to block inflammatory hyperalgesia in both rodents (Joris et al. 1987; Stein and Lang 2009) and monkeys (Ko et al. 2002). Tissue inflammation sensitizes and increases excitability of primary afferent neurons causing an upregulation of MOP, KOP, DOP and NOP on the primary afferent nerve fiber terminals and the non-neuronal cells like lymphocytes (Chen and Sommer 2006; Stein and Lang 2009). Hence, opioids can directly reduce peripheral afferent nociceptive signals (Sawynok 2003; Stein et al. 2009). Inflammation in the periphery also causes spinal neuroplasticity such as the activation of glia, which in turn enhance pain by releasing proinflammatory mediators (Raghavendra et al. 2004; Watkins and Maier 2003). Therefore, centrally acting analgesics such as the MOP agonist morphine can block nociceptive transmission at the level of spinal cord (Hameed et al. 2010; Hutchinson et al. 2007). In addition, MOP, KOP and DOP agonists are shown to cause disinhibition of neurons in the periaqueductal gray which then activate spinally projecting neurons in the rostroventral medulla. This leads to attenuation of nociceptive signals originating from the dorsal horn as a result of tissue inflammation (Basbaum and Fields 1984; Fraser et al. 2000).
Ketorolac and naproxen are clinically available NSAIDs that non-selectively inhibit COX enzyme activity and the metabolites like prostaglandin E2 thereby blocking inflammation-induced pain (Buckley and Brogden 1990; Krekels et al. 2011). The third part of the studies showed that both ketorolac and naproxen were effective in attenuating carrageenan-induced hyperalgesia in a dose dependent manner. Ketorolac was more potent than naproxen (figures 4 and 6). Both drugs, however, failed to block acute thermal nociception indicating that the state of inflammation is required to obtain the antihyperalgesic effects of these NSAIDs. Studies in monkeys using algogens to induce hyperalgesia demonstrate that ketorolac effectively blocked thermal hyperalgesia induced by bradykinin but not capsaicin and prostaglandin E2. This suggests that ketorolac is ineffective in blocking hyperalgesia induced by agents that directly activate, rather than sensitize, the primary afferent nerve fibers (Brandt et al. 2001; Negus et al. 1995). It is reported that opioid receptor subtypes MOP, KOP and DOP as well as their endogenous agonists like β-endorphin, dynorphin and enkephalin are involved in the antihyperalgesic effects of some NSAIDs such as celecoxib and flunixin in the rodent carrageenan model (Correa et al. 2010; Herrero and Headley 1996). In order to determine if antihyperalgesic effects of ketorolac are also mediated by opioid receptors, naltrexone was administered as a pretreatment. At both low and high doses, naltrexone failed to block the antihyperalgesic effects of ketorolac. This indicates that the antihyperalgesic effects of ketorolac are not mediated by opioid receptor subtypes MOP, KOP and DOP. Hence, the present data strengthen the notion that NSAIDs attenuate pain predominantly by anti-inflammatory mechanisms in primates.
As previously reported, DOP agonists blocked chemically-altered thermal hypersensitivity (Brandt et al. 2001; Butelman et al. 1995) but were not effective in blocking acute thermal nociception (Dykstra et al. 1993; Negus et al. 1998). It has been speculated that for DOP agonists to be effective, a state of inflammation may be required. Inflammatory mediators induce trafficking of normally intracellular DOP receptors to the surface of sensory nerve fibers and make them accessible to DOP agonists (Cahill et al. 2003; Zhang et al. 1998). Together, these findings suggest that in primates the profile of DOP agonists is similar to NSAIDs as both types of drugs can reverse thermal hyperalgesia but not acute nociception. Therefore, DOP agonists have great potential to be developed to treat chronic inflammatory pain conditions like burn injuries.
Interestingly, Ro 64-6198 was almost 10 times more potent than fentanyl in blocking carrageenan-induced hyperalgesia (figure 6). Similarly, centrally administered NOP agonist UFP-112 was more potent than morphine in blocking capsaicin-induced allodynia in monkeys (Hu et al. 2010). There was a 10 times potency difference in the ability of Ro 64-6198 to block carrageenan-induced thermal hyperalgesia compared to acute thermal nociception. Studies in rodents show that peripheral inflammation induced by carrageenan or agents like complete Freund's Adjuvant increase expression of nociceptin/orphanin FQ and NOP receptors in primary sensory neurons and spinal cord (Itoh et al. 2001; Jia et al. 1998). It is also reported in rodents that spinally administered NOP agonists block carrageenan-induced hyperalgesia and nerve-injury induced allodynia in rodents (Obara et al. 2005; Sukhtankar et al. 2013). This indicates that the state of injury or inflammation largely upregulates the NOP system which may lead to the increased potency of NOP agonists under these conditions. To our knowledge, this is the first study to characterize potency and effectiveness of systemically administered NOP agonist against inflammation-induced thermal hyperalgesia versus acute thermal nociception. Given that NOP-related agonists have a much wider therapeutic window than MOP and DOP agonists in preclinical pain models (Calo and Guerrini 2013; Lin and Ko 2013; Sukhtankar and Ko 2013), it is worth developing NOP-related ligands as safe and effective analgesics.
Together, the present study demonstrates that long lasting hyperalgesia can be modeled in rhesus monkeys by tail injection of carrageenan. In this model, not only the two major classes of analgesics, i.e., MOP agonists and NSAIDs, were distinguished under different types of pain states, but also agonists selective for four opioid receptor subtypes displayed differential effectiveness and potency in the same group of subjects. Our findings support that clinically available MOP agonists such as fentanyl block pain from a wide range of stimuli as compared to NSAIDs. As an alternative to the MOP agonists as analgesics, the NOP agonists show a promising profile against both hyperalgesia and acute pain and need to be further investigated for their potential use in humans. The present model can also be further utilized to determine and compare the levels of drug metabolites in cerebrospinal fluids or plasma following their administration. Therefore, this model has a prominent preclinical value and serves as a valuable translational bridge for analgesic drug development.
Acknowledgments
We thank Tristan Edwards and John Busenbark for technical assistance of data collection. This study was supported by U.S. Public Health Service Grants R01-AR-059193 and R01-DA-032568.
Footnotes
The authors declare no conflict of interest.
The authors declare that the experiments comply with the current laws of the United States of America.
References
- 1.Barber A, Bartoszyk GD, Bender HM, Gottschlich R, Greiner HE, Harting J, Mauler F, Minck KO, Murray RD, Simon M, et al. A pharmacological profile of the novel, peripherally-selective kappa-opioid receptor agonist, EMD 61753. British journal of pharmacology. 1994;113:1317–1327. doi: 10.1111/j.1476-5381.1994.tb17142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual review of neuroscience. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi BR, Zhang XF, Reilly RM, Kym PR, Yao BB, Chen J. Species comparison and pharmacological characterization of human, monkey, rat, mouse TRPA1 channels. The Journal of pharmacology and experimental therapeutics. 2012;341:360–368. doi: 10.1124/jpet.111.189902. [DOI] [PubMed] [Google Scholar]
- 4.Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. The Journal of pharmacology and experimental therapeutics. 2001;296:939–946. [PubMed] [Google Scholar]
- 5.Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86–109. doi: 10.2165/00003495-199039010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Butelman ER, Ball JW, Harris TJ, Kreek MJ. Topical capsaicin-induced allodynia in unanesthetized primates: pharmacological modulation. The Journal of pharmacology and experimental therapeutics. 2003;306:1106–1114. doi: 10.1124/jpet.103.052381. [DOI] [PubMed] [Google Scholar]
- 7.Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH. GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 2001;298:1049–1059. [PubMed] [Google Scholar]
- 8.Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1993;267:1269–1276. [PubMed] [Google Scholar]
- 9.Butelman ER, Negus SS, Gatch MB, Chang KJ, Woods JH. BW373U86, a delta-opioid receptor agonist, reverses bradykinin-induced thermal allodynia in rhesus monkeys. European journal of pharmacology. 1995;277:285–287. doi: 10.1016/0014-2999(95)00134-7. [DOI] [PubMed] [Google Scholar]
- 10.Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 11.Calo G, Guerrini R. Medicinal Chemistry, Pharmacology, and Biological Actions of Peptide Ligands Selective for the Nociceptin/Orphanin FQ Receptor. In: Ko M-C, Husbands SM, editors. Research and Development of Opioid-Related Ligands. ACS; 2013. pp. 275–325. [Google Scholar]
- 12.Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, Sturkenboom M, Perez-Gutthann S, Safety of Non-Steroidal Anti-Inflammatory Drugs P. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project) Drug safety : an international journal of medical toxicology and drug experience. 2012;35:1127–1146. doi: 10.1007/BF03261999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Sommer C. Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. Journal of the peripheral nervous system : JPNS. 2006;11:232–240. doi: 10.1111/j.1529-8027.2006.0093.x. [DOI] [PubMed] [Google Scholar]
- 14.Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. The Journal of pharmacology and experimental therapeutics. 1993;267:888–895. [PubMed] [Google Scholar]
- 15.Correa JD, Paiva-Lima P, Rezende RM, Dos Reis WG, Ferreira-Alves DL, Bakhle YS, Francischi JN. Peripheral mu-, kappa- and delta-opioid receptors mediate the hypoalgesic effect of celecoxib in a rat model of thermal hyperalgesia. Life sciences. 2010;86:951–956. doi: 10.1016/j.lfs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. The Journal of pharmacology and experimental therapeutics. 1998;285:1031–1038. [PubMed] [Google Scholar]
- 17.Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt R, Chang KJ. A novel delta opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. The Journal of pharmacology and experimental therapeutics. 1993;267:875–882. [PubMed] [Google Scholar]
- 18.Ellis DJ, Millar WL, Reisner LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after cesarean section. Anesthesiology. 1990;72:981–986. doi: 10.1097/00000542-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. British journal of pharmacology. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hameed H, Hameed M, Christo PJ. The effect of morphine on glial cells as a potential therapeutic target for pharmacological development of analgesic drugs. Current pain and headache reports. 2010;14:96–104. doi: 10.1007/s11916-010-0093-y. [DOI] [PubMed] [Google Scholar]
- 21.Hawkinson JE, Szoke BG, Garofalo AW, Hom DS, Zhang H, Dreyer M, Fukuda JY, Chen L, Samant B, Simmonds S, Zeitz KP, Wadsworth A, Liao A, Chavez RA, Zmolek W, Ruslim L, Bova MP, Holcomb R, Butelman ER, Ko MC, Malmberg AB. Pharmacological, pharmacokinetic, and primate analgesic efficacy profile of the novel bradykinin B1 Receptor antagonist ELN441958. The Journal of pharmacology and experimental therapeutics. 2007;322:619–630. doi: 10.1124/jpet.107.120352. [DOI] [PubMed] [Google Scholar]
- 22.Herrero JF, Headley PM. Reversal by naloxone of the spinal antinociceptive actions of a systemically-administered NSAID. British journal of pharmacology. 1996;118:968–972. doi: 10.1111/j.1476-5381.1996.tb15494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends in pharmacological sciences. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 24.Hu E, Calo G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature reviews Neuroscience. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 27.Itoh M, Takasaki I, Andoh T, Nojima H, Tominaga M, Kuraishi Y. Induction by carrageenan inflammation of prepronociceptin mRNA in VR1-immunoreactive neurons in rat dorsal root ganglia. Neuroscience research. 2001;40:227–233. doi: 10.1016/s0168-0102(01)00230-9. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Y, Linden DR, Serie JR, Seybold VS. Nociceptin/orphanin FQ binding increases in superficial laminae of the rat spinal cord during persistent peripheral inflammation. Neuroscience letters. 1998;250:21–24. doi: 10.1016/s0304-3940(98)00430-3. [DOI] [PubMed] [Google Scholar]
- 30.Joris JL, Dubner R, Hargreaves KM. Opioid analgesia at peripheral sites: a target for opioids released during stress and inflammation? Anesthesia and analgesia. 1987;66:1277–1281. [PubMed] [Google Scholar]
- 31.Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- 32.Ko MC, Husbands SM. Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. The Journal of pharmacology and experimental therapeutics. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. The journal of pain : official journal of the American Pain Society. 2009;10:509–516. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko MC, Naughton NN, Traynor JR, Song MS, Woods JH, Rice KC, McKnight AT. Orphanin FQ inhibits capsaicin-induced thermal nociception in monkeys by activation of peripheral ORL1 receptors. British journal of pharmacology. 2002;135:943–950. doi: 10.1038/sj.bjp.0704535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko MC, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. The Journal of pharmacology and experimental therapeutics. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 36.Krekels EH, Angesjo M, Sjogren I, Moller KA, Berge OG, Visser SA. Pharmacokinetic-pharmacodynamic modeling of the inhibitory effects of naproxen on the time-courses of inflammatory pain, fever, and the ex vivo synthesis of TXB2 and PGE2 in rats. Pharmaceutical research. 2011;28:1561–1576. doi: 10.1007/s11095-011-0389-6. [DOI] [PubMed] [Google Scholar]
- 37.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- 38.Kupers RC, Chen CC, Bushnell MC. A model of transient hyperalgesia in the behaving monkey induced by topical application of capsaicin. Pain. 1997;72:269–275. doi: 10.1016/s0304-3959(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 39.Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS chemical neuroscience. 2013;4:214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Negus SS, Butelman ER, Al Y, Woods JH. Prostaglandin E2-induced thermal hyperalgesia and its reversal by morphine in the warm-water tail-withdrawal procedure in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1993;266:1355–1363. [PubMed] [Google Scholar]
- 41.Negus SS, Butelman ER, Gatch MB, Woods JH. Effects of morphine and ketorolac on thermal allodynia induced by prostaglandin E2 and bradykinin in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1995;274:805–814. [PubMed] [Google Scholar]
- 42.Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1998;286:362–375. [PubMed] [Google Scholar]
- 43.Obara I, Przewlocki R, Przewlocka B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain. 2005;116:17–25. doi: 10.1016/j.pain.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- 45.Reich A, Szepietowski JC. Opioid-induced pruritus: an update. Clinical and experimental dermatology. 2010;35:2–6. doi: 10.1111/j.1365-2230.2009.03463.x. [DOI] [PubMed] [Google Scholar]
- 46.Sawynok J. Topical and peripherally acting analgesics. Pharmacological reviews. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50:345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- 48.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain research reviews. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Current opinion in pharmacology. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Sukhtankar DD, Ko M-C. Pharmacological Investigation of NOP-Related Ligands as Analgesics without Abuse Liability. In: Ko M-C, Husbands SM, editors. Research and Development of Opioid-Related Ligands. ACS; 2013. pp. 393–416. [Google Scholar]
- 51.Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. Effects of Spinally Administered Bifunctional Nociceptin/Orphanin FQ Peptide Receptor/mu-Opioid Receptor Ligands in Mouse Models of Neuropathic and Inflammatory Pain. The Journal of pharmacology and experimental therapeutics. 2013;346:11–22. doi: 10.1124/jpet.113.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace MS, Rowbotham M, Bennett GJ, Jensen TS, Pladna R, Quessy S. A multicenter, double-blind, randomized, placebo-controlled crossover evaluation of a short course of 4030W92 in patients with chronic neuropathic pain. The journal of pain : official journal of the American Pain Society. 2002a;3:227–233. doi: 10.1054/jpai.2002.123650. [DOI] [PubMed] [Google Scholar]
- 53.Wallace MS, Rowbotham MC, Katz NP, Dworkin RH, Dotson RM, Galer BS, Rauck RL, Backonja MM, Quessy SN, Meisner PD. A randomized, double-blind, placebo-controlled trial of a glycine antagonist in neuropathic pain. Neurology. 2002b;59:1694–1700. doi: 10.1212/01.wnl.0000036273.98213.34. [DOI] [PubMed] [Google Scholar]
- 54.Warner EA. Opioids for the treatment of chronic noncancer pain. The American journal of medicine. 2012;125:1155–1161. doi: 10.1016/j.amjmed.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nature reviews Drug discovery. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 56.Whiteside GT, Boulet JM, Walker K. The role of central and peripheral mu opioid receptors in inflammatory pain and edema: a study using morphine and DiPOA ([8-(3,3-diphenyl-propyl)-4-oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-3-yl]-acetic acid) The Journal of pharmacology and experimental therapeutics. 2005;314:1234–1240. doi: 10.1124/jpet.105.088351. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]