Abstract
Rationale
Although widely prescribed, little is known about selective serotonin reuptake inhibitors (SSRIs) effects on social behavior and cerebrospinal fluid (CSF) monoamines in female primates.
Objective
To determine the effects of sertraline on agonistic and affiliative behavior.
Methods
21 adult female cynomolgus monkeys were housed in small, stable social groups, trained to participate in oral dosing, and began a 5-week cumulative dose response study. Serial doses of 0, 5, 10, 15, and 20 mg/kg of sertraline were administered orally for one week each. Behavior was recorded daily during 10-minute observations before and 4 hours after dosing. On the 7th day of dosing, circulating sertraline/desmethylsertraline and CSF monoamines/metabolites were determined 4 hours after the last dose.
Results
At 20 mg/kg, circulating sertraline/desmethylsertraline was in the therapeutic range. CSF 5-hydroxyindole acetic acid decreased 33% (p<0.05). Overall aggression, submission, locomotion and time alone decreased, whereas affiliative behaviors (body contact, grooming) increased (all p’s<0.05). Effects of sertraline on aggression and submission were social status-dependent, reducing aggression in dominants and submission in subordinates.
Conclusions
A clinically relevant oral dose of sertraline resulted in CSF metabolite changes similar to those observed in patients, and altered the socioemotional behavior of female monkeys. Changes in CSF 5-HT and dopamine are novel observations that may be sex-specific. The robust effects of sertraline on aggression and affiliation may explain the efficacy of SSRIs on a range of human behavioral pathologies that share the characteristics of increased aggression and decreased sociality.
Keywords: Nonhuman primates, females, SSRI, sertraline, CSF, social and emotional behavior
Introduction
Data from the National Health and Nutrition Examination Survey 2005–2008 suggest that antidepressants are the third most common prescription drug taken by Americans of all ages, and the most frequently used by persons aged 18–44 years. About one in 10 Americans aged 12 years and over take antidepressant medication. Women are 2.5 times more likely than men to take antidepressants and 23% of women aged 40–59 take antidepressants. More than 60% of Americans taking antidepressant medication have taken it for 2 years or longer; 14% having taken the medication for 10 years or more. Less than one-third of Americans taking one antidepressant medication and less than one-half of those taking multiple antidepressants have seen a mental health professional in the past year (Pratt et al. 2011). Side effects are common including sexual dysfunction (Segraves 2007). A selective serotonin reuptake inhibitor (SSRI)- induced apathy syndrome has been identified (Barnhart et al. 2004), and discontinuation of treatment can be difficult and uncomfortable, and is referred to as the SSRI-discontinuation syndrome (Schatzberg et al. 2006).
Anderson and colleagues (Anderson et al. 2005) used sertraline to study the time course of the drug’s biological activity in the context of the commonly reported, though questioned (Taylor et al., 2006), time lag for the clinical effects of the SSRIs. Few primate studies have assessed sertraline effects on social behavior, however, one study examining treatments for type 2 alcoholism showed that sertraline reduces alcohol intake and at the same time reduces aggression (Higley et al. 1998). Other SSRIs have been evaluated for self-injurious and stereotypic behavior in nonhuman primates (Fontenot et al. 2009). Given sertraline’s level of use and its side effects, as well as the known involvement of the central 5-HT system in most major classes of behavior, it is surprising that studies of the effects of SSRIs on broad classes of social behavior in animal models, and particularly primate models, are not available.
Here we report the effects of a commonly prescribed SSRI, sertraline HCl (Zoloft ®) on the agonistic and affiliative behavior of socially-housed adult female cynomolgus monkeys (Macaca fascicularis).
Methods
Subjects
Twenty-two adult, reproductive-aged female cynomolgus monkeys were imported directly from Indonesia (Institut Pertanian Bogor, Bogor, Indonesia) and quarantined in single cages for one month. Following quarantine, the monkeys were housed in social groups (n = 4–5), in indoor pens (3.05m × 3.05m × 3.05m), in a climate-controlled building, with 12/12 light/dark, and water ad libitum. All monkeys were fed a Western-like diet containing 44% of calories from fat and 0.29 mg/Cal cholesterol, approximately equal to a human consumption of 500 mg cholesterol/2000 calories (Table 1). One animal had an adverse reaction (hemorrhaging) to the sertraline and was removed from treatment leaving a final sample size of 21. The incidence of bleeding in one animal is consistent with increased risk of bleeding in patients treated with sertraline (Andrade et al. 2010).
Table 1.
Diet Ingredients
| g/100 grams | |
|---|---|
| Casein | 6.00 |
| Lactalbumin | 6.00 |
| Wheat Germ | 3.00 |
| Soy Protein | 2.00 |
| Wheat Flour, self-rising | 35.00 |
| Dextrin | 9.00 |
| Sucrose | 7.00 |
| Alphacel (non-nutrient bulk) | 8.18 |
| Lard | 5.00 |
| Beef Tallow | 3.00 |
| Butter, lightly salted | 3.10 |
| Safflower Oil | 3.00 |
| Dried Egg Yolk | 1.80 |
| Complete Vitamin Mix | 2.50 |
| Modified Ausman-Hayes Mineral Mix #2 | 5.00 |
| Calcium Carbonate | 0.400 |
| Calcium Phosphate, Monobasic | 0.02 |
| Composition | |
| Protein (% of calories) | 17 |
| Carbohydrate (% of calories) | 46.5 |
| Lipid (% of calories) | 35.9 |
| Saturated (% of calories) | 14.4 |
| Monounsaturated (% of calories) | 12.8 |
| Polyunsaturated (% of calories) | 8.7 |
| Cholesterol (mg/Calorie)* | 0.17 |
| Calcium (mg/1800 Calories) | 268.9 |
| Phosphorus (mg/1800 Calories) | 271.1 |
Experimental Design
Eighteen months following formation of social groups, the monkeys were trained to run out of their social group pens into a dosing cage where they were individually offered fat-free vanilla pudding orally from a metal syringe. After consuming the pudding orally, the monkeys were immediately released into their home cage. This initial dose-training was completed in about two weeks, and a cumulative dose-response study of sertraline HCl (Zoloft®) was initiated. Doses of 0, 5, 10, 15, and 20 mg/kg were administered orally in the pudding for one week each at about 0800 hours each day. On day 7 of each dose and 4 hours after dosing, the monkeys were sedated with 10–15 mg/kg ketamine HCl and blood samples were taken to measure plasma levels of sertraline and desmethylsertraline. In addition, cerebrospinal fluid (CSF) samples were taken every two weeks at the 0, 10, and 20 mg/kg doses for determination of 5-hydroxy indole acetic acid (5-HIAA, a metabolite of 5-HT) concentrations. CSF samples were taken by inserting a 22- gauge needle percutaneously into the cisternal space while the ketamine-sedated animal was maintained in a lateral recumbent position. Approximately 1–1.5 cc of spinal fluid was obtained and frozen at −70°C until metabolite determinations were made.
Assays
Sertraline and desmethylsertraline were analyzed by gas chromatography (NMS Labs, Willow Grove, PA). An internal standard (8–chloroperphenazine) was added to 1.0 ml serum aliquots that were buffered to pH 10 and extracted with toluene and back extracted into dilute H2SO4. The acid was made basic and extracted with toluene. Sertraline and metabolite were derivatized with butyric anhydride and analyzed on a DB-5 capillary column, using an Agilent 6890 Gas Chromatograph with nitrogen selective detection. This GC/NPD method was calibrated over a range of 1 to 200 ng/mL, with inter-assay precision for sertraline/desmethylsertraline of 14/22% and 10/14% at 10 and 140 ng/mL, respectively. Elution times were approximately 7.2 and 6.9 minutes for sertraline and desmethylsertraline, respectively. 5-HIAA was analyzed using high-pressure liquid chromatography (HPLC) with electrochemical detection as previously described (Shively et al. 2007). Intra- and interassay coefficients of variation were <10%.
Behavior Observations
Seven behaviors, aggression, submission, locomotion, passive body contact, grooming, being groomed, and alone were recorded at 20-second intervals, in 10-minute group scans, 6 days/week, twice/day: Once in the morning prior to the daily dose at approximately 0800 hours (PRE), and again 4 hours post-dosing (POST) at approximately 1200 hours. During the group scan, the 7 behaviors were recorded as 7 1-letter codes. One would be entered for each 20 sec period for each monkey. Thus, every 20 sec the observer had to write down 1 of 7 codes for each of 4 monkeys. The number of intervals in which each behavior was observed was divided by the total number of 20-second scans X 100, resulting in a percent of 20-second scans in which the behavior was observed (Altmann 1974). Thus, a total of 30 samples of behavior, twice a day for 6 days (360 samples) were completed at each of 5 doses, for a total of 1800 samples of behavior. The specific aggressive behaviors recorded included open mouth threat, stare threat, chase, charge, lunge, and cage shake display, the frequency of which were summed for a total measure of aggression. The specific submissive behaviors recorded included submissive present, fear grimace, lip smack, move away, crouch, flee, scream, and scream threat, the frequency of which were summed for a total measure of submission. Locomotion was recorded if the monkey traversed space. Alone was recorded if the monkey was out of monkey arm’s reach of another monkey. Social status was also determined weekly based on the direction of the outcome of agonistic interactions, not on frequencies of behaviors. Social status hierarchies were linear and stable over the course of the experiment. Operational definitions of all behaviors have been previously published (Shively et al. 1986). For analysis weekly averages for the Pre-Dose and weekly averages for the Post –Dose time periods were calculated. Interobserver reliability was r≥0.92.
Statistical Analysis
The circulating levels of sertraline and desmethylsertraline, and CSF metabolite concentrations were analyzed by a one-way repeated measures analysis of variance (ANOVA). Initially, the behavioral data were analyzed by a 2 (dominant, subordinate) × 2 (pre, post-dosing) × 5 (doses) nested repeated measures ANOVA. ANOVA was also used for follow up analyses of subsets of the data to clarify the effects found in the initial analyses. The significance level was set at p=0.05.
Results
Circulating Levels of Sertraline and Desmethylsertraline and CSF Levels of 5-HIAA (Table 2)
Table 2.
Circulating Sertraline, Desmethylsertraline, and CSF 5-HIAA
| Dose mg/kg | |||||
|---|---|---|---|---|---|
| ng/ml | 0 | 5 | 10 | 15 | 20 |
| Sertraline | 0.0 (0.0) | 29.8 (2.55) | 58.3 (8.84) | 72.2 (15.24) | 75.7 (13.75) |
| Desmethylsertraline | 0.0 (0.0) | 41.0 (4.07) | 71.5 (9.78) | 86.5 (8.60) | 97.4 (16.97) |
| CSF 5-HIAA | 189.4 (11.61) | 115.9 (5.52) | 126.0 (9.45) | ||
Plasma levels of sertraline (F[3,57]=78.4, p=0.001) and desmethylsertraline (F[3,57]=79.7, p=0.0002) rose significantly over the course of the study to levels comparable to those found in patients treated with sertraline. 5-HIAA levels decreased with increasing sertraline (F[2,40]=49.8, p<0.0001) confirming that the drug entered the central nervous system.
Social Status and Time of Day Effects on Behavior
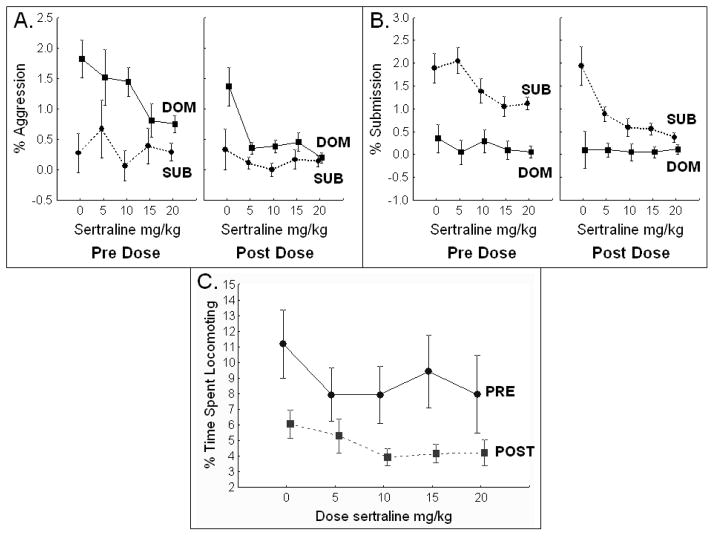
Social status is known to affect social behavior. Aggressive behavior (Figure 1A) was higher in dominants than subordinates (Social Status F[1,19]=11.5, p=0.003), and submissive behavior (Figure 1B) was lower in dominants than subordinates (Social Status F[1,19]=38.8, p<0.0001).
Figure 1. Aggression, Submission & Locomotion.
The behavior units represent the percent of the scan observations in which the behavior was observed. A. Aggressive behavior was higher in dominants than subordinates. Aggression was lower after dosing (POST) than before dosing (PRE) in dominant monkeys. Aggressive behavior decreased with increasing dose of sertraline in dominant monkeys. B. Submissive behavior was higher in subordinates than dominants. Submissive behavior was lower after dosing (POST) than before dosing (PRE) in subordinates. Submissive behavior decreased with increasing dose of sertraline in subordinate monkeys. C. Locomotion was lower after dosing (POST) than before (PRE), and decreased with increasing sertraline dose. SUB=subordinate; DOM=dominant; PRE=prior to daily dosing; POST=4 hours after daily dosing.
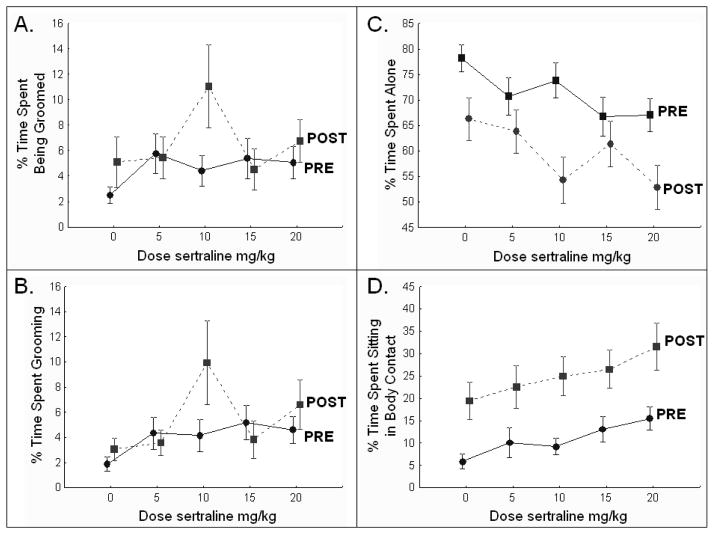
Circadian variations in behavior are common. In general, active behaviors decline in the midday, and passive behaviors increase. Here, behavior was recorded daily before dosing (PRE), at about 0800 hours, and four hours later after the monkeys received their daily dose of sertraline, at about 1200 hours (POST). Aggressive behavior was lower after dosing than before dosing (PrePost F[1,19]=19.8, p<0.0003), especially in dominant monkeys (Social Status X PrePost interaction: F[1,19]=6.8, p<0.02) (Figure 1A), and submissive behavior was lower after dosing than before dosing (PrePost F[1,19]=15.6, p<0.0009), especially in subordinate monkeys (Social Status X PrePost interaction: F[1,19]=8.9, p<0.008) (Figure 1B). As expected, locomotion (Figure 1C) was lower after dosing than before dosing (PrePost F[1,19]=6.6, p<0.02). Likewise, time alone (Figure 2C) was lower (PrePost F[1,19]=20.1, p<0.0003), and sitting in passive body contact (Figure 2D) was higher after dosing than before (PrePost F[1,19]=34.4, p=0.00001).
Figure 2. Affiliation.
The behavior units represent the percent of the scan observations in which the behavior was observed. A. Being Groomed: There was a significant dose by time of day interaction, however subsequent analysis showed no significant effect of sertraline. B. Grooming increased with increasing dose. C. Alone: The frequency in which the monkeys were observed alone was lower after (POST) than before (PRE) dosing. Time alone decreased with increasing dose of sertraline. D. Sitting in passive body contact was higher after (POST) than before (PRE) dosing, and increased with increasing dose of sertraline. PRE=prior to daily dosing; POST=4 hours after daily dosing.
Effects of Sertraline on Behavior (main and interaction effects of Dose)
Aggression and Submission
Aggressive behavior decreased with increasing dose of sertraline (Dose F[4,76]=3.7, p=0.009), but only in dominant monkeys (Social Status X Dose interaction: F[4,76]=2.9, p<0.03) (Figure 1A). Submissive behavior also decreased with increasing dose of sertraline (Dose F[4,76]=4.6, p=0.002), but only in subordinate monkeys (Social Status X Dose interaction: F[4,76]=2.2, p<0.02). Because of the circadian difference in submissive behavior reported above, a significant Social status X Dose X PrePost interaction (F[4,76]=2.6, p=0.04) was observed (Figure 1B). Further analysis comparing levels of submission pre- and post dose at the 0 versus 20 mg dose using subordinate data only (2 X 2 ANOVA) revealed a significant main effect of dose (F[1,20]=5.7, p<0.03), confirming that sertraline reduced submissive behavior in subordinates.
Activity Levels (Figure 1C)
Locomotion decreased with increasing dose (Dose F[4,76]=3.9, p<0.006).
Affiliation: Grooming and Being Groomed
Grooming (Figure 2B) increased with increasing dose (Dose F[4,76]=3.3, p<0.02); the dose effect on being groomed (Figure 2A) was not significant. The Dose X PrePost interaction was significant for grooming (F[4,76]=3.2, p<0.02), and being groomed (F[4,76]=3.0, p=0.02). Visual inspection suggests these interactions were due to the post-dose increase in grooming/being groomed at the 10 mg/kg dose (Figure 2B). The analysis was repeated without the 10 mg dose week, and a main effect of dose was observed on grooming (Dose: (F[3,57]= 3.1, p=0.03) but not being groomed.
Time alone (Figure 2C) decreased with increasing dose (Dose F[4,76]=6.5, p=0.0001). The Dose X PrePost interaction was significant (Dose X PrePost F[4,76]=4.4, p=0.003). This may reflect a lack of difference between the pre- and post-dose observations during the 5 mg/kg week and the 15 mg/kg week or the widely disparate amounts of time spent alone at the 10 mg dose. Further analysis comparing time alone pre- and post dose at the 0 versus 20 mg dose (2 × 2 ANOVA) confirmed that sertraline decreased time spent alone (main effect of Dose F[1,19]=29.8, p<0.0001). The main effect of time of day was still significant (PrePost F[1,19]=16.2, p=0.0007) and there was no dose by time of day interaction (Dose X PrePost F[1,19]=0.23, p=0.63).
Sitting in passive body contact (Figure 2D) increased with increasing sertraline dose (Dose F[4,76]=6.2, p=0.0002).
Discussion
Summary
In the study reported here the administration of sertraline HCl had wide ranging effects on the social behavior of adult female nonhuman primates. The circulating concentrations of sertraline and desmethylsertraline demonstrate that the drug was successfully administered by training the animals for oral dosing. The circulating concentrations of sertraline and desmethylsertraline observed at the 20 mg/kg dose fell within the clinical range of efficacy observed in patients (Reis et al. 2004). The CSF concentration changes in 5-HIAA demonstrate that the sertraline reached the central nervous system and affected the 5-HT system. The observed decline in CSF 5-HIAA levels was of the approximate magnitude observed in prior studies of macaques (Anderson et al. 2005; Clarke et al. 1999) and is consistent with the 40–60% declines typically seen in patients following sustained SSRI treatment (Sheline et al. 1997). Thus, the observed decreases in agonistic behavior and increases in affiliative behavior with sertraline administration appear to be directly due to the drug.
It may be that SSRI efficacy in a range of human behavioral pathologies may be due, in part, to their specific beneficial effects on aggression and affiliation. The best recognized pathology affected by SSRIs is depression, which is often accompanied by anger, irritability and aggression, as well as social isolation (Painuly et al. 2011). To the extent that the observations made here are generalizable to human beings, it is possible that the antidepressant effects of SSRIs in patients may be due as much to their effects on agonism and affiliation as their effects on mood. There are some indications that SSRIs may be helpful in reducing aggression and increasing positive social behaviors in dementias and autism spectrum disorder which support this hypothesis (Kolevzon et al. 2006; Pollock et al. 2007).
Design Considerations
The purpose of this cumulative dose-response experiment was to document the effects of a commonly prescribed SSRI on broad aspects of primate social behavior. The cumulative dose-response design has both strengths and weaknesses. The strengths are that the design mimics clinical care in that doses are typically adjusted without washout periods. Another major strength is that studies can be completed with smaller sample sizes in shorter time periods, both critical concerns in nonhuman primate studies. The weaknesses are that data are only collected at each dose for 1 week and neurobiological effects of antidepressants continue to change over a several week period, thus the effects of dose and duration cannot be cleanly separated. However, many of the observed effects of sertraline involved increasing magnitude of change in behavior with increasing dose and time which is another indicator of a true effect of sertraline on behavior. The cumulative dose-response design also does not include a placebo group which would provide an additional control and also facilitate blinding behavioral technicians to treatment.
Translational Value of the Model
The serotonergic system of macaques shares more similarities with human beings than rodent systems with regard to nuclear organization, projection pathways, innervation patterns, axonal morphologies, and 5-HT 1A receptor localization, making macaques optimal models for investigations involving central nervous system 5-HT (Amaral and Lavenex 2007; Azmitia and Gannon 1986; Buckmaster and Amaral 2001). The subjects were chosen to model the age-sex group of human beings that are prescribed the most antidepressants in the US. The monkeys were also fed a diet modeled on a typical Western diet as macronutrient availability may alter neurobiological systems, and because the usual laboratory monkey chow does not reflect either human consumption or feeding practices of monkeys in the wild, and is replete with neurobiologically active isoflavones which alter 5-HT neurotransmission in macaques (Shively et al. 2003; Stroud et al. 2006).
Social Modulation of Pharmacological Effects
A wealth of studies demonstrate important effects of social factors e.g. social support, social status, and social stress on depression. Because there is little information about the effects of SSRIs on socially-housed primates, and changes in aggression levels have been shown previously, we reasoned that in this initial study of socially-housed monkeys, it was prudent to assign all animals in a social group to the same treatment (Higley et al. 1998; Carrillo et al. 2009; Yanowitch and Coccaro 2011).
The effects on agonistic behavior were social status-dependent. Social status, determined by the outcomes of agonistic interactions and not the frequency, was stable throughout this experiment. Sertraline decreased aggressive behavior but only in dominants; subordinate aggressive behavior was already low and remained low throughout the study. Likewise, sertraline decreased submissive behavior but only in subordinates; submissive behavior of dominants was already low and remained so throughout the study. It is likely that the submissive behavior of subordinates decreased because the aggressive behavior of dominants decreased. This is an important consideration because when monkeys change social status from being subordinate to dominant, or vice versa, their agonist behavior follows and becomes appropriate for their social status. This suggests that the effects of sertraline, and perhaps other SSRIs, may be dependent on social variables, and could change within a subject with changing social conditions. The fact that a social attribute of the monkeys, social status, modified the effects of sertraline on behavior suggests that studying the effects of pharmacologic agents in social-living subjects may better predict the range of effects that might be expected in human beings.
The Role of Serotonin
Among their many neurobiological effects, SSRIs make more serotonin (5-HT) available in the synapse, altering central serotonergic neurotransmission. The observed decreases in CSF 5-HIAA following SSRI administration are presumed to be due to increased extracellular levels of 5-HT causing increased autoreceptor-mediated inhibitory feedback on synthesis/turnover of 5-HT. A wealth of preclinical and clinical data suggest that abnormally low serotonergic activity results in increased aggression (Carrillo et al 2009; Moskowitz et al., 2003). However, the majority of these studies necessarily rely either on cerebrospinal fluid concentrations of 5-HIAA, or acute depletion of tryptophan, the percursor for 5-HT synthesis in the brain, and the interpretation of such studies is not straightforward. For example, CSF 5-HIAA concentrations reflect the sum of 5-HT metabolism in the CNS. CSF 5-HIAA may be low because 5-HT is low, which is how it is often interpreted, or it may be low because 5-HT metabolism is low leaving more 5-HT available at the site of action, as in the case of SSRI administration. Likewise, interpreting the neurobiological cause of behavioral responses to acute depletion of a neurotransmitter that modulates many neurotransmitter systems and processes in the brain is also fraught with difficulty. The sudden removal of function of such a ubiquitous modulator is bound to have overt acute effects that may be temporally bound. Increasing serotonin function in clinical populations appears to reduce aggression, and SSRIs are prescribed for aggressive disorders. However, whether increasing 5-HT decreases aggression in healthy individuals is less clear (Young et al., 2013). Likewise, while excessive aggression associated with low 5-HT is incompatible with friendly or affiliative behavior, it is also unclear whether raising 5-HT concentrations actually promotes affiliation, or just creates a nonantagonistic situation that allows it (Young et al., 2013).
Circadian Changes in Social Behavior
Observations were done daily just prior to and 4 hours following sertraline dosing, at 0800 and 1200 hrs respectively. The purpose was to capture potential differences in acute versus chronic changes in behavior, and also to document the accumulation of effects which might begin to appear prior to daily dosing with the passage of time and increasing dose. However, circadian rhythms in behavior complicate the interpretation of the observations. In general, active behaviors (aggression, submission, locomotion) decline in the midday, and passive behaviors (sitting in body contact) increase as a normal part of circadian cycles in behavior. Here, main effects of time of day (i.e. PrePost) reflect consistent changes in behavior at every dose including baseline (0 mg sertraline). These are reasonably interpreted as circadian changes. However change in behavior pre- versus post-dosing that were different at different doses required follow up analyses to determine whether there was a true effect of sertraline on behavior. Follow up analyses confirmed effects of sertraline on submission (in subordinates only), and grooming but not being groomed.
Unexpected Variations in Behavior After Dosing
At 10 mg/kg three unusual effects on behavior were observed after dosing: There was a sharp increase in time spent grooming, which was accompanied by a sharp increase in time spent being groomed, and decrease in time spent alone. Grooming is associated with reduced urinary cortisol metabolites in the groomer, and reduced heart rate in the individual being groomed, suggesting that it reduces physiological stress responses (Shutt et al. 2007; Boccia et al. 1989). It may be that increased grooming by some of the monkeys was in response to a transient agitation which is often reported early in treatment in patients.
Summary and Future Research
Thus, sertraline, a commonly prescribed SSRI, has significant effects on spontaneous socioemotional behavior in female primates living in stable long-term social groups, decreasing agonism and increasing affiliation, two major axes of social behavior. The effects may well be amplified by all animals within a social group having the same treatment (sertraline or placebo). However, since SSRIs are among the most prescribed drugs in the US, it is important to consider their global effects on behavior and social interaction in small groups such as families. The behavioral effects reported here reflect 5 weeks of exposure at gradually increasing doses until a dose corresponding to a therapeutic dose in human beings was reached. Since the majority of human beings who take antidepressants do so for more than two years, it will be important to determine the longer term effects of commonly prescribed antidepressants on social interaction. Likewise, since a large proportion of antidepressant users are middle-aged women, it will be important to study these long-term effects in women and female animal models.
Acknowledgments
This work was supported by the National Institutes of Health RO1HL087103 and R21MH086731 (to CAS). We are grateful to Matthew McMullin and NMS Labs for his help with the sertraline and desmethylsertraline analyses. All procedures involving primates were conducted using protocols approved by the Institutional Animal Care and Use Committee of Wake Forest University and were in compliance with all institutional, state, and federal laws for the usage of primates in laboratory settings.
Footnotes
Financial Disclosures
Drs. Shively, Register, Higley, and Willard reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Carol A. Shively, Department of Pathology, Section on Comparative Medicine, Wake Forest School of Medicine, Winston-Salem, NC
Thomas C. Register, Department of Pathology, Section on Comparative Medicine, Wake Forest School of Medicine, Winston-Salem, NC.
J. Dee Higley, Department of Psychology, Brigham Young University, Salt Lake City, UT
Stephanie L. Willard, Integrative Neuroscience Graduate Program, Wake Forest School of Medicine, Winston-Salem, NC
References
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The hippocampus book. Oxford University Press; New York: 2007. pp. 37–114. [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49(3):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Barr CS, Lindell S, Durham AC, Shifrovich I, Higley JD. Time course of the effects of the serotonin-selective reuptake inhibitor sertraline on central and peripheral serotonin neurochemistry in the rhesus monkey. Psychopharmacology (Berl) 2005;178(2–3):339–346. doi: 10.1007/s00213-004-2011-7. [DOI] [PubMed] [Google Scholar]
- Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiol Behav. 1989 Mar;45(3):667–70. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Amaral DG. Intracellular recording and labeling of mossy cells and proximal CA3 pyramidal cells in macaque monkeys. J Comp Neurol. 2001;430:264–281. doi: 10.1002/1096-9861(20010205)430:2<264::aid-cne1030>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl) 2009;205(3):349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry. 1999;46:221–228. doi: 10.1016/s0006-3223(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2009;48(2):176–84. [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. The serotonin reuptake inhibitor sertraline reduces excessive alcohol consumption in nonhuman primates. Neuropsychopharmacology. 1998;18:431–443. doi: 10.1016/S0893-133X(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Mathewson KA, Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J Clin Psychiatry. 2006;67(3):407–414. doi: 10.4088/jcp.v67n0311. [DOI] [PubMed] [Google Scholar]
- Moskowitz DS, Pinard G, Zuroff DC, Annable L, Young SN. Tryptophan, serotonin and human social behavior. Adv Exp Med Biol. 2003;527:215–24. doi: 10.1007/978-1-4615-0135-0_25. [DOI] [PubMed] [Google Scholar]
- Painuly NP, Grover S, Gupta N, Mattoo SK. Prevalence of anger attacks in depressive and anxiety disorders: implications for their construct? Psychiatry Clin Neurosci. 2011;65(2):165–174. doi: 10.1111/j.1440-1819.2010.02177.x. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry. 2007;15(11):942–952. doi: 10.1097/JGP.0b013e3180cc1ff5. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. Hyattsville, MD: National Center for Health Statistics; 2011. NCHS data brief, no 76. [Google Scholar]
- Reis M, Aberg-Wistedt A, Agren H, Höglund P, Akerblad AC, Bengtsson F. Serum disposition of sertraline, N-desmethylsertraline and paroxetine: a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum Psychopharmacol. 2004;19(5):283–291. doi: 10.1002/hup.599. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Blier P, Delgado PL, Fava M, Haddad PM, Shelton RC. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(Suppl 4):27–30. [PubMed] [Google Scholar]
- Segraves RT. Sexual dysfunction associated with antidepressant therapy. Urol Clin North Am. 2007;34(4):575–579. doi: 10.1016/j.ucl.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR, Adams MR. Effects of ovariectomy, social instability and social status on female Macaca fascicularis social behavior. Physiol Behav. 1986;36(6):1147–1153. doi: 10.1016/0031-9384(86)90492-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3(2):114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, et al. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32(8–10):981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biol Lett. 2007 Jun;22;3(3):231–3. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud FC, Appt SE, Wilson ME, Franke AA, Adams MR, Kaplan JR. Concentrations of isoflavones in macaques consuming standard laboratory monkey diet. Am Assoc Lab Anim Sci. 2006;45(4):20–23. [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–23. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- Young SN. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;25;368(1615):20110375. doi: 10.1098/rstb.2011.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]