Abstract
Rationale
Despite widespread abuse there are few validated methods to study the rewarding effects of inhalants. One model that that may have utility for this purpose is intracranial self-stimulation (ICSS).
Objectives
We wished to compare and contrast the ICSS reward-facilitating effects of abused inhalants to other classes of abused drugs. Compounds were examined using two different ICSS procedures in mice to determine the generality of each drug's effects on ICSS and the sensitivity of the procedures.
Methods
Male C57BL/6J mice with electrodes implanted in the medial forebrain bundle were trained under a three component rate-frequency as well as a progressive ratio (PR) ICSS procedure. The effects of nitrous oxide, toluene vapor, cocaine and diazepam on ICSS were then examined.
Results
Concentrations of 1360-2900 ppm inhaled toluene vapor significantly facilitated ICSS in the rate frequency procedure and 1360 ppm increased PR breakpoint. A concentration of 40% nitrous oxide facilitated ICSS in the rate-frequency procedure but reduced PR breakpoint. Doses of 3-18 mg/kg cocaine facilitated ICSS in the rate frequency procedure and 10 and 18 mg/kg increased PR breakpoint. Doses of 1 and 3 mg/kg diazepam facilitated ICSS in the rate frequency procedure and 3 mg/kg increased PR breakpoint.
Conclusions
The reinforcement facilitating effect of toluene in ICSS is at least as great as diazepam. In contrast, nitrous oxide weakly enhances ICSS in only the rate frequency procedure. The data suggest that the rate frequency procedure may be more sensitive than the PR schedule to the reward facilitating effects of abused inhalants.
Keywords: rate frequency, ICSS, Intracranial self-stimulation, mice, inhalant, toluene, nitrous oxide, diazepam, progressive ratio, cocaine
Introduction
Nitrous oxide and toluene are two commonly abused inhalants. Toluene is a volatile aromatic hydrocarbon found in a wide range of consumer and industrial products including paint thinner, glues, and gasoline. Nitrous oxide is a gas used clinically as an anesthetic adjunct in medicine and dentistry (Balster et al. 2009; Brouette and Anton 2001). Inhaled toluene produces a rapid onset of psychoactive effects, including excitation, euphoria, incoordination, and hallucinations (Cruz and Domínguez 2011; Elkoussi and Bakheet 2011). Nitrous oxide also produces a myriad of psychoactive effects including euphoria, fatigue, numbing sensations, and detachment (Walker and Zacny 2003; Zacny et al. 2008; Cho et al. 1997; Collado et al. 2007). Chronic toluene and nitrous oxide abuse each have severe medical consequences. Toluene exposure causes neurotoxicity as well as reproductive, pulmonary, renal, cardiovascular, and hepatic organ system damage (Bowen and Hannigan 2006; Gupta et al. 2011; Hannigan and Bowen 2010; Hoet and Lison 2008; Yücel et al. 2008). Chronic nitrous oxide abuse has been associated with vitamin B12 deficiency and myeloneuropathies (Lin et al. 2011; Tatum et al. 2010).
Despite being inexpensive, readily available and of high abuse liability, the mechanism or mechanisms underlying the rewarding effects of toluene and nitrous oxide are poorly understood (Bowen and McDonald 2009; Garland et al. 2009). One reason this may be the case is absence of validated in vivo procedures for examining their abuse-related effects. While the intravenous self-administration procedure is the most widely utilized behavioral paradigm to assess drug reinforcement, it is difficult to conduct self-administration studies with inhalants. To date only one study has demonstrated intravenous self-administration of an inhalant in rodents and that experiment used a unique between-subject single day acquisition procedure in mice which is poorly suited to longitudinal behavioral studies (Blokhina et al. 2004).
One procedure which may be adaptable to examining the reward-related effects of inhalants is intracranial self-stimulation (ICSS). Most classes of abused drugs facilitate ICSS-reinforced behavior (Kornetsky et al. 1979; Wise 1980; Fish et al. 2010; Straub et al. 2010). The development of ICSS procedures to examine the reward-related effects of inhalants in mice is of particular interest given the powerful genetic tools available only in mice. For example, knock-in mice with subtype specific GABAA point site mutations have been utilized to investigate which subtypes contribute to the behavioral effects of alcohol and benzodiazepines (Blednov et al. 2011; Reynolds et al. 2012). These same strategies could also be applied to inhalants to delineate receptor systems and subtypes responsible for mediating their reward-like effects.
Two studies in rats have demonstrated that, similar to other drugs of abuse, toluene vapor facilitates ICSS (Yavich and Zvartau 1994; Bespalov et al. 2003). It has also recently been demonstrated that I.P. injected toluene reduces ICSS thresholds in mice in a discrete-trial current-threshold procedure (Chan et al. 2012). Mimicking the route a drug is abused in humans has traditionally been thought to be of limited importance in preclinical studies, therefore the most convenient injection route is typically utilized. However, c-Fos expression in rats showed administration route-dependent differences in neuronal activation produced by toluene (Perit et al. 2012). Among the important neuronal systems differentially affected by route were areas associated with reward and motor control, with inhaled toluene producing significant increases in c-Fos expression in the nucleus-accumbens core and caudate-putamen relative to the findings of a previous study using injected toluene (Lo and Chen 2005). While c-Fos is only a proxy for neuronal activation this data coupled with the fact that toluene is abused exclusively by inhalation supports studying the reward altering effects of liquid volatile inhalants by the inhalation route. In addition the use of inhalation exposure is the only route through which abused gaseous compounds such as nitrous oxide can be examined.
The goals of the present study were to develop and characterize ICSS procedures to assess the reward-related effects of inhalational delivery of abused inhalants in mice. The effects of toluene and nitrous oxide on ICSS were examined using both rate-frequency and progressive ratio procedures to determine the generality of any effects on reinforcement as well as the relative sensitivity of both procedures. As a positive control we examined cocaine as it produces robust facilitation of ICSS (Fish et al. 2010; Straub et al. 2010). Lastly, given that toluene and nitrous oxide both have positive GABAA modulatory effects for comparison we also examined diazepam (Bowen et al. 1996; Cruz et al. 1998; Emmanouil et al. 1994; Hapfelmeier et al. 2000).
Materials and Methods
Subjects
A total of 23 adult C57BL/6J mice were used (Jackson Laboratory, Bar Harbor, Maine). Mice were individually housed under a 12h light/dark cycle (lights on a 0600). Mice were tested M-F between 0800 and 1800. Chow (Harlan, Madison, WI) and water were available ad libitum except during experimental sessions. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and were in accordance with the NIH “Guide for the Care and Use of Laboratory Animals: Eighth edition” (National Research Council 2011).
Compounds
HPLC grade toluene was purchased from Fisher Scientific. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse drug supply program and prepared in 0.9% sterile saline. Diazepam was obtained in an injectable commercial formulation and diluted in 0.9% sterile saline. Medical grade nitrous oxide and oxygen cylinders were purchased from National Welders (Richmond, VA).
Surgical Procedure
Mice were anesthetized with isoflurane vapor and implanted with 6 mm bipolar electrodes (Plastics One, Roanoke, Virginia) into the right medial forebrain bundle within the lateral hypothalamus (Paxinos and Franklin 2001). Coordinates relative to Bregma were: −1.5 anterior-posterior, 1.0 medial-lateral, and −5.0 dorsal-ventral. Three stainless steel screws were affixed in holes in the skull to serve as anchors for a dental acrylic headpiece. Mice were treated daily for 3 days post-surgery with 0.5 mg/kg of the analgesic carprofen. Mice were allowed six days to recover prior to beginning ICSS training sessions.
Dynamic Exposure System and Testing Apparatus
The inhalant exposure ICSS system consisted of four 20 liter acrylic cubicles each of which contained a two-lever operant conditioning chamber (Med-Associates, St. Albans, VT). A bipolar lead tether connection (Plastics One, Roanoke, VA) and mercury commutator (Mercotac 205L, Carlsbad CA) was suspended above the operant conditioning chamber by a counterbalanced arm. Toluene vapor was produced using a dynamic vapor generator composed of a filtered, pressure regulated air supply routed via tubing to two mass flow proportional valves controlled by a Matheson 8284 dynamic gas mixer (Matheson, Albuquerque, NM). The air output from one valve passed through a fritted glass bubbler inserted in a 3-neck round flask filled with liquid toluene, generating a toluene-laden air stream. The toluene-laden air was mixed with metered fresh air from the second mass control valve permitting a broad range of highly reproducible inhalant concentrations to be generated. The blended vapor stream was routed through the upper rear wall of the operant conditioning chambers via Tygon tubing. The effluent mixture was vented from bottom rear wall of the exposure chamber through a Miran 1A single wavelength infrared spectrophotometer coupled to a computerized chart recorder (DATAQ, Akron, OH), providing real time inhalant concentration measurement relative to a previously generated standard curve. Toluene concentrations within each ICSS chamber were also quantified by headspace gas chromatography. To deliver nitrous oxide, the system was similar except the inhalant and mix gas sources were provided by compressed cylinders. ICSS stimulation and operant schedule control was provided by commercially-available components (Med-Associates, St. Albans, VT). A schematic of the operant conditioning chamber housed within the gas exposure system is shown in figure 1.
Figure 1.
Schematic representation of the experimental apparatus constructed to expose mice to toluene vapor. A dynamic gas mixer controlled the flow rate of compressed air directed through a 1L glass bubbler container partially filled with liquid toluene. The toluene vapor laden air stream thus generated was then proportionally mixed with a clean air stream before being directed into a 20L exposure cubicle housing an operant conditioning chamber. Effluent from the exposure chamber was routed through an IR spectrometer connected to a computerized chart recorder to monitor real-time exposure chamber concentrations. N2O delivery was identical except that nitrous oxide gas and medical oxygen were delivered from compressed cylinders and the bubbler was not utilized.
Rate Frequency ICSS Procedure
After surgical recovery mice were trained to respond in daily 1 hr. sessions for brain stimulation under a fixed ratio 1 (FR1) schedule of reinforcement. Each active lever-press resulted in electrical stimulation consisting of a 500 ms, 158 Hz pulse train. Current amplitude for each subject was adjusted within a range of 50 to 300 uA in order to generate maximal rates of responding. When responding stabilized under these conditions the mice progressed to the three component sessions used for rate-frequency testing. Training and test sessions were 70 minutes in duration, divided into three 10-min response components separated by two 20-min timeouts. The timeout periods served as pretreatment windows prior to the second and third ICSS response components. Vehicle or air was administered during the first 20-min timeout. Drugs were injected or inhalant exposures initiated during the second 20-min timeout. In this manner baseline control ICSS data (second response component) as well as test data (third response component) could be generated within the same subject on the same test day. Treatment exposures of inhalants initiated at the start of the second timeout continued through the third response component resulting in 30 minutes of total inhalant exposure. Response components were signaled by the illumination of the chamber house light. During initial training, each active lever press during the 10-min response components delivered a 500 ms pulse train. After responding under the multiple component schedule stabilized animals were transitioned to sessions in which the frequency available for self-stimulation began at 158 Hz and decreased each min thereafter by 0.05 log units ending at 56 Hz on the last min of each component. After behavior again stabilized, as defined by consistent response rates ≥30 responses per minute and lever pressing for at least four self-stimulation frequencies of the rate-frequency curve, testing began. At any point in the progression of training, animals which failed to respond reliably were removed from the study and replaced.
Toluene concentrations of 480, 1360, 2900, 3300 and- 5000 parts-per-million (PPM) were examined. Nitrous oxide concentrations of 5, 20, 40, 60 and 80% combined with oxygen were also examined in an identical exposure design. Sessions in which injected drugs were given were identical to inhalant test sessions with the exception that following the second response component the mice were given an IP injection of drug. Cocaine was tested at doses of 3, 10, and 18 mg/kg and diazepam was tested at doses of 0.3, 1, 3, 6 and 10 mg/kg.
Inhalant concentrations and test drug doses were generally tested in an ascending order. In mice which advanced most rapidly through testing it was sometimes necessary to then test lower doses/concentrations to adequately characterize the full dose/concentration-effect curve. The ascending dose series was then updated for subsequent subjects. Upon completion of a dose/concentration-effect curve, subjects were assigned to the next planned experimental condition requiring additional subjects. Drug naïve subjects which replaced those animals lost to electrode failures or other causes were counterbalanced across test conditions with subjects with previous drug exposure to ensure that individual conditions contained similar numbers of naïve and experienced subjects.
Progressive Ratio ICSS Procedure
Following completion of rate frequency testing, the animals were transitioned to a chain PR/FR schedule (Depoortere et al. 1999). Under the PR/FR schedule, the number of lever-presses required to increment the schedule was increased after each completed component. The PR step-size was increased according to an exponential step size function (5* e(trial# * 0.1)- 5) (Sharma et al. 2012). After each PR was completed the animal received five, 500 ms ICSS pulse trains over five sec and then entered a second component in which the next ten FR1 responses were each reinforced by one 500 ms ICSS pulse train. Following the completion of the FR1 component the PR value incremented to the next higher ratio value and the PR/FR sequence repeated until a PR requirement was not completed within 6 min, terminating the session. During PR/FR training and testing the ICSS frequency was fixed at 158 Hz and the current amplitude for each mouse was the same as that used in the rate frequency procedure. Concentrations of 100, 480, 1360, and 2900 ppm toluene and 20, 40 and 80% nitrous oxide combined with oxygen were administered 20 min prior to and for the duration of the PR/FR session. Diazepam was tested at doses of 0.3, 1, 3, and 6 mg/kg administered 15 min prior to the start of the PR/FR session. Cocaine was tested at doses of 3, 10 and 18 mg/kg administered 5 min prior to the start of the PR/FR session.
Quantification of Exposure Concentrations and Inhalant Blood Levels
Blood toluene concentrations were determined by submandibular blood sampling following 20 min of exposure to 480, 1360, 3300, and 5000 ppm toluene and after 30 minutes exposure to 3300 ppm. The headspace gas chromatography analytical methods were similar to those previously reported (Shelton 2009; Shelton and Nicholson 2012; Shelton and Nicholson 2010).
Data Analysis
In the three component procedure, data from the first 10-min response component was discarded as it has been shown to exhibit more variability compared to subsequent components (Carlezon and Chartoff 2007). Stimulations earned during each 1-min trial of the second 10-min air/vehicle exposure control component were compared to the equivalent trial in the third 10-min drug test component. For each mouse, data from each trial were expressed as a percentage of the number of stimulations in the 1-min trial with the greatest number of responses, regardless of frequency, in the second baseline component. The values are reported as percentage of the maximum control responses (%MCR). This data normalization procedure allowed grouping of the data across subjects despite differences in individual maximum response rates. Data from the second control and third test component were compared individually for each drug dose or inhalant concentration by two-way within subject repeated measures analysis of variance (ANOVA) according to previous literature (Altarifi and Negus 2011; Bauer et al. 2013; Bonano et al. 2013). GraphPad version 6.01 for Windows (La Jolla, CA) was used for all analyses. Significant (p < 0.05) main effects and interactions were subsequently examined by Holm-Sidak post-hoc tests comparing identical frequencies between the control and test component.
Rate-frequency data was also analyzed to calculate M50 values for each treatment condition using a variant of the method outlined in Carlezon and Chartoff 2007. M50 values represent the interpolated frequency that maintains 50% maximum response rates. The estimated linear portion of the rate-frequency curve from 20%-80% of %MCR was analyzed for each subject using a least-squares regression line of best fit to estimate M50. Group treatment condition M50s were then compared by student's paired t-test to group vehicle control baseline to determine whether the %M50 shift was significant (p < 0.05).
In the progressive ratio procedure, test condition breakpoint data were normalized by expressing them as a change in breakpoint from each subject's baseline control breakpoint. The average breakpoint of the two mid-week vehicle (air or saline) test sessions was defined as the baseline breakpoint. Change in breakpoint resulting from drug exposure was calculated by subtracting the baseline breakpoint from the test breakpoint for each subject. Group mean changes in breakpoints were then compared across drug or inhalant doses by one-way within subject repeated measures ANOVA. Significant (p < 0.05) main effects were examined by Holm-Sidak post-hoc tests comparing changes in breakpoint for drug and inhalant exposure conditions to either air or saline.
Results
Rate Frequency Procedure
Doses of 3, 10 and 18 mg/kg cocaine significantly facilitated ICSS (Figure 2a-c). There was a significant main effect of 3 mg/kg cocaine [F (1,10) = 15.68, p = 0.0027] (Figure 2a) on ICSS responding but no drug × frequency interaction [F (1,10) = 1.121, p = 0.3561]. There was a significant drug × frequency interaction [F (9, 90) = 2.480, p = 0.0141] of 10 mg/kg cocaine on %MCR (Figure 2b) with responding at the 6 lowest frequencies significantly increased over control. There was a significant drug x frequency interaction [F (9, 90) = 4.278, p = 0.0001] of 18 mg/kg cocaine on %MCR (Figure 2c) with the 7 lowest frequencies showing significantly higher rates of responding compared to control. Cocaine doses of 3 mg/kg [t (10) = 2.961, p = 0.0143] and 10 mg/kg [t (10) = 3.101, p = 0.0112] significantly lowered the M50 (table 1). A M50 value for 18 mg/kg cocaine could not be calculated as responding was too robustly increased across the entire ICSS frequency range to permit the analysis.
Figure 2.
Mean (±SEM) percent maximal control ICSS response rate following 20 minute pretreatment with I.P. cocaine. Closed symbols represent the cocaine treatment condition and open symbols the saline control condition. (a) 3 mg/kg cocaine (b) 10 mg/kg cocaine (c) 18 mg/kg cocaine. * denotes significant differences compared to saline control (p < 0.05).
Table 1.
Percentage M50 shift and percentage maximum rate of baseline for Diazepam (n=9), Cocaine (n=11), Nitrous Oxide (n=7) and Toluene (n=11).
| Drug | Dose/Conc. | %M50 Shift (±SEM) | %Max | Rate (±SEM) | |||
|---|---|---|---|---|---|---|---|
| Diazepam | 0.3 mg/kg | 6.0 | 7.5 | 88.9 | 9.2 | ||
| 1 mg/kg | ↓ | −18.9 | 4.0 | 117.9 | 8.8 | ||
| 3 mg/kg | ↓ | −19.9 | 5.4 | 110.8 | 8.6 | ||
| 10 mg/kg | NC | 47.0 | 20.9 | ||||
| Cocaine | 3 mg/kg | ↓ | −10.2 | 3.4 | ↑ | 110.9 | 4.2 |
| 10 mg/kg | ↓ | −19.3 | 5.3 | 106.0 | 5.9 | ||
| 18 mg/kg | NC | ↑ | 122.3 | 9.0 | |||
| Nitrous Oxide | 5% | 2.7 | 9.4 | 102.8 | 7.3 | ||
| 20% | 1.6 | 4.3 | 101.2 | 5.8 | |||
| 40% | ↓ | −16.0 | 4.2 | 94.7 | 4.6 | ||
| 60% | −4.4 | 6.9 | ↓ | 82.4 | 3.4 | ||
| 80% | NC | ↓ | 71.7 | 4.1 | |||
| Toluene | 480 PPM | −4.6 | 5.2 | ↑ | 107.3 | 5.2 | |
| 1360 PPM | ↓ | −26.7 | 6.0 | 99.6 | 5.3 | ||
| 2000 PPM | ↓ | −21.6 | 5.7 | 97.5 | 5.7 | ||
| 2900 PPM | −11.9 | 8.6 | 88.4 | 4.9 | |||
| 3300 PPM | NC | ↓ | 79.9 | 7.2 | |||
| 5000 PPM | NC | ↓ | 42.4 | 7.2 | |||
↑↓indicate direction of significance from vehicle baseline (p < 0.05).
NC, not calculated
In the rate frequency procedure 1 and 3 mg/kg diazepam significantly facilitated and 10 mg/kg significantly depressed ICSS (Figure 3a-d). There was a significant main effect of 1 mg/kg diazepam [F (1, 8) = 5.478, p = 0.0474] (Figure 3b) but no drug × frequency interaction [F (9, 72) = 1.374, p = 0.2160]. There was a significant drug × frequency interaction [F (9, 72) = 3.473, p = 0.0013] of 3 mg/kg diazepam on %MCR (Figure 3c) with responding at 5 of 6 of the lowest frequencies significantly increased over control. There was a significant drug × frequency interaction of 10 mg/kg diazepam on %MCR [F (9, 72) = 2.624, P = 0.0111] (Figure 2d) with the 2 highest frequencies showing a significant decrease of ICSS responding compared to control. Diazepam doses of 1 mg/kg [t (8) = 4.314, p = 0.0026] and 3 mg/kg [t (8) = 3.406, p = 0.0093] also significantly lowered M50 values (table 1).
Figure 3.
Mean (±SEM) percent maximal control ICSS response-rate following 15 minute pretreatment with I.P. diazepam. Closed symbols represent the diazepam treatment condition and open symbols the saline control condition. (a) 0.3 mg/kg diazepam (b) 1 mg/kg diazepam (c) 3 mg/kg diazepam (d) 10 mg/kg diazepam. * denotes significant differences compared to saline control (p < 0.05).
There was a linear relationship between toluene vapor exposure concentration and toluene blood concentration (Figure 4). The lowest exposure concentration of 480 ppm toluene produced a mean blood concentration of 3.7 ug/ml after 20 min of exposure increasing to 61.2 ug/ml at the highest, 5000 ppm toluene exposure concentration. To determine the extent to which toluene blood concentration may have increased during the third ICSS response component we also examined toluene blood levels after 30 min of exposure to the intermediate concentration of 3300 ppm. Exposure to 3300 ppm toluene for 30 min resulted in only a 15% increase compared to exposure for 20 min (data not shown).
Figure 4.
Mean (±SEM) C57BL/6J mice toluene blood concentrations measured after 20 minutes of exposure in the dynamic chamber per exposure concentration (n=3-4).
Concentrations of 1360-5000 ppm toluene all produced significant effects on ICSS (figure 5a-f). There was a significant interaction [F (9, 90) = 7.134, p < 0.0001] of 1360 ppm toluene on ICSS with 6 of 10 frequencies showing significantly greater %MCR (Figure 5b) compared to control. There was significant interaction [F (9, 90) = 2.455, p = 0.0151] of 2000 ppm toluene on ICSS with 7 of 10 frequencies showing significantly greater %MCR (Figure 5c) compared to control.
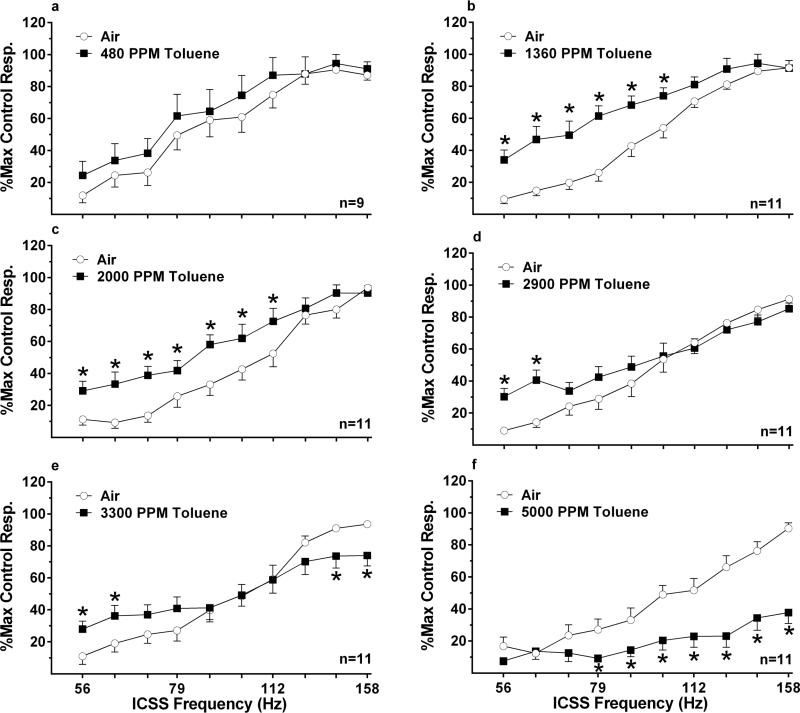
Figure 5.
Mean (±SEM) percent maximal control ICSS response rate following 20 minute of exposure to toluene vapor. Closed symbols represent the toluene vapor treatment condition and open symbols the air control condition. (a) 480 ppm toluene (b) 1360 ppm toluene (c) 2900 ppm toluene (d) 3300 ppm toluene 3e) 5000 ppm toluene. * denotes significant differences compared to saline control (p < 0.05).
There was a significant interaction of 2900 ppm toluene [F (9, 90) = 5.200, p < 0.0001] on %MCR (Figure 5d) with the 2 lowest frequencies showing a significantly enhancement of ICSS responding compared to control. There was a significant interaction of 3300 ppm toluene [F (9, 90) = 7.136, p < 0.0001] on %MCR (Figure 5e) on ICSS with the two highest frequencies demonstrating a significant depression and the two lowest frequencies a significant enhancement of ICSS responding compared to control. The 5000 ppm toluene concentration produced a significant interaction [F (9, 90) = 8.499, p < 0.0001] on %MCR (Figure 5f) with 7 of 10 frequencies exhibiting a significant suppression of ICSS responding compared to control. Toluene concentrations of 1360 ppm [t (10) = 4.946, p = 0.0006] (table 1) and 2000 ppm significantly decreased the M50 [t (10) = 3.652, p = 0.0045] (table 1).
Nitrous oxide concentrations of 40, 60 and 80% all significantly altered ICSS responding (Figures 6a-d). There was a significant interaction [F (9, 54) = 2.400, p = 0.0228] of 40% nitrous oxide on ICSS with 2 of 10 frequencies showing significantly greater %MCR than control (Figure 6b). The 60% nitrous oxide concentration produced a significant interaction [F (9, 54) = 3.101, p = 0.0045] with the highest frequency showing a significantly lower %MCR than control (Figure 6c). The highest, 80% nitrous oxide concentration produced a significant interaction [F (9, 54) = 9.944, p < 0.0001] on %MCR (Figure 6d) with 5 of 10 frequencies showing suppressed rates of ICSS responding compared to control. 40% Nitrous oxide also significantly lowered the M50 [t (6) = 3.771, p = 0.0093] (table 1).
Figure 6.
Mean (±SEM) percent maximal control ICSS response rate following 20 minute of exposure to nitrous oxide gas combined with 100% oxygen. Closed symbols represent the nitrous oxide treatment condition and open symbols the air control condition. (a) 20% nitrous oxide (b) 40% nitrous oxide (c) 60% nitrous oxide (d) 80% nitrous oxide. * denotes significant differences compared to saline control (p < 0.05).
Progressive Ratio Procedure
Cocaine significantly increased progressive-ratio breakpoint [F (1.280, 7.681) = 9.534, p = 0.0126] (Figure 7a). Post hoc tests revealed that 10 and 18 mg/kg cocaine significantly (p < 0.05) increased breakpoint compared to saline control. Diazepam significantly increased [F (2.657, 18.60) = 4.447, p = 0.0189] progressive-ratio breakpoint (Figure 7b). Post hoc tests revealed that 3 mg/kg diazepam significantly increased breakpoint compared to saline control. Toluene (Figure 7c) also significantly increased breakpoint [F (2.462, 17.23) = 7.964, p = 0.0023]. Post hoc tests revealed that the 1360 ppm toluene concentration significantly increased breakpoint compared to air control. Nitrous oxide (Figure 6d) significantly decreased ICSS breakpoints [F (1.719, 10.32) = 5.400, p = 0.0282]. Post hoc tests revealed that the 40% and 80% nitrous oxide concentrations significantly (p < 0.05) decreased breakpoint compared to the air control.
Figure 7.
(a) Mean (±SEM) change in progressive-ratio breakpoint relative to saline control for 3, 10 and 18 mg/kg I.P. injected cocaine (b) Mean (±SEM) change in progressive-ratio breakpoint relative to saline control for 0.3, 1, 3 and 6 mg/kg I.P. injected diazepam (c) Mean change in progressive-ratio breakpoint relative to air control for exposure to 110, 480, 1360, and 2900 ppm toluene (d) Mean change in progressive-ratio breakpoint relative to air control for exposure to 20, 40 and 80% nitrous oxide. * denotes significant differences compared to saline control (p < 0.05).
Discussion
As expected, cocaine produced a robust dose-dependent facilitation of ICSS responding at all three tested doses (Figure 2a-c). These data are in agreement with previous literature examining cocaine treated C57BL/6J mice in the rate frequency ICSS procedure (Fish et al. 2010; Straub et al. 2010). The positive GABAA allosteric modulator diazepam (Figure 3a-d) produced a less robust biphasic effect on ICSS in the rate-frequency procedure. The 0.3 mg/kg diazepam dose did not alter ICSS, 1 and 3 mg/kg doses facilitated ICSS and the 10 mg/kg dose suppressed ICSS. Similarly, the 3 mg/kg dose of diazepam also significantly increased ICSS breakpoint in the progressive ratio procedure (Figure 7a). Our data are consistent with previous ICSS studies in mice that utilized a different curve-shift ICSS procedure. That study in C57BL/6J mice showed no effect of 0.3 mg/kg diazepam and a significant facilitation of ICSS by 1-4 mg/kg diazepam (Reynolds et al. 2012; Straub et al. 2010). Taken together these data confirm that diazepam reliably facilitates ICSS in mice across multiple ICSS procedure variants. Overall our data with cocaine and diazepam demonstrate that the three component rate-frequency and progressive ratio ICSS test conditions we employed are sensitive to the reward-facilitating effects of abused drugs.
The present data substantially extend the conditions under which it can be demonstrated that toluene will facilitate ICSS to include both the rate-frequency and progressive-ratio ICSS procedure variants. However, in contrast to cocaine which facilitated ICSS across the entire tested dose range, toluene produced a complex pattern of rate increasing and decreasing effects more comparable to our second positive control diazepam. Specifically, intermediate toluene concentrations facilitated ICSS in both the rate frequency and the PR procedure. Moderately high toluene concentrations produced a biphasic effect, facilitating ICSS at low frequencies and suppressing ICSS at high frequencies while having no effect on PR responding. The highest toluene concentration suppressed ICSS regardless of stimulation frequency in the rate-frequency procedure.
In ICSS, an enhancement of responding for stimulation following drug pretreatment is usually interpreted as a facilitation of brain reward circuitry (Wise et al. 1992). In contrast, a depression of ICSS responding by a test drug has multiple interpretations (Carlezon and Chartoff 2007). The first is that the pretreatment drug is producing anhedonic-like effects, reducing the subject's sensitivity to the reinforcing effects of brain stimulation. For example, it has been shown that the kappa-opioid receptor agonist U69,593 produces a characteristic anhedonic-like effect when administered alone as well as blocking the reinforcement enhancing effects of ICSS produced by cocaine (Tomasiewicz et al. 2008). It is unclear if toluene is producing anhedonic-like effects in the present study although it is possible given previous data demonstrating that extended exposure to 1650-3300 ppm toluene vapor for 4 hours can produce a conditioned taste aversion (Miyagawa et al. 1984). However, in a conditioned place preference study, a significant preference for an environment paired with a shorter 30 minutes of exposure to 2000 and 3000 ppm toluene was observed while exposure to 5000 ppm toluene failed to produce a place preference, possibly indicating the recruitment of anhedonic-like effects at this higher concentration (Lee et al. 2006). Interestingly, at the same 5000 ppm toluene concentration we observed a significant depression in responding in our ICSS rate-frequency procedure.
A second interpretation of depression of ICSS responding is that the test drug interferes with the ability of the subjects to physically perform the operant. For example, the sedative zolpidem has been shown to decrease operant performance in ICSS in the rate frequency procedure (Reynolds et al. 2012). It is certainly possible that this could be responsible for the suppression of ICSS at 5000 ppm toluene given that exposure to 15 min of 8000 ppm toluene vapor suppresses locomotor activity in C57BL/6J mice (Bowen et al. 2010). Exposure to concentrations of toluene vapor greater than 2000-3000 ppm suppress operant responding under fixed ratio as well as fixed interval schedules in mice (Moser and Balster 1985; Bowen and Balster 1998) suggesting that operant responding is even more sensitive to motor incoordination effect of toluene than locomotor activity. However, in the present study the effect of toluene at 3300 ppm was ICSS frequency dependent with responding at low ICSS frequencies increased by toluene whereas responding was suppressed at high ICSS frequencies. This effect could have been due to motor incoordination manifesting itself primarily at the high frequencies which supported the greatest rates of responding but it may also suggest a classic rate-dependent effect (Dews 1977). This latter interpretation is possible, although under fixed interval schedules for food maintained responding response-rate increasing effects of toluene in mice are either not apparent (Bowen and Balster 1998) or extremely modest (Moser and Balster 1986). Additional studies will be necessary to further explore these possibilities as they relate to ICSS performance.
The facilitation of ICSS by nitrous oxide was much less pronounced than that produced by toluene, diazepam or cocaine. In the rate-frequency procedure 40% nitrous oxide facilitated ICSS, although only significantly so at two intermediate stimulation frequencies (Figure 6b). The 60% and 80% nitrous oxide concentrations suppressed ICSS in the rate-frequency procedure and both 40% and 80% nitrous oxide suppressed PR responding. Prior preclinical studies examining the rewarding effects of nitrous oxide have also have generated conflicting results. Squirrel monkeys will self-administered a combination of 60% N2O / 40% O2 (Wood et al. 1977). However, in a rat place conditioning and self-administration study, repeated pairings of an environment with 40 minute of exposures to 30% and 60% nitrous oxide produced a conditioned place aversion (Ramsay et al. 2003) and inconsistent results in self-administration. Human self-administration and subjective tests of N2O exposure have been equally divergent among healthy subjects. In human subjects given the option to self-administer 30% N2O, oxygen vehicle or air, but blinded to the identity of their choice, 5 of 12 subjects consistently chose N2O, 4 consistently avoided N2O, and 3 subjects were divided between N2O and the oxygen/placebo alternatives (Walker and Zacny 2001). Taken together the present results and previous studies suggest that the rewarding effects of nitrous oxide at least as measured by self-administration, ICSS and conditioned place preference are at best fairly modest compared to other drugs of abuse including other inhalants such as toluene.
In summary, our findings indicate that toluene robustly facilitates progressive ratio ICSS breakpoint and enhances the reinforcing efficacy of low to mid-range frequencies of stimulation in the rate-frequency procedure. In contrast N2O only weakly facilitates ICSS responding in the rate-frequency procedure and produced a concentration dependent suppression of ICSS breakpoint. These results suggest that while demonstrating some reinforcement enhancing effects on brain reward systems, N2O may also produce effects which more strongly compete with behaviors necessary to progress in the PR procedure relative to rate-frequency schedule. In the rate-frequency procedure every lever-press results in some degree of stimulation, the production of interfering behaviors may have a much more limited effect on response output as compared to the progressive ratio procedure which requires successively greater numbers of responses in the absence of reinforcer presentation.
Given the relative difficulty of quantifying the reinforcing effects of inhaled substances using standard measures of drug reinforcement, developing alternative procedures is important for advancing our knowledge in the area. Our data with both toluene and nitrous oxide in mice suggest that ICSS is a viable method to probe the neurobiological mechanisms underlying the rewarding effects of abused inhalants.
Acknowledgments
Funding: Provided by National Institute of Drug Abuse grant R01DA-020553 and F31DA034469.
Footnotes
Conflict of Interest: none.
References
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Cruz SL, Howard MO, et al. Classification of abused inhalants. Addiction. 2009;104:878–882. doi: 10.1111/j.1360-0443.2008.02494.x. doi: 10.1111/j.1360-0443.2008.02494.x. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Sukhotina I, Medvedev I, et al. Facilitation of electrical brain self-stimulation behavior by abused solvents. Pharmacol Biochem Behav. 2003;75:199–208. doi: 10.1016/s0091-3057(03)00071-6. doi: 10.1016/S0091-3057(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, et al. Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABA(A) receptors. J Pharmacol Exp Ther. 2011;336:145–154. doi: 10.1124/jpet.110.171645. doi: 10.1124/jpet.110.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina EA, Dravolina OA, Bespalov AY, et al. Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol. 2004;485:211–218. doi: 10.1016/j.ejphar.2003.11.068. doi: 10.1016/j.ejphar.2003.11.068. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, et al. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3223-5. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol. 1998;6:235–247. doi: 10.1037//1064-1297.6.3.235. doi: 10.1037/1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH. Developmental toxicity of prenatal exposure to toluene. AAPS J. 2006;8:E419–424. doi: 10.1007/BF02854915. doi: 10.1007/BF02854915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Kimar S, Irtenkauf S. Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol Biochem Behav. 2010;95:249–257. doi: 10.1016/j.pbb.2010.01.014. doi: 10.1016/j.pbb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, McDonald P. Abuse pattern of toluene exposure alters mouse behavior in a waiting-for-reward operant task. Neurotoxicol Teratol. 2009;31:18–25. doi: 10.1016/j.ntt.2008.09.002. doi: 10.1016/j.ntt.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. Eur J Pharmacol. 1996;312:131–136. doi: 10.1016/0014-2999(96)00459-1. doi: 10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10:79–94. doi: 10.1080/105504901750160529. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chan M-H, Chung S-S, Stoker AK, et al. Sarcosine attenuates toluene-induced motor incoordination, memory impairment, and hypothermia but not brain stimulation reward enhancement in mice. Toxicol Appl Pharmacol. 2012;265:158–165. doi: 10.1016/j.taap.2012.10.004. doi: 10.1016/j.taap.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AM, Coalson DW, Klock PA, et al. The effects of alcohol history on the reinforcing, subjective and psychomotor effects of nitrous oxide in healthy volunteers. Drug Alcohol Depend. 1997;45:63–70. doi: 10.1016/s0376-8716(97)01346-x. [DOI] [PubMed] [Google Scholar]
- Collado V, Nicolas E, Faulks D, Hennequin M. A review of the safety of 50% nitrous oxide/oxygen in conscious sedation. Expert Opin Drug Saf. 2007;6:559–571. doi: 10.1517/14740338.6.5.559. doi: 10.1517/14740338.6.5.559. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Domínguez M. Misusing volatile substances for their hallucinatory effects: a qualitative pilot study with Mexican teenagers and a pharmacological discussion of their hallucinations. Subst Use Misuse. 2011;46(Suppl 1):84–94. doi: 10.3109/10826084.2011.580222. doi: 10.3109/10826084.2011.580222. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, et al. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- Depoortere, Perrault G, Sanger DJ. Intracranial self-stimulation under a progressive-ratio schedule in rats: effects of strength of stimulation, d-amphetamine, 7-OH-DPAT and haloperidol. Psychopharmacology (Berl) 1999;142:221–229. doi: 10.1007/s002130050883. doi: 10.1007/s002130050883. [DOI] [PubMed] [Google Scholar]
- Dews PB. Rate-dependency hypothesis. Science. 1977;198:1182–1183. doi: 10.1126/science.563103. doi: 10.1126/science.563103. [DOI] [PubMed] [Google Scholar]
- Elkoussi A, Bakheet S. Volatile substance misuse among street children in Upper Egypt. Subst Use Misuse. 2011;46(Suppl 1):35–39. doi: 10.3109/10826084.2011.580202. doi: 10.3109/10826084.2011.580202. [DOI] [PubMed] [Google Scholar]
- Emmanouil DE, Johnson CH, Quock RM. Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors. Psychopharmacology (Berl) 1994;115:167–172. doi: 10.1007/BF02244768. doi: 10.1007/BF02244768. [DOI] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, et al. Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol Clin Exp Res. 2010;34:81–89. doi: 10.1111/j.1530-0277.2009.01069.x. doi: 10.1111/j.1530-0277.2009.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Howard MO, Perron BE. Nitrous oxide inhalation among adolescents: prevalence, correlates, and co-occurrence with volatile solvent inhalation. J Psychoactive Drugs. 2009;41:337–347. doi: 10.1080/02791072.2009.10399771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SR, Palmer CA, Curé JK, et al. Toluene optic neurotoxicity: magnetic resonance imaging and pathologic features. Hum Pathol. 2011;42:295–298. doi: 10.1016/j.humpath.2010.08.005. doi: 10.1016/j.humpath.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Bowen SE. Reproductive toxicology and teratology of abused toluene. Syst Biol Reprod Med. 2010;56:184–200. doi: 10.3109/19396360903377195. doi: 10.3109/19396360903377195. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier G, Zieglgänsberger W, Haseneder R, et al. Nitrous oxide and xenon increase the efficacy of GABA at recombinant mammalian GABA(A) receptors. Anesth Analg. 2000;91:1542–1549. doi: 10.1097/00000539-200012000-00045. doi: 10.1097/00000539-200012000-00045. [DOI] [PubMed] [Google Scholar]
- Hoet P, Lison D. Ototoxicity of toluene and styrene: state of current knowledge. Crit Rev Toxicol. 2008;38:127–170. doi: 10.1080/10408440701845443. doi: 10.1080/10408440701845443. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Lee DE, Gerasimov MR, Schiffer WK, Gifford AN. Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. Drug Alcohol Depend. 2006;85:87–90. doi: 10.1016/j.drugalcdep.2006.03.013. doi: 10.1016/j.drugalcdep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Lin R-J, Chen H-F, Chang Y-C, Su J-J. Subacute combined degeneration caused by nitrous oxide intoxication: case reports. Acta Neurol Taiwan. 2011;20:129–137. [PubMed] [Google Scholar]
- Lo P-S, Chen H-H. Immunohistochemical localization of toluene-induced c-Fos protein expression in the rat brain. Toxicology Letters. 2005;157:151–160. doi: 10.1016/j.toxlet.2005.01.014. doi: 16/j.toxlet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Miyagawa M, Honma T, Sato M, Hasegawa H. Conditioned taste aversion induced by toluene administration in rats. Neurobehav Toxicol Teratol. 1984;6:33–37. [PubMed] [Google Scholar]
- Moser VC, Balster RL. Effects of toluene, halothane and ethanol vapor on fixed-ratio performance in mice. Pharmacol Biochem Behav. 1985;22:797–802. doi: 10.1016/0091-3057(85)90530-1. doi: 10.1016/0091-3057(85)90530-1. [DOI] [PubMed] [Google Scholar]
- Moser VC, Balster RL. The effects of inhaled toluene, halothane, 1,1,1-trichloroethane, and ethanol on fixed-interval responding in mice. Neurobehav Toxicol Teratol. 1986;8:525–531. [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. 8th ed. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; San Diego: 2001. [Google Scholar]
- Perit KE, Gmaz JM, Caleb Browne JD, et al. Distribution of c-Fos immunoreactivity in the rat brain following abuse-like toluene vapor inhalation. Neurotoxicol Teratol. 2012;34:37–46. doi: 10.1016/j.ntt.2011.10.007. doi: 10.1016/j.ntt.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Watson CH, Leroux BG, et al. Conditioned place aversion and self-administration of nitrous oxide in rats. Pharmacol Biochem Behav. 2003;74:623–633. doi: 10.1016/s0091-3057(02)01048-1. doi: 10.1016/S0091-3057(02)01048-1. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Engin E, Tantillo G, et al. Differential roles of GABA(A) receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology. 2012;37:2531–2540. doi: 10.1038/npp.2012.115. doi: 10.1038/npp.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C, Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Vis Exp. 2012:e3754. doi: 10.3791/3754. doi: 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL. Discriminative stimulus effects of inhaled 1,1,1-trichloroethane in mice: comparison to other hydrocarbon vapors and volatile anesthetics. Psychopharmacology (Berl) 2009;203:431–440. doi: 10.1007/s00213-008-1380-8. doi: 10.1007/s00213-008-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Nicholson KL. GABAA-positive modulator selective discriminative stimulus effects of 1,1,1-trichloroethane vapor. Drug Alcohol Depend. 2012;121:103–109. doi: 10.1016/j.drugalcdep.2011.08.016. doi: 10.1016/j.drugalcdep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Nicholson KL. GABA(A) positive modulator and NMDA antagonist-like discriminative stimulus effects of isoflurane vapor in mice. Psychopharmacology (Berl) 2010;212:559–569. doi: 10.1007/s00213-010-1979-4. doi: 10.1007/s00213-010-1979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub CJ, Carlezon WA, Jr, Rudolph U. Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav Brain Res. 2010;206:17–20. doi: 10.1016/j.bbr.2009.08.025. doi: 10.1016/j.bbr.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Tatum WO, Bui DD, Grant EG, Murtagh R. Pseudo-guillain-barre syndrome due to “whippet”-induced myeloneuropathy. J Neuroimaging. 2010;20:400–401. doi: 10.1111/j.1552-6569.2009.00388.x. doi: 10.1111/j.1552-6569.2009.00388.x. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, et al. The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers. Drug Alcohol Depend. 2001;64:85–96. doi: 10.1016/s0376-8716(00)00234-9. doi: 10.1016/S0376-8716(00)00234-9. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Bitonic dose-response functions for reinforcing and self-reported effects of nitrous oxide in humans. Pharmacol Biochem Behav. 2003;74:851–857. doi: 10.1016/s0091-3057(03)00015-7. [DOI] [PubMed] [Google Scholar]
- Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13(Suppl 1):213–223. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Wood RW, Grubman J, Weiss B. Nitrous oxide self-administration by the squirrel monkey. J Pharmacol Exp Ther. 1977;202:491–499. [PubMed] [Google Scholar]
- Yavich L, Zvartau E. A comparison of the effects of individual organic solvents and their mixture on brain stimulation reward. Pharmacol Biochem Behav. 1994;48:661–664. doi: 10.1016/0091-3057(94)90328-x. doi: 10.1016/0091-3057(94)90328-X. [DOI] [PubMed] [Google Scholar]
- Yücel M, Takagi M, Walterfang M, Lubman DI. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci Biobehav Rev. 2008;32:910–926. doi: 10.1016/j.neubiorev.2008.01.006. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Walker DJ, Derus LM. Choice of nitrous oxide and its subjective effects in light and moderate drinkers. Drug Alcohol Depend. 2008;98:163–168. doi: 10.1016/j.drugalcdep.2008.06.001. doi: 10.1016/j.drugalcdep.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]