Abstract
Physiologic changes during pregnancy alter the pharmacokinetics, safety, and efficacy of many drugs. For clinicians, there is often uncertainty regarding the safety of these drugs due to a scarcity of published data. This study aimed to comprehensively evaluate the characteristics and publication patterns of obstetric studies registered in ClinicalTrials.gov from 2007-2012. Primary outcome measures, funding sources, inclusion criteria, and the reporting of study results were evaluated. A manual review of Medline/PubMed was performed to identify publications associated with studies registered in ClinicalTrials.gov. Of 93,709 total studies, there were 5,203 (6%) obstetric studies registered in ClinicalTrials.gov. Interventional studies accounted for 70% and 30% were observational. Clinical trials of drugs (49%), procedures (13%), and behavioral interventions (12%) were most common. Among interventional drug trials, 84% featured randomized allocation to study arms and 93% included measures of safety and/or efficacy as primary endpoints. Of 946 (18%) studies completed more than two years ago, only 11% had reported results and less than 7% had been published. In an area with a great need for evidence of safe and effective therapies, the low publication rate of completed studies incorporating elements of high-quality trial design is concerning. The sources of this trend should be closely investigated.
Keywords: pregnancy, children, obstetric, pharmacology, infant
INTRODUCTION
Many pregnant women have medical conditions that require treatment with prescription medications and nearly all women take at least one prescription drug or supplement during the course of their pregnancy.1-3 More than two-thirds of women take a prescription drug other than their antenatal vitamins during pregnancy.4 Despite these statistics, few studies have sought to delineate those medications that are safe for use during pregnancy and those that should be avoided.5 The great majority of medications enter the market with no data to support their use among pregnant women.6-8
Pregnant women have historically been excluded from clinical trials, primarily due to concerns regarding the safety of early fetal exposure to investigational drugs.9 As a result, pregnant women have become “therapeutic orphans”, severely limiting the ability of obstetricians to practice evidence-based prescribing.10, 11 Recognition of this data shortage has led to regulatory changes over the last two decades.12, 13 Since 1993, the U.S. National Institutes of Health (NIH) has recommended that pregnant women be included in clinical trials, provided that there is not a compelling physiologic reason for their exclusion.14 However, despite these calls for clinical trials involving pregnant women, only one drug (hydroxyprogesterone caproate) has been approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012.15
Although data on the safety of drugs prescribed to pregnant women are scarce, the teratogenic effects of thalidomide on limb formation, alcohol on development of the central nervous system, and diethylstilbestrol on genital development serve as cautionary reminders of the imperative to study the safety of drugs used in pregnant women.16-18 This study examines the characteristics of obstetric studies registered within a national clinical trials database from 2007-2012. As a secondary objective, we sought to evaluate the reporting of study results and patterns of publication among completed trials.
MATERIALS AND METHODS
Selection of Obstetric Studies
ClinicalTrials.gov is a publicly-available registry of clinical research studies that is maintained by the U.S. National Library of Medicine.19 The registry includes data on federally-funded and privately-sponsored observational and interventional studies. As of mid-2013, there were more than 145,000 studies on a wide array of diseases and conditions registered in ClinicalTrials.gov.20
A query of ClinicalTrials.gov was performed using a registry search function with any of the following key words, including: “obstetric”, “maternal”, “fetal”, “pregnancy”, “pregnant”, “congenital”, “prenatal”, “antenatal”, or “teratogen”. To coincide with the enactment of a federal law in 2007 that mandated the registration and reporting of study results for phase 2-4 interventional studies involving drugs, biological agents, and medical devices,21 we selected studies registered between 01 October 2007 and 31 December 2012 for analysis. No restrictions were applied on the basis of study inclusion / exclusion criteria or the availability of study results. All data were downloaded on 08 June 2013.
Study Characteristics and Quality Indicators
Data elements extracted from ClinicalTrials.gov included: a unique trial identifier, study title, recruitment status, condition(s) studied, primary purpose of the study, interventional or observational status, interventional type (if appropriate), primary funding source, age group and gender eligibility criteria, trial phase (0-4), anticipated enrollment size, study design, primary endpoint, blinding status, and the availability of study results. Primary funding sources were classified as government, industry, or non-profit according to methods described by Bourgeois et al.22
Analysis of Clinical Trial Publications
Publications of registered obstetric studies were identified using methods adapted from Ross et al.23 Briefly, to provide a minimum of at least two years for investigators to analyze their data, write a manuscript, and have the paper published, we identified obstetric studies that were completed by 31 December 2010. Publications registered in ClinicalTrials.gov were accessed by reviewing the “publications” field in ClinicalTrials.gov. Citations provided by the study sponsors and those that were automatically indexed to the trial registry by the ClinicalTrials.gov identifier (NCT Number) were reviewed. If more than one publication was identified, all listed publications were reviewed. For trials that were completed and did not have any publications indexed in ClinicalTrials.gov, we manually searched Medline / PubMed with the condition studied, the intervention studied, and the name of the principal investigator. All searches were updated and finalized as of 08 June 2013.
Statistical Analyses
Descriptive statistics were used to characterize the studies extracted from the ClinicalTrials.gov registry. Comparisons between study types were conducted using the χ2-test or Fisher’s exact test, as appropriate. Continuous variables were compared with non-parametric Wilcoxon-Mann-Whitney tests. All statistical analyses were performed using Stata 11.2 (StataCorp LP, College Station, TX, USA).
RESULTS
Study Characteristics
From October of 2007 through December of 2012 there were 93,709 studies registered in ClinicalTrials.gov. Of these, 5203 (6%) were identified in our search for obstetric studies. Overall, 38% of these obstetric studies are actively recruiting participants, 34% have been completed, and 12% are not yet actively recruiting (Table 1). A majority (62%) of these studies involve research on treatments, followed by research on prevention (23%), diagnostics (4%), supportive care (4%), and basic science investigations (3%). Interventional studies accounted for 70% of obstetric studies, 30% were observational, and <1% were expanded-access trials. Among interventional studies, research involving drugs (49%), procedures (13%), and behavioral interventions (12%) were most common (Table 2). Studies investigating medical devices (9%), dietary supplements (6%), and biological agents (6%) were less common. The median number of estimated study participants was 103 (interquartile range [IQR]: 42-300).
Table 1.
Characteristics of obstetreic studies registered in ClinicalTrials.gov from 2007-2012.
| Characteristic | Category | All Studies (n = 5,203) |
|---|---|---|
| Recruitment Status, n (%) | Recruiting | 1,972 (38%) |
| Completed | 1,766 (34%) | |
| Active, not recruiting | 625 (12%) | |
| Not yet recruiting | 378 (7%) | |
| Terminated | 217 (4%) | |
| Enrolling by invitation | 131 (3%) | |
| Withdrawn | 73 (1%) | |
| Suspended | 34 (1%) | |
| Study Design, n (%) | Interventional | 3,655 (70%) |
| Observational | 1,541 (30%) | |
| Expanded access | 7 (<1%) | |
| Primary Purpose, n (%) | Treatment | 2,138 (62%) |
| Prevention | 807 (23%) | |
| Diagnostic | 140 (4%) | |
| Supportive care | 131 (4%) | |
| Basic science | 111 (3%) | |
| Health services research | 108 (3%) | |
| Screening | 28 (1%) |
Table 2.
Study design characteristics of interventional obstetric studies registered in ClinicalTrials.gov.
| Characteristic | Category | Interventional Studies (n = 3,655) |
|---|---|---|
| Intervention, n (%) * | Drug | 1,807 (49%) |
| Other | 597 (16%) | |
| Procedure | 484 (13%) | |
| Behavioural change | 446 (12%) | |
| Device | 341 (9%) | |
| Dietary supplement | 215 (6%) | |
| Biologic | 207 (6%) | |
| Allocation status, n (%) | Randomized | 2,577 (71%) |
| Non-randomized | 459 (13%) | |
| Unknown / missing | 619 (17%) | |
| Blinding, n (%) | Open | 2050 (56%) |
| Single blind | 509 (14%) | |
| Double blind | 1,058 (29%) | |
| Unknown / missing | 38 (1%) | |
| Interventional group, n (%) | Single group | 1,001 (27%) |
| Parallel | 2,388 (65%) | |
| Cross-over | 152 (4%) | |
| Factorial | 77 (2%) | |
| Unknown / missing | 37 (1%) | |
| Endpoint classification, n (%) | Bioavailability | 13 (0%) |
| Bioequivalence | 24 (1%) | |
| Efficacy | 1,370 (37%) | |
| Pharmacokinetics and/or pharmacodynamics | 104 (3%) | |
| Safety | 205 (6%) | |
| Safety / efficacy | 1,294 (35%) | |
| Unknown / missing | 645 (18%) | |
| Study phase, n (%) | Phase 0, 1, 1/2 | 476 (13%) |
| Phase 2, 2/3 | 622 (17%) | |
| Phase 3, 4 | 1,037 (28%) | |
| Unknown / missing | 1,520 (42%) | |
| Expected sample size, median (IQR) | 100 (40 – 266) | |
| Lead funding source, n (%) | Industry | 568 (16%) |
| Government | 153 (4%) | |
| Non-profit | 2,886 (79%) | |
| Unknown / missing | 48 (1%) |
NOTE: Cumulative percentage exceeds 100% due to studies classified in multiple interventional categories.
Of 3655 interventional obstetric studies, 1807 (49%) investigated drugs. Among these interventional drug trials, 84% featured randomized allocation to study arms and 93% included measures of safety and/or efficacy as primary endpoints. The majority of interventional drug trials focused on therapeutics (75%). Prevention (15%) and basic science (2%) investigations were less commonly registered. The median number of estimated participants was 76 (IQR: 30-200). Phase 3 studies accounted for (39%) of interventional drug trials. Overall, 73% of interventional drug trials were sponsored primarily by non-profit organizations (including universities and medical centers), 20% by industry, 5% by the NIH, and <1% by other governmental agencies.
Availability of Study Results
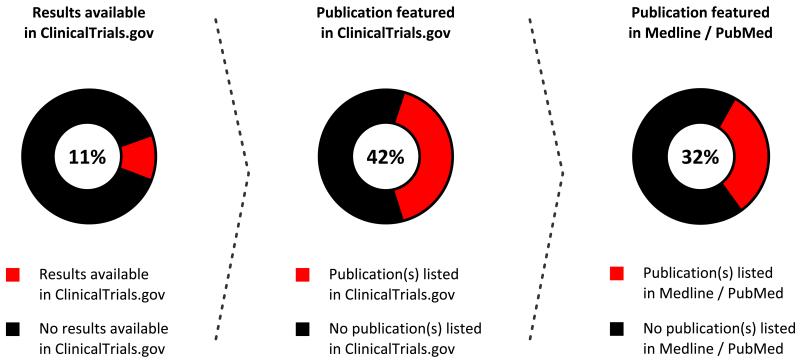
Completed studies accounted for 34% of the total number of obstetric studies registered on ClinicalTrials.gov. Of these, 946 (54%) were completed more than two years before our search for study results and publications. A minority (11%) of these completed studies had reported the results of preliminary or final analyses on ClinicalTrials.gov (Figure 1). Slightly less than half of the studies with results available also had one or more publications indexed on ClinicalTrials.gov. A manual review of Medline / PubMed found an additional 20 (32%) studies that had published their results and were not associated with their ClinicalTrials.gov registry entry. The combination of these two methods resulted in the identification of a published article in association with 65 (60%) of the 108 studies that reported results in ClinicalTrials.gov. However, this represents only 7% of the 946 trials completed more than two years before our search. Publication rates were similar among studies that enrolled mother-infant pairs (8%) when compared to studies that enrolled pregnant women exclusively (6%) (P = 0.4).
Figure 1.
Reporting and publication of obstetric study results in ClinicalTrials.gov and Medline / PubMed.
DISCUSSION
Fetal exposure to most maternally-administered drugs has not been well studied.24 As a result, there is a pressing need to conduct obstetric studies that can serve to inform prescribing patterns for pregnant women and their unborn children.7 Although obstetric studies accounted for less than 10% of the overall number of trials registered in ClinicalTrials.gov, more than 80% incorporated elements of high-quality trial design, including randomization and safety/efficacy endpoints. However, there was a large discrepancy between the number of completed obstetric studies and the reporting of their study results and subsequent publication. Fewer than 1 in 10 obstetric studies completed more than two years ago have been published.
Human teratogens, such as thalidomide and rubella, can exert markedly different effects on the developing fetus.25, 26 In the clinical setting, there is no direct method for determining the extent of fetal exposure to a drug.24 However, factors that define the extent of fetal exposure are likely to include: the concentration-time profile of the drug in the maternal circulation; the size, solubility, ionization, and protein binding of the drug; transfer of the drug in and out of the placental circulation; the extent of placental first-pass metabolism; and the rate of fetal clearance.24 Furthermore, physiological changes that occur throughout pregnancy, such as increased plasma volume and variations in protein binding, are known to alter the pharmacokinetic properties of many commonly prescribed medications.27 All of these factors have the potential to influence the safety and efficacy of maternally-administered drugs at different gestational ages.28 However, the indiscriminate withholding of certain medications could pose more risk to the fetus than prescribing the drug.29 Regardless, data obtained from well-designed, high-quality studies will enable clinicians to make informed decisions about prescribing medications during pregnancy.11
Defining the quality of clinical studies is challenging, owing to varying study designs, multiple stakeholders, debate regarding the selection of appropriate endpoints, and many other factors.30 To address this issue, the Institute of Medicine held a Roundtable on Research and Development of Drugs, Biologics, and Medical Devices, in which they defined “high-quality data” as “data strong enough to support conclusions and interpretations equivalent to those derived from error-free data”.31 In the current study, 70% of obstetric studies registered in ClinicalTrials.gov were interventional trials. More than 80% of these interventional trials incorporated a randomization procedure, which compares favorably with the 69% randomization rate reported among all 40,970 interventional trials registered in ClinicalTrials.gov from 2007-2010.32 Among pediatric trials, 86% were classified as safety and/or efficacy studies, which is similar to the 93% reported here among interventional obstetric trials.22 In aggregate, these findings suggest that obstetric studies are of a similar or higher quality than studies conducted solely among adults or children.
The primary obligation of study sponsors and investigators is to their study participants, in this case pregnant women, who provided informed consent to participate in an experiment designed to create generalizable knowledge.33 A second obligation is to their clinical colleagues who make medical decisions regarding the care of pregnant women based on evidence from high-quality studies, often in the form of peer-reviewed publications.34 In this study, we evaluated obstetric studies that had been completed for more than two years to allow for the preparation of data for analysis, writing of the manuscript, and revisions during the peer-review process. Among nearly a thousand completed studies that met this definition, less than 7% had been published in a journal indexed on Medline / PubMed. A slightly larger proportion (11%) provided a summary of their results in ClinicalTrials.gov, which is a complementary non-peer-reviewed method of results reporting that is publicly accessible. These statistics provide a compelling argument to bolster the timely reporting of study results, which are needed to accurately characterize the balance of risks and benefits associated with obstetric therapies.
A limitation of this study is that ClinicalTrials.gov does not include all clinical research studies conducted in the U.S., as the legal requirements for the registration and reporting of results do not extend to Phase 0-1 trials or to non-interventional studies.32 Moreover, the accuracy, validity, and completeness of the data entered in ClinicalTrials.gov depend upon self-reporting by study sponsors and investigators.19 However, ClinicalTrials.gov uses an automated alert system to notify sponsors when required data fields are missing or internally inconsistent.35 Additionally, it was not possible to distinguish studies that were actively recruiting participants from those that have completed their enrollment and are conducting long-term follow-up. In ClinicalTrials.gov both of these groups are classified as “recruiting”. Lastly, it was not possible to evaluate the conditions or diseases investigated in these obstetric studies.
CONCLUSION
The safety and effectiveness of many medications administered to pregnant women have not been systematically investigated.7 This paucity of data routinely forces clinicians to prescribe, or withhold, medications from pregnant women without a clear idea of how they may affect the fetus. To meet this need, more than 5000 obstetric studies have been registered within ClinicalTrials.gov. A majority of these studies are interventional trials of drugs or biologics, most of which were randomized and featured one or more measures of safety and/or efficacy as a primary or secondary endpoint. However, among studies completed more than two years ago, only 11% had reported their results in the ClinicalTrials.gov registry and less than 7% had been published. Additional steps are warranted to encourage the publication of these studies, which fulfills critical obligations to study participants and the medical community. Dissemination of these findings is essential to create a healthcare system that embraces research and continuously improves the quality of care delivered to pregnant women and their unborn children.
Acknowledgements
None.
Funding Disclosure: This work was supported by a grant from the Primary Children’s Hospital Foundation (to CMTS). There was no involvement by study sponsors in the: (1) design of the study; (2) collection, analysis, and interpretation of the data; (3) the writing of the paper; and (4) the decision to submit the paper for publication.
Abbreviations
- FDA
United States Food and Drug Administration
- NIH
National Institutes of Health
Footnotes
Presented in Part: Pediatric Academic Societies’ (PAS) Annual Meeting, Washington DC, USA, May 4-7, 2013 (publication number 3820.298).
Author Contributions: Mr. Stockmann had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- Study Concept and Design: Stockmann
- Acquisition of Data: Stockmann
- Analysis and Interpretation of the Data: Stockmann, Sherwin, Koren, Campbell, Constance, Linakis, Balch, Varner, Spigarelli
- Drafting of the Manuscript: Stockmann, Campbell, Constance, Linakis
- Critical Revision of the Manuscript for Important Intellectual Content: Stockmann, Sherwin, Koren, Campbell, Constance, Linakis, Balch, Varner, Spigarelli
- Statistical Analysis: Stockmann
- Obtained Funding: Sherwin, Spigarelli
- Administrative, Technical, or Material Support: Sherwin, Balch, Varner, Spigarelli
- Study Supervision: Spigarelli
REFERENCES
- 1.Koren G, Pastuszak A, Ito S. Drugs in pregnancy. The New England journal of medicine. 1998;338(16):1128–1137. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- 2.Lee E, Maneno MK, Smith L, et al. National patterns of medication use during pregnancy. Pharmacoepidemiology and drug safety. 2006;15(8):537–545. doi: 10.1002/pds.1241. [DOI] [PubMed] [Google Scholar]
- 3.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. American journal of obstetrics and gynecology. 2004;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Headley J, Northstone K, Simmons H, Golding J. Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. European journal of clinical pharmacology. 2004;60(5):355–361. doi: 10.1007/s00228-004-0775-7. [DOI] [PubMed] [Google Scholar]
- 5.Lo WY, Friedman JM. Teratogenicity of recently introduced medications in human pregnancy. Obstetrics and gynecology. 2002;100(3):465–473. doi: 10.1016/s0029-7844(02)02122-1. [DOI] [PubMed] [Google Scholar]
- 6.Mastoianni AC, Faden R, Federman D. Women and health research: a report from the Institute of Medicine. Kennedy Inst Ethics J. 1994;4:55–62. doi: 10.1353/ken.0.0121. [DOI] [PubMed] [Google Scholar]
- 7.Zajicek A, Giacoia GP. Obstetric clinical pharmacology: coming of age. Clinical pharmacology and therapeutics. 2007;81(4):481–482. doi: 10.1038/sj.clpt.6100136. [DOI] [PubMed] [Google Scholar]
- 8.Baylis F. Pregnant women deserve better. Nature. 2010;465(7299):689–690. doi: 10.1038/465689a. [DOI] [PubMed] [Google Scholar]
- 9.National Research Act . 1974. Vol. Public Law 93-348. [Google Scholar]
- 10.Doering PL. Drug use during pregnancy. National Association of Retail Druggists J. 1992:53–57. [Google Scholar]
- 11.Doering PL, Boothby LA, Cheok M. Review of pregnancy labeling of prescription drugs: is the current system adequate to inform of risks? American journal of obstetrics and gynecology. 2002;187(2):333–339. doi: 10.1067/mob.2002.125740. [DOI] [PubMed] [Google Scholar]
- 12.Boothby LA, Doering PL. FDA labeling system for drugs in pregnancy. The Annals of pharmacotherapy. 2001;35(11):1485–1489. doi: 10.1345/aph.1A034. [DOI] [PubMed] [Google Scholar]
- 13.Teratology public affairs committee position paper: Pregnancy labeling for prescription drugs: Ten years later. Birth defects research Part A, Clinical and molecular teratology. 2007;79(9):627–630. doi: 10.1002/bdra.20389. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health Revitalization Act of 1993 . Science and Technology. 1993. Vol. Public Law 103-43. [Google Scholar]
- 15.Endicott S, Haas DM. The current state of therapeutic drug trials in pregnancy. Clinical pharmacology and therapeutics. 2012;92(2):149–150. doi: 10.1038/clpt.2012.81. [DOI] [PubMed] [Google Scholar]
- 16.Ward SP. Thalidomide and congenital abnormalities. British medical journal. 1962;2(5305):646–647. doi: 10.1136/bmj.2.5305.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson JW, Jones KL, Smith DW. Fetal alcohol syndrome. Experience with 41 patients. JAMA: the journal of the American Medical Association. 1976;235(14):1458–1460. [PubMed] [Google Scholar]
- 18.Gill WB, Schumacher GF, Bibbo M, Straus FH, 2nd, Schoenberg HW. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. The Journal of urology. 1979;122(1):36–39. doi: 10.1016/s0022-5347(17)56240-0. [DOI] [PubMed] [Google Scholar]
- 19.Zarin DA, Tse T, Ide NC. Trial Registration at ClinicalTrials.gov between May and October 2005. The New England journal of medicine. 2005;353(26):2779–2787. doi: 10.1056/NEJMsa053234. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. National Institutes of Health [Accessed 28 June 2013];The National Library of Medicine. ClinicalTrials.gov. at: http://clinicaltrials.gov/
- 21.Tse T, Williams RJ, Zarin DA. Reporting “basic results” in ClinicalTrials.gov. Chest. 2009;136(1):295–303. doi: 10.1378/chest.08-3022. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JP, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. doi: 10.1136/bmj.d7292. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimmer BP, Parker KL. Contraception and pharmacotherapy of obstetrical and gynecological disorders. In: Brunton LL, Blumenthal DK, Murri N, Dandan RH, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th Edition McGraw-Hill; New York: 2011. [Google Scholar]
- 25.Froster UG, Baird PA. Maternal factors, medications, and drug exposure in congenital limb reduction defects. Environmental health perspectives. 1993;101(Suppl 3):269–274. doi: 10.1289/ehp.93101s3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dally A. Thalidomide: was the tragedy preventable? Lancet. 1998;351(9110):1197–1199. doi: 10.1016/S0140-6736(97)09038-7. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clinical pharmacokinetics. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 28.Loebstein R, Koren G. Clinical relevance of therapeutic drug monitoring during pregnancy. Therapeutic drug monitoring. 2002;24(1):15–22. doi: 10.1097/00007691-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kutcher JS, Engle A, Firth J, Lamm SH. Bendectin and birth defects. II: Ecological analyses. Birth defects research Part A, Clinical and molecular teratology. 2003;67(2):88–97. doi: 10.1002/bdra.10034. [DOI] [PubMed] [Google Scholar]
- 30.Kleppinger CF, Ball LK. Building quality in clinical trials with use of a quality systems approach. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51(Suppl 1):S111–116. doi: 10.1086/653058. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine [Accessed 12 June 2013];Assuring data quality and validity in clinical trials for regulatory decision making: workshop report. 1999 from: http://www.nap.edu/openbook.php?record_id=9623. [PubMed]
- 32.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA: the journal of the American Medical Association. 2012;307(17):1838–1847. doi: 10.1001/jama.2012.3424. ClinicalTrials.gov [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration [Accessed 12 June 2013];Comparison of FDA and HHS human subject protection regulations. 46.102. Definitions. Available at: http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/educationalmaterials/ucm112910.htm.
- 34.Califf RM. Better late than never: a welcome publication of tardy clinical trial results. JACC: Heart Failure. 2013;1(2):112–114. doi: 10.1016/j.jchf.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. The New England journal of medicine. 2011;364(9):852–860. doi: 10.1056/NEJMsa1012065. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]