Abstract
Measures of central pain processing like conditioned pain modulation (CPM), and suprathreshold heat pain response (SHPR) have been described to assess different components of central pain modulatory mechanisms. Central pain processing potentially play a role in the development of postsurgical pain, however, the role of CPM and SHPR in explaining postoperative clinical pain and disability is still unclear.
Seventy eight patients with clinical shoulder pain were included in this study. Patients were examined before shoulder surgery, at 3 months, and 6 months after surgery. The primary outcome measures were pain intensity and upper extremity disability. Analyses revealed that the change score (baseline – 3 months) of 5th pain rating of SHPR accounted for a significant amount of variance in 6 month postsurgical clinical pain intensity and disability after age, sex, preoperative pain intensity, and relevant psychological factors were considered.
The present study suggests that baseline measures of central pain processing were not predictive of 6 month postoperative pain outcome. Instead, the 3 month change in SHPR might be a relevant factor in the transition to elevated 6-month postoperative pain and disability outcomes.
In patients with shoulder pain, the 3 month change in a measure of central pain processing might be a relevant factor in the transition to elevated 6-month postoperative pain and disability scores.
Keywords: Conditioned pain modulation, suprathreshold heat pain response, postoperative pain, disability, shoulder surgery
INTRODUCTION
There has been growing evidence that chronic postsurgical pain is a major problem. 1-3 However, little is known about the factors that mark the transition from “normal” acute pain after surgery to chronic postsurgical pain. It is thought that certain patients may be at greater risk for the development of pain after surgery than others, where multiple potential predictive factors for persistent postsurgical pain such as age, sex, neurophysiological factors, preoperative pain and psychological factors have been described. 3-6
Chronic pain states are commonly associated with alterations in the central processing of noxious stimuli, and may share similar alterations in mechanisms of central pain processing. 7-11 Specific measures of central pain processing have been described to assess different components of central pain modulatory mechanisms. Conditioned pain modulation (CPM) represents a reduction in the magnitude of one stimulus in response to a second stimulus site;12, 13 therefore, it is believed to be an indication of endogenous pain inhibition or an “inhibitory” measure. In contrast, temporal summation of suprathreshold heat pain response (SHPR) represents the perception of increased pain despite constant or even reduced peripheral afferent input,8, 14 therefore, it is considered to be a manifestation of enhanced central excitability or a “facilitatory” measure. These measures represent two constructs whose balance might influence the risk for chronic pain development, but are not often studied together. For example, the combination of high pain sensitivity (higher “facilitatory” measure) and low endogenous pain inhibition (lower “inhibitory” measure) may confer the greatest risk for clinical pain conditions. 15-17
Recent research has focused on the assessment of experimental pain sensitivity as a predictor for persistent postsurgical pain;6, 18, 19 however the question of order is still unanswered by these studies. There are two likely scenarios with direct relevance to the current study. Patients with chronic postoperative pain may 1) experience elevated facilitation and decreased inhibition at baseline or 2) develop detrimental changes in facilitation and inhibition during the postoperative course. The present study extends our previous studies 20, 21 by determining whether changes between preoperative and postoperative assessment of SHPR and CPM adds variance in predicting postoperative pain reports and disability after consideration of established predictive factors for persistent postsurgical pain (such as age, sex, preoperative pain, and relevant psychological factors).22-27
We hypothesized that changes in SHPR and CPM will contribute to predicting postoperative clinical pain and disability after potential predictive factors are considered in the model. If this hypothesis is supported it will establish these 2 measures of central pain processing as unique contributors to postoperative pain intensity, and provide further indication of how they are potentially involved in the transition from acute to chronic post-operative pain states.
MATERIALS AND METHODS
Participants
The University’s institutional review board for human participants approved this study. This prospective design includes data from consecutive patients seeking operative treatment of shoulder pain where procedures were limited to arthroscopic procedures affecting the glenohumeral joint or clavicle. Specific operative procedures included rotator cuff repair, adhesive capsulitis, acromioplasty, and labral repair (Table 1). Patients were recruited from University of Florida’s Orthopedics Sports Medicine Institute (OSMI) by participating physicians, and provided informed consent before participating in this study.
Table 1.
Demographic characteristics and summary of medical history for the sample
| Subject’s characteristics | Improved Group (pain) N=59 Mean (SD) |
Not improved Group (pain) N=14 Mean (SD) |
P- Value |
Improved Group (disability) N=55 Mean (SD) |
Not improved Group (disability) N=23 Mean (SD) |
P- Value |
|---|---|---|---|---|---|---|
| Age | 45.07 (19.24) | 48(17.86) | 0.61 | 43.25 (17.98) | 51.35 (20.73) | 0.09 |
| Gender | 0.91 | 0.17 | ||||
| - Male | 43 (72.9%) | 10 (71.4%) | 37 (67.3%) | 19 (82.6%) | ||
| - Female | 16 (27.1%) | 4 (28.6%) | 18 (32.7%) | 4 (17.4%) | ||
| Dominant Side | 0.81 | 0.62 | ||||
| - Right | 52 (88.1%) | 12 (85.7) | 48 (87.3%) | 21 (91.3%) | ||
| - Left | 7 (11.9%) | 2 (14.3) | 7 (12.7%) | 2 (8.7%) | ||
| Ethnicity | 0.73 | 0.22 | ||||
| - Hispanic or Latino | 5 (8.5%) | 0 | 5 (9.1%) | 0 | ||
| - Non Hispanic or Latino | 51 (86.4%) | 14 (100%) | 48 (87.3%) | 22 (95.7%) | ||
| - Unknown or not reported | 3 (5.1%) | 0 | 2 (3.6%) | 1 (4.3%) | ||
| Race | 0.56 | 0.80 | ||||
| - Asian | 0 | 0 | 0 | 0 | ||
| - Native Hawaiian or Other Pacific Islander |
0 | 0 | 0 | 0 | ||
| - Black or African American |
3 (5.1%) | 1 (7.1%) | 4 (7.3%) | 0 | ||
| - White | 50 (84.7%) | 12 (85.7%) | 45 (81.8%) | 22 (95.7%) | ||
| - More Than One Race | 4 (6.8%) | 1 (7.1%) | 4 (7.3%) | 1 (4.3%) | ||
| - Unknown or Not Reported |
2 (3.4%) | 0 | 2 (3.6%) | 0 | ||
| Surgery side | 0.85 | 0.28 | ||||
| - Right | 32 (54.2%) | 8 (57.1%) | 26 (47.3%) | 14 (60.9%) | ||
| - Left RPI | 27 (45.8%) | 6 (42.9%) | 29 (52.7%) | 9 (39.1%) | ||
| - Baseline | 3.61 (2.28) | 2.48 (1.96) | 0.09 | 4.04 (2.18) | 1.77 (1.49) | <0.001 |
| - 3 months after surgery | 1.54 (1.56) | 2.29 (2.03) | 0.13 | 1.69 (1.49) | 1.71 (2.00) | 0.98 |
| - 6 months after surgery | 0.80 (0.90) | 2.81 (2.12) | <0.001 | 1.15 (1.45) | 1.39 (1.45) | 0.51 |
| DASH | ||||||
| - Baseline | 40.89 (18.64) | 38.75 (16.16) | 0.69 | 46.23 (16.54) | 25.43 (12.35) | <0.001 |
| - 3 months after surgery | 29.39 (16.92) | 18.72 (5.00) | 0.22 | 28.31 (15.90) | 36.14 (18.22) | 0.07 |
| - 6 months after surgery | 15.08 (10.59) | 28.39 (12.81) | <0.001 | 14.86 (11.16) | 26.08 (11.12) | <0.001 |
| Pain duration (weeks) | 71.17(86.69) | 100.36 (129.82) |
0.31 | 68.98 (68.59) | 88.78 (137.13) |
0.39 |
| Previous rehabilitation | 0.06 | 0.41 | ||||
| - Yes | 31 (52.5%) | 4 (28.6%) | 23 (41.8%) | 13 (56.5%) | ||
| - No | 23 (39%) | 10 (71.4%) | 27 (49.1%) | 10 (43.5%) | ||
| - Missing | 5 (8.5%) | 0 | 5 (9.1%) | 0 | ||
| Shoulder pathology (Acromioplasty) |
28 (47.5%) | 6 (42.9%) | 0.76 | 25 (45.5%) | 10 (43.5%) | 0.88 |
| Shoulder pathology (Bursae resection) |
20 (33.9%) | 2 (14.3%) | 0.16 | 17 (30.9%) | 6 (26.1%) | 0.68 |
| Shoulder pathology (Labrum repair) |
28 (47.5%) | 5 (35.7%) | 0.43 | 25 (45.5%) | 11 (47.8%) | 0.85 |
| Shoulder pathology (Other) |
49 (83.1%) | 10 (71.4%) | 0.33 | 46 (83.6%) | 16 (69.6%) | 0.16 |
Abbreviation: BPI, Brief Pain Inventory; DASH, Disability of the Arm, Shoulder, and Hand questionnaire.
Inclusion Criteria
The inclusion criteria for being a participant were: (a) between 18 and 85 years of age, (b) complaints of pain limited to anterior, lateral, or posterior shoulder, (c) documented or suspected rotator cuff tendinopathy (evidence from clinical examination or imaging studies) including small (<1 cm), medium (1-3 cm), and large (3-5 cm) tears, (d) documented or suspected adhesive capsulitis (evidence from clinical examination or imaging studies), (e) documented or suspected SLAP (Superior Labrum from Anterior to Posterior) lesion (evidence from clinical examination or imaging studies), and (f) scheduled for arthroscopic surgery.
Exclusion Criteria
The exclusion criteria were: (a) current complaints of pain lasting longer than the past 3 months involving neck, elbow, hand, low back, hip, knee, or ankle, (b) massive rotator cuff tear (>5 cm), (c) documented shoulder OA or RA, (d) prior shoulder surgery within the past year or currently complaining of pain from prior shoulder surgery, (e) current shoulder fracture, tumor, or infection, (f) previously diagnosed chronic pain disorder (including, but not limited to IBS, fibromyalgia, TMD, CLBP, etc), (g) current psychiatric management, and (h) current gastrointestinal or renal illness 20.
Overall Procedure
Study participants completed a standard intake information form after signing the informed consent. Patients underwent baseline assessments, which included psychological questionnaires, patients self-reported pain intensity and disability, and quantitative sensory testing for experimental pain sensitivity 24 to 48 hours before patient’s shoulder surgery. The experimental pain sensitivity assessment was performed in both hands and includes the assessment of SHPR (at 3 different temperatures), and heat threshold. The order of assessment between surgical and non-surgical side was controlled to prevent order effects during experimental pain sensitivity testing. Finally patients underwent CPM assessment (described below). Patients were re-assessed on all measures at 3-month, and 6-month follow up time points. All assessments were performed by evaluators trained in the assessment protocol who were blinded to psychological measure and recovery data.
Measures
Demographic and Historical Information
Study participants completed a standard intake information form. Demographic data collected at initial evaluation include gender, age, employment status, litigation status, marital status, educational level, and health history. Historical data include the type of onset of symptoms, the length of time of the symptoms, the number of previous episodes of musculoskeletal pain, and previous treatments for pain.
Shoulder Pain Intensity
Shoulder pain intensity was assessed with the Brief Pain Inventory (BPI), 28 which includes a numerical rating scale (NRS) for pain intensity. Subjects rated their pain intensity over three conditions, the present pain intensity, the worst pain intensity over the past 24 hours, and the least pain intensity over the past 24 hours. These 3 ratings were summed and divided by 3 for use in data analyses. 29 Studies have shown that this aggregate measure has sufficient psychometric strengths.29, 30
Shoulder Disability
Disability was assessed with the Disability of the Arm, Shoulder, and Hand questionnaire (DASH).31 The DASH includes 30 items to measure the extent to which patients’ pain or limited activity affects their ability to perform certain functions, to sleep, to carry on routine daily activities, and social activities. DASH has been validated for the assessment of shoulder disorders. 32-34
Experimental Pain Sensitivity
a) Suprathreshold heat pain response (SHPR)
Suprathreshold heat pain response (SHPR) was tested at the thenar eminence of the surgical and non-surgical sides (side of shoulder surgery and opposite side of shoulder surgery) with a thermode of 27 mm surface area by a Contact Heat Evoked Potential Stimulator (CHEPS) (Medoc Advanced Medical Systems, Ramat Yishai, Israel). This apparatus is composed of an HP-thermode that provides extremely fast heating rates of up to 70°C.s−1 and cooling rates of up to 40°C.s−1.
The CHEPS was programmed to deliver 5 consecutive heat pulses that rapidly rise from an adapting temperature to a peak temperature of 46, 48 or 50°C (depending on the test) at a rate of 30°C.s−1, remain at this level for 0.5 second, and then return to baseline at a rate of 30°C.s−1, with an interpulse intervals of 2.5 seconds. 35, 36
Subjects verbally rated the intensity of each thermal pulse on a numerical rating scale from 0 = “no pain” to 100 = “the worst pain imaginable”. 36 Additionally, subjects were asked to rate the magnitude of the delayed pain intensity following each heat pulse. The procedure was performed three times in a consecutive order, the first one using 46°C, the second using 48°C, and the third one using 50°C as a thermal stimulus, to determine the patient’s moderate level of pain to be used in a following assessment (for specifics see CPM assessment below).
This study used the “5th pain rating” which was the pain rating from the fifth pulse of each trial, 37-40 which is considered to represent a simple measure of SHPR assessment.41 In addition, we included it in the current study because our previous study indicated that the 5th pain rating of a SHPR train accounted for a significant proportion of variance in shoulder pain intensity. 20
b) Heat pain threshold
Subjects received a continuously ascending heat stimulus on their surgical and non-surgical sides (forearms). The stimulus started at 35°C and increased at a rate of 0.5°C.s−1. Subjects were asked to press a button and then rate their pain using a 0 (no pain) −100 (worst pain imaginable) NRS at the first sensation of pain. Two different trials were performed with a resting period of 2 minutes in between, and the average of the two temperatures was calculated as the heat pain threshold.
c) Conditioned pain modulation (CPM)
This study included CPM because is believed to provide an indication of the potential for endogenous pain inhibition, and because studies show its predictive utility in chronic post-operative pain development. 18, 42
Test stimulus (SHPR)
SHPR was tested at the thenar eminence of the uninvolved hand, using CHEPS (described above). Sequences of 5 consecutive heat pulses with interpulse intervals of 2.5 seconds were delivered.35, 36 The temperature used for the test stimulus (SHPR) was determined from the previous SHPR assessment, and was the temperature that reached a moderate level of pain (pain rating of 50 or closer to 50 from 0 to 100 on numerical rating scale) as an average of five heat pulses.
Subjects verbally rated the intensity of each thermal pulse on a numerical rating scale from 0 = “no pain” to 100 = “the worst pain imaginable”. 36 Additionally, subjects were asked to rate the magnitude of the delayed pain intensity following each heat-tap. Subjects were also asked to provide ratings of heat sensations 15 s and 30 s after the last heat stimulus (aftersensation). 43 We selected SHPR as the test stimulus because evidence suggests that CPM effects are largest for C-fiber mediated pain. 44, 45
Conditioning stimulus (Cold-pressor pain)
Subjects were instructed to immerse their surgical side hand up to the wrist into a cold water bath for up to one minute. The water was maintained at a constant temperature of 8°C, and was constantly circulated to prevent warming around the hand.
Conditioned pain modulation procedure
After baseline experimental pain assessment participants underwent the CPM assessment with the application of the test stimulus (described above) on the non-surgical side. After 30s from the last heat stimulus, subjects were instructed to immerse their surgical side hand up to the wrist into the cold water bath (conditioning stimulus). Thirty seconds after hand immersion, subjects were asked to rate the pain from the immersed hand, and were instructed to maintain their hands in the water bath for as long as they could tolerate for a maximum of one minute. One minute after the immersion of the hand, a new test stimulus was delivered on the non-surgical side. The protocol was created with consecutive stimuli (test stimulus, then conditioning stimulus, hand removed from water, and then test stimulus), and was the same procedure used in our previous study 21 (Figure 1).
Figure 1.
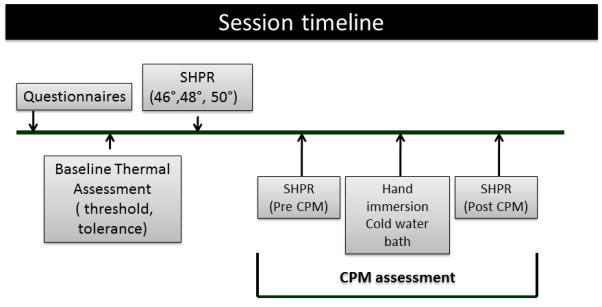
Schematic representation of each testing session
Psychological Factors
Our preliminary study in a different sample has shown that pain catastrophizing and depressive symptoms were the most relevant psychological factors in predicting postoperative clinical pain intensity in a population with similar characteristics. 20 Therefore for our current study we selected the same psychological factors to explore the contribution of them in postoperative clinical pain intensity in a longer follow up period (6 months).
a) Depressive symptoms
Self-report of depressive symptoms were measured using the Patient Health Questionnaire (PHQ-9).46 The PHQ-9 is a 9-item self-reported questionnaire designed to evaluate the presence of depressive symptoms during the prior 2 weeks. As a severity measure, scores can range from 0 (absence of depressive symptoms) to 27 (severe depressive symptoms). Each of the 9 items, asking for each of the DSM-IV diagnostic criteria, can be scored from 0 (not at all) to 3 (nearly every day). As a diagnostic measure, major depression is diagnosed if 5 or more of the 9 depressive symptom criteria have been present at least “more than half the days” (a score of 2) in the past 2 weeks, and one of the symptoms is depressed mood or anhedonia.
Previous studies support its validity, feasibility, and its capacity to detect changes of depressive symptoms over time. 47
b) Pain catastrophizing
Pain catastrophizing was measured by the Pain Catastrophizing Scale (PCS).48 The PCS has 13 descriptions of pain experience assessing catastrophic cognitions, for example: “I feel I cańt go on”, “Therés nothing I can do to reduce the intensity of the pain”. Subjects were asked to indicate whether they agreed with these statements by using a 5-point rating scale (0, “not at all” to 4, “all the time”) to rate the frequency of these cognitions. A PCS sum score was calculated for all items (range, 0 – 52), with a high score indicating a high level of pain catastrophizing.
Outcome Measures
Outcome measures were collected for all patients at baseline (24 to 48 hours before patient’s shoulder surgery), at 3-month after the surgery, and 6-month after the surgery. All assessments were performed by evaluators who were blinded to psychological measure data. Continuous data was used for the primary analyses of this manuscript.
For our planned secondary analyses, patients were classified according to their BPI scores for postoperative pain intensity (6 months after surgery) into two groups “improved” and “not improved”. Improvement was defined as a decrease of at least 30% in shoulder pain from baseline to 6 months after surgery. Patients who did not improve had a decrease of less than 30% in shoulder pain, and patients who improved had a decrease of at least 30% in shoulder pain from baseline to 6 months after surgery. We have selected 30% reduction in shoulder pain from baseline, because literature suggests that 30% reduction is associated with individuals reporting notable improvement on the patient global impression of change. 49, 50 In addition, it has been suggested that in studies with baseline pain variability, the clinical relevance should be defined in terms of percent change. 50
For upper extremity disability improvement, a difference score of 15 points on the DASH from baseline to 6 months was considered a minimal clinically important difference (MCID). Patients were classified into two groups “improved” (a difference score ≥ than 15 points on DASH) and “not improved” (a difference score < than 15 points on DASH). We have selected 15 points difference from baseline to 6 months, because literature suggest that a change in DASH score exceeding 15 points is the most accurate change score for discriminating between improved and unimproved patients. 51, 52
Data reduction and analysis
Data analysis was conducted over a series of steps using SPSS, Version 18.0. Significance levels were set a priori at p<0.05 for all comparison and Cohen’s d is reported as a measure of effect size. Descriptive statistics (mean, standard deviation) were calculated for all variables. The distributions of variables were tested for normality by visual examination and with Kolmogorov-Smirnov test before used in analysis. For analysis purposes measurements from both arms were averaged into one score, because paired t-test shows non-significant differences (p > 0.05) between measures in the right side versus left side, or surgical side versus non-surgical side, which is consistent with findings from an earlier shoulder pain cohort.53
For analysis purposes on CPM, we followed recent recommendations 54 on presenting results and calculation of CPM using the absolute difference for CPM and the percent change. Specifically, the report suggested that the change in the test stimulus before and after the induction of conditioning stimulus should be reported using change in the absolute value and percent change of the sensation.54 The “absolute difference” for CPM, was calculated by the difference between test stimulus before the application of conditioning stimulus (pre CPM), minus the test stimulus after the application of conditioning stimulus (post CPM). The “percent change” for CPM was calculated as follows:
Pearson correlations were calculated between clinical shoulder pain intensity, disability, experimental pain measurement (absolute difference of CPM, percent change of CPM, and 5th pain rating), and psychological factors at baseline and 6 months after surgery. ANOVA models were used to determine baseline differences on 5th pain rating, absolute difference of CPM, percent change of CPM, and the magnitude of pain threshold between patients who improved and patients who do not improve their level of pain and disability at 6 months.
Primary Analyses
Hierarchical regression models were conducted to assess which QST measure accounted for significant amount of variance in clinical pain intensity and disability. These models determined whether baseline, 6 months, and raw change score (baseline-3 months) of QST measures accounted for significant variance in clinical pain intensity and disability at baseline and 6 months. Raw change score (baseline-3 months) was used to have predictive ability of 6 months outcome and easier clinical interpretation. Regression models included age, sex, shoulder pain duration, and pre-operative clinical pain and disability in the first step to control for these potentially confounding factors, and QST measures (5th pain rating of SHPR, CPM, percent change of CPM, and pain threshold) in the second step of the regression model in a stepwise manner. Stepwise regression was used in the second step to create a parsimonious model consisting of QST measures with the strongest association with clinical pain intensity.
A different regression model was built to determine the contribution of the clinically relevant QST (determined in previous analysis) in explaining 6 months clinical pain intensity and disability, after potential predictive factors for persistent postsurgical pain were considered. Age, sex, shoulder pain duration, baseline clinical pain and disability and psychological factors (PCS and PHQ-9) were considered in the first step, and the appropriate QST measure from previous analysis was entered in the second step. VIF was reported for the final model to investigate potential multicollinearity among the independent variables.
Secondary Analyses
Repeated measures ANOVA was used to assess the effect of time (baseline, and 3 months after surgery) on the absolute difference of CPM, the percent change of CPM, 5th pain rating, and pain threshold by condition (improved vs. not improved for pain and disability). For this analysis the between groups factor was condition (improved vs. not improved), and the within subjects factor was time (baseline, and 3 months after surgery). Bonferroni correction was used to protect against Type I errors, and simple contrasts were used in case the interaction terms were significant to determine differences on CPM, 5th pain rating, or pain threshold between groups.
RESULTS
Subjects
This study had 78 patients with 6 months follow up completed. From those, 3 patients did not have information on their baseline BPI, 2 patients had missing information on BPI at 6 months, and one patient did not have information on their DASH score at 6 months. Therefore cases with only completed data on BPI and DASH were included in this analysis, resulting in a total sample size of 73 subjects for the pain analyses and 77 subjects for the disability analyses. No difference between included and excluded patients was noted in any of the variables (p>0.05).
Descriptive statistics for the demographic, clinical pain and medical history are summarized in Table 1. Experimental pain sensitivity assessment and psychological characteristics from the sample at baseline, 3 months, and 6 months post-surgery are summarized in Table 2. All continuous dependent variables were found to approximate a normal distribution by visual examination and were appropriate for our planned multiple regression analyses and ANOVA’s.
Table 2.
Experimental pain assessment and psychological characteristics for the sample
| Sample characteristics | Improved Group (pain) N=59 Mean (SD) |
Not improved Group (pain) N=14 Mean (SD) |
P- Value |
Improved Group (disability) N=55 Mean (SD) |
Not improved Group (disability) N=23 Mean (SD) |
P- value |
|---|---|---|---|---|---|---|
| 5th pulse 50°C baseline | 38.54 (25.29) | 24.93 (16.78) | 0.06 | 40.68 (24.72) | 27.22 (19.29) | 0.02 |
| 5th pulse 50°C at 3 months | 31.11 (26.42) | 33.75 (23.58) | 0.75 | 32.93 (27.12) | 32.65 (24.59) | 0.97 |
| 5th pulse 50°C at 6 months | 25.53 (21.65) | 25.23 (21.64) | 0.96 | 27.46 (23.86) | 26.20 (20.96) | 0.84 |
| Pre CPM baseline | 32.17 (25.70) | 23.57 (22.66) | 0.26 | 34.03 (26.01) | 22.02 (19.11) | 0.05 |
| Pre CPM at 3 months | 24.97 (17.91) | 20.37 (14.31) | 0.41 | 26.94 (18.79) | 20.04 (14.53) | 0.14 |
| Pre CPM at 6 months | 20.74 (15.28) | 25.68 (18.46) | 0.32 | 22.60 (16.45) | 22.15 (16.22) | 0.92 |
| Post CPM baseline | 24.65 (24.84) | 15.29 (18.17) | 0.21 | 26.06 (25.16) | 16.02 (17.39) | 0.09 |
| Post CPM at 3 months | 18.65 (17.06) | 16.78 (19.15) | 0.74 | 19.87 (18.26) | 17.26 (17.43) | 0.59 |
| Post CPM at 6 months | 14.56 (13.31) | 15.86 (11.35) | 0.75 | 16.63 (14.64) | 12.14 (9.39) | 0.21 |
| Absolute difference CPM baseline | 7.50 (12.76) | 8.40 (8.68) | 0.81 | 7.99 (12.00) | 6.00 (11.51) | 0.51 |
| Absolute difference CPM 3 months | 6.53 (9.25) | 3.58 (10.59) | 0.33 | 7.25 (9.44) | 2.78 (8.85) | 0.08 |
| Absolute difference CPM 6 months | 5.97 (7.82) | 9.81 (10.79) | 0.15 | 5.79 (8.28) | 10.01 (11.43) | 0.09 |
| Percent change CPM baseline | 22.72% | 24.89% | 0.88 | 26.02% | 14.06% | 0.29 |
| Percent change CPM at 3 months | 31.15% | 32.03% | 0.94 | 32.54% | 25.93% | 0.51 |
| Percent change CPM at 6 months | 31.24% | 29.37% | 0.85 | 27.67% | 37.59% | 0.25 |
| Pain threshold baseline | 43.86 (2.28) | 44.78 (2.00) | 0.17 | 43.90 (2.14) | 44.59 (2.28) | 0.22 |
| Pain threshold 3 months | 44.30 (2.39) | 45.11 (2.58) | 0.29 | 44.43 (2.37) | 44.51 (2.39) | 0.89 |
| Pain threshold 6 months | 44.58 (2.32) | 45.23 (1.87) | 0.35 | 44.84 (2.21) | 44.53 (2.29) | 0.60 |
| PCS baseline | 9.86 (6.97) | 8.07 (6.72) | 0.39 | 9.98 (7.00) | 8.14 (6.28) | 0.29 |
| PCS 3 months | 8.42 (7.99) | 9.21 (9.39) | 0.75 | 7.63 (7.65) | 10.13 (8.86) | 0.21 |
| PCS 6 months | 5.74 (5.42) | 10.00 (7.85) | 0.02 | 6.25 (6.24) | 7.39 (5.97) | 0.46 |
| PHQ-9 baseline | 2.81 (3.98) | 4.50 (2.62) | 0.14 | 3.02 (3.91) | 3.22 (3.19) | 0.83 |
| PHQ-9 3 months | 2.65 (3.31) | 4.50 (3.50) | 0.07 | 2.42 (2.91) | 3.96 (4.04) | 0.06 |
| PHQ-9 6 months | 2.52 (3.51) | 5.29 (3.69) | 0.01 | 2.43 (3.22) | 4.04 (4.26) | 0.07 |
Abbreviation: CPM, Conditioned Pain Modulation; PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire
Only the 5th pain rating at 50°C was used in these analyses, to keep consistency with our previous work21 and because this temperature represents a supra-threshold stimulus for almost all subjects we have tested in the past. The other temperatures were not reported in this paper because the 5th pain rating at 48°C and the 5th pain rating at 50°C were highly correlated at baseline and at 6 months (r’s between 0.90 and 0.97), and the 5th pain rating at 46°C is too low to reach supra-threshold levels of pain for most subjects.
After 6 months, 81% of our sample had an improvement in their clinical pain intensity and 19% did not improve. For upper extremity disability, 74% of our sample reached the MCID 6 months after the surgery (improved group), and 26% did not reach the MCID (not improved group). No difference was noted for the baseline demographic and medical history between the improved and not improved groups for pain and disability (p>0.05).
For pain groups (improved vs. not improved), simple ANOVAs showed no significant baseline differences in the absolute difference of CPM [F(1,68) = 0.06; p=0.81; d= 0.08], in the percent change of CPM [F(1,67) = 0.024; p=0.88; d= 0.04], in the 5th pain rating at 50°C [F(1,70) = 3.65; p=0.06; d= 0.65], or in pain threshold [F(1,69) = 1.918; p=0.17; d= 0.43] between groups.
For disability groups (improved vs. not improved), analyses showed no significant baseline differences between groups in the absolute difference of CPM [F(1,72) = 0.44; p=0.51; d= 0.17], in the percent change of CPM [F(1,72) = 1.12; p=0.29; d= 0.25], or in pain threshold [F(1,72) = 1.55; p=0.22; d= 0.31]. However there was a significant difference between groups in the 5th pain rating at 50°C [F(1,73) = 5.40; p=0.02; d= 0.61], such that the improved group had a higher mean disability (mean=40.68; SD=24.72) compared to the not improved group (mean=27.22; SD=19.29). These results did not change after controlling for baseline pain intensity.
There were no significant baseline differences in PCS [F(1,71) = 0.76; p=0.39; d= 0.26], or in PHQ-9 [F(1,71) = 2.27; p=0.14; d= 0.51] between pain groups, or in disability groups (PCS [F(1,73) = 1.15; p=0.29; d= 0.28]; PHQ-9 [F(1,73) = 0.05; p=0.83; d= 0.05] even after controlling for baseline pain intensity.
Primary Analyses
Pearson correlations among clinical shoulder pain intensity, disability, experimental pain measurement, and psychological factors at baseline and 6 months after surgery are summarized in Table 3.
Table 3.
Correlations among clinical shoulder pain intensity, experimental pain measurement, and psychological factors at baseline and 6 months after surgery
| BPI | BPI | Cfiber50 | Cfiber50 | CPM | CPM | %change | %change | PCS | PCS | PHQ-9 | PHQ-9 | Threshold | Threshold | DASH | DASH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 6m | B | 6m | B | 6m | CPM B | CPM 6m | B | 6m | B | 6m | B | 6m | B | 6m | |
| BPI B | 1 | .331 | .234 | .047 | −.129 | −.177 | −.132 | −.227 | .248 | .053 | −.088 | −.193 | −.037 | −.050 | .628** | .127 |
| BPI 6m | 1 | −.080 | .009 | −.137 | .046 | −.220 | −.045 | .151 | .385** | .158 | .177 | .099 | .068 | .226 | .559** | |
| Cfiber50 B | 1 | .581** | .129 | .274* | −.062 | .042 | .051 | −.139 | .176 | −.147 | −.465** | −.154 | .217 | −.043 | ||
| Cfiber50 6m | 1 | −.025 | .230 | −.092 | −.203 | −.221 | −.143 | .092 | −.072 | −.330** | −.366** | .046 | .129 | |||
| CPM B | 1 | .146 | .594** | .348** | .049 | −.165 | −.065 | −.105 | −.009 | .079 | −.078 | −.211 | ||||
| CPM 6m | 1 | −.006 | .680** | .086 | .015 | .300* | .084 | −.065 | .006 | −.048 | .150 | |||||
| %changeCPM B | 1 | .158 | −.005 | −.199 | −.046 | .000 | .069 | .167 | −.067 | −.249* | ||||||
| %changeCPM 6m | 1 | .179 | −.013 | .197 | .046 | .102 | .253* | −.069 | .042 | |||||||
| PCS B | 1 | .334** | .385** | .236* | −.117 | −.067 | .349** | .225* | ||||||||
| PCS 6m | 1 | .269* | .343** | −.006 | −.026 | .152 | .230* | |||||||||
| PHQ-9 B | 1 | .699** | −.186 | .032 | .338** | .245* | ||||||||||
| PHQ-9 6m | 1 | −.009 | .004 | .089 | .287* | |||||||||||
| Threshold B | 1 | .549** | −.043 | .047 | ||||||||||||
| DASH B | 1 | .062 | −.037 | |||||||||||||
| DASH 6m | 1 | .203 |
B=Baseline; 6m=6 months after surgery
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
Abbreviation: BPI, Brief Pain Inventory; CPM, Conditioned Pain Modulation; PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire DASH; Disability of the Arm, Shoulder, and Hand questionnaire.
Hierarchical regression analyses were conducted to determine the contribution of baseline, 6 months, and 3 months change score (baseline-3 months) of QST measures to 6 months clinical shoulder pain intensity and disability. The regression models predicting 6 months pain intensity and disability with baseline and concurrent factors did not have significant QST predictors (p>0.05).
However 3 months change score of QST explained an additional 10% of the variance for 6 months clinical pain intensity (R2=0.18, p= 0.009), where change score of the 5th pain rating (beta= −0.34, p=0.004) was the unique significant predictor. These results did not change after accounting for baseline clinical pain, and other potentially confounding factors (such as age, sex, shoulder pain duration, and pre-operative disability) indicating that 3 months change score of the 5th pain rating was still the unique significant predictor of 6 months clinical pain intensity even after controlling for baseline clinical pain. In other words, for every unit increase in 6 months clinical pain, there is 0.02 unit decrease in 3 months change score of the 5th pain rating (B= −0.02, Std. Error = 0.01) The model explaining 6 months disability using change score of QST measures was not significant (p>0.05).
In addition, different regression models were built to determine the contribution of the 3 months change score of 5th pain rating in explaining 6 months clinical pain intensity and disability, after potential predictive factors were considered. After accounting for age, sex, pain duration, baseline clinical pain, and baseline psychological factors (PCS, PHQ-9), the 3 months change score of 5th pain rating (beta= −0.35, p=0.003) account for an extra 11% of the variance in 6 months clinical pain intensity with a significant addition to the model (R2=0.27, p=0.01) (Table 4). The same trend of results was obtained when accounting for psychological factors at 3 months. Additionally, the 3 months change score of 5th pain rating predicting 6 months clinical pain intensity was investigated after accounting for the 3 months change score of psychological factors (PCS, PHQ-9). The same trend of results was obtained, where the 3 months change score of 5th pain rating (beta= −0.37, p=0.002) was the unique significant predictor, and account for an extra 13% of the variance in 6 months clinical pain intensity with a significant addition to the model (R2=0.22, p=0.01). The change score of 5th pain rating (beta= −0.30, p=0.01), account for an extra 9% of the variance in 6 months disability, however the addition to the model was non-significant (R2=0.23, p=0.02) (Table 5). The same trend of results was obtained when including 3 months change score of psychological factors (PCS, PHQ-9), where the 3 months change score of 5th pain rating (beta= −0.29, p=0.01) was the unique significant predictor, and account for an extra 9% of the variance in 6 months disability, however the addition to the model was non-significant (R2=0.20, p=0.04).
Table 4.
Explaining 6 months post operative clinical pain intensity with 3 months change score of 5th pain rating after controlling for potential predictive factors for persistent postsurgical pain
| Variable | R2 | B | SE | β | P-Value |
|---|---|---|---|---|---|
| 1st Model | 0.16 | 0.09 | |||
| Age | 0.01 | 0.01 | 0.15 | 0.25 | |
| Sex | 0.86 | 0.41 | 0.03 | 0.83 | |
| Pain duration | 0.002 | 0.002 | 0.12 | 0.33 | |
| Baseline PCS | 0.01 | 0.03 | 0.04 | 0.78 | |
| Baseline PHQ-9 | 0.05 | 0.05 | 0.15 | 0.27 | |
| Baseline clinical pain | 0.18 | 0.09 | 0.29 | 0.04 | |
| 2nd Model | 0.27 | 0.01 | |||
| Constant | −0.08 | 0.64 | 0.90 | ||
| Age | 0.01 | 0.01 | 0.11 | 0.37 | |
| Sex | −0.16 | 0.39 | −0.05 | 0.68 | |
| Pain duration | 0.002 | 0.002 | 0.15 | 0.19 | |
| Baseline PCS | 0.01 | 0.03 | 0.05 | 0.70 | |
| Baseline PHQ-9 | 0.07 | 0.05 | 0.18 | 0.15 | |
| Baseline clinical pain | 0.17 | 0.08 | 0.28 | 0.04 | |
| Change score of 5th | −0.02 | 0.01 | −0.35 | 0.003 | |
| pain rating |
Abbreviation: PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire
Table 5.
Explaining 6 months post operative disability with 3 months change score of 5th pain rating after controlling for potential predictive factors for persistent postsurgical pain
| Variable | R2 | B | SE | β | P-Value |
|---|---|---|---|---|---|
| 1st Model | 0.14 | 0.12 | |||
| Age | 0.15 | 0.08 | 0.25 | 0.07 | |
| Sex | 1.34 | 3.42 | 0.05 | 0.69 | |
| Pain duration | 0.01 | 0.01 | 0.11 | 0.36 | |
| Baseline PCS | 0.32 | 0.25 | 0.19 | 0.19 | |
| Baseline PHQ-9 | 0.46 | 0.42 | 0.15 | 0.27 | |
| Baseline DASH | 0.07 | 0.09 | 0.11 | 0.48 | |
| 2nd Model | 0.23 | 0.02 | |||
| Constant | 2.02 | 6.05 | 0.74 | ||
| Age | 0.13 | 0.08 | 0.22 | 0.09 | |
| Sex | 0.48 | 3.29 | 0.02 | 0.88 | |
| Pain duration | 0.02 | 0.01 | 0.14 | 0.23 | |
| Baseline PCS | 0.38 | 0.24 | 0.23 | 0.11 | |
| Baseline PHQ-9 | 0.48 | 0.39 | 0.16 | 0.24 | |
| Baseline DASH | 0.08 | 0.09 | 0.13 | 0.37 | |
| Change score of 5th | −0.15 | 0.06 | −0.30 | 0.01 | |
| pain rating |
Abbreviation: PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire; DASH; Disability of the Arm, Shoulder, and Hand questionnaire.
Additional analyses were ran entering in our regression models the change score of 5th pain rating as a residualized change score (z-change score). Our previous results did not change when entering z-change score so only raw results were reported in the paper for ease of interpretation. VIF showed minimal multicollinearity concerns among the independent variables in all regression models.
Secondary Analyses
To assess whether central pain modulatory measures had differential changes between pain and disability improvement groups repeated measures ANOVA was conducted. For pain groups, changes in absolute difference on CPM [F(1,65) = 0.74; p=0.39; d= 0.20], and percent change of CPM [F(1, 63) = 0.001; p=0.98; d= 0.04] over time (pre surgical stage and 3 months after surgery) had no significant effect on condition 6 months after surgery (improved vs. not improved) (Figures 2a, and 2c). The interaction term (time*condition) was also non-significant for pain threshold [F(1,67) = 1.12; p=0.74; d=0.37].
Figure 2.
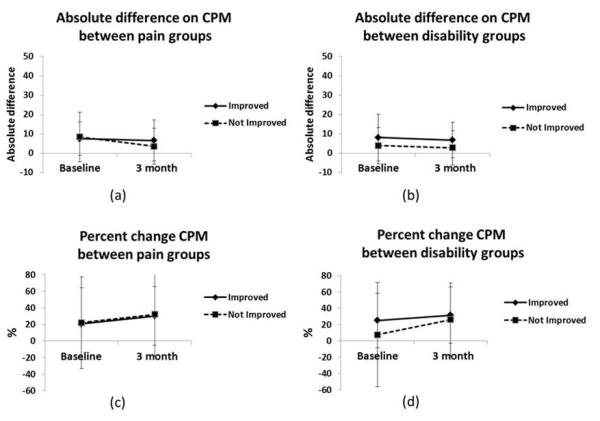
Change over time on absolute difference on CPM and percent change of CPM between groups. Error bars represented by standard deviation. (a) Change on absolute difference on CPM between pain improvement groups. (b) Change on absolute difference on CPM between disability improvement groups. (c) Change on percent change on CPM between pain improvement groups. (d) Change on percent change on CPM between disability improvement groups.
However, the interaction term (time*condition) for the 5th pain rating at 50°C was significant [F(1,68) = 4.92; p=0.03; d= 0.35], meaning that the change of 5th pain rating over time (pre surgical stage and 3 months after the surgery) differed based on 6 months pain intensity improvement (Figure 3). After decomposing the interaction term, results showed that the improved pain intensity group significantly decreased their mean of the 5th pain rating at 3 months post-surgery (baseline mean= 38.54 (25.29); 3 months mean= 31.30 (26.61); p= 0.02). The not improved group increased their mean of the 5th pain rating at 3 months but not in a significant manner (baseline mean= 25.46 (16.44); 3 months mean= 33.75 (23.58); p= 0.20). The same trend of results was obtained when baseline clinical pain was entered as a covariate.
Figure 3.
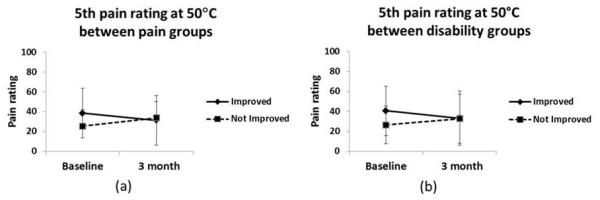
Change over time on 5th pain rating between groups. Error bars represented by standard deviation. (a) Change on absolute difference on 5th pain rating between pain improvement groups. (b) Change on absolute difference on 5th pain rating between disability improvement groups.
For disability groups, changes in absolute difference on CPM [F(1,69) = 0.001; p=0.97; d= 0.41], and percent change of CPM [F(1, 67) = 0.66; p=0.42; d= 0.25] over time (pre surgical stage and 3 months after surgery) did not differ based on disability group 6 months after surgery (improved vs. not improved) (Figures 2b, and 2d). The interaction term (time*condition) was also non-significant for pain threshold [F(1,71) = 1.55; p=0.22; d= 0.16].
However, the interaction term (time*condition) for the 5th pain rating at 50°C for disability groups was significant [F(1,72) = 5.44; p=0.02; d= 0.34], (Figure 3). After decomposing the interaction term, results showed that the “improved group” significantly decreased their mean of the 5th pain rating at 3 months post-surgery (baseline mean= 40.68 (24.72); 3 months mean= 33.18 (27.32); p= 0.02). The not improved group increased their mean of the 5th pain rating at 3 months but not in a significant manner (baseline mean= 26.50 (18.94); 3 months mean= 32.65 (24.59); p= 0.22). The same trend of results was obtained when baseline disability was entered as a covariate.
DISCUSSION
This study investigated the effect of central pain modulatory mechanisms (CPM and SHPR), on postoperative clinical shoulder pain and disability. These results indicate that the 3-month change score of SHPR made a unique contribution to 6 months pain intensity and disability scores even after controlling for other potential predictive factors for persistent postsurgical pain. Our study provides evidence that measures of central sensitization and psychological factors may play a differential role in determining risk of post-operative shoulder outcomes, with changes on SHPR being the unique contributor of pain intensity and disability in this particular sample. In complimentary analyses using categorical outcomes, SHPR had differential change over time, where the group showing 6 month improvement in clinical outcomes had significantly decreased SHPR at 3 months. In contrast, CPM and pain threshold did not have differential changes over time between patients with different 6 month postsurgical outcomes for pain intensity and disability. Although our conclusions are speculative, these data suggest that a post-operative decrease in an excitatory measure of pain sensitivity could be predictive of favorable clinical outcomes.
The present study presents novel data that extends our previous work20, 21 and the work of others15, 18, 19, 42 in several ways. First, few longitudinal studies have investigated changes in central pain modulatory measures. Second, the present study attempted to differentiate inhibitory and facilitatory experimental pain sensitivity measures as precursors of continued postoperative pain and disability. Third, patients included in this study underwent shoulder surgery; therefore this study extends the literature investigating development of postoperative chronic pain and disability to a broader range of surgical procedures than typically reported.15, 18, 19, 42, 55 Fourth, the exploration of QST, after controlling for potential predictive factors such as psychological factors,, makes this study a novel contribution to the literature as past studies typically incorporate either QST or psychological measures.
In our previous study 21 the 5th pain rating of SHPR and the percent change of CPM were elevated for patients with shoulder pain compared to healthy controls, however the absolute difference of CPM did not differ (suggesting an altered pain sensitivity for the clinical cohort before having shoulder surgery). These findings were in agreement with other studies in clinical populations showing that continued nociceptive input from musculoskeletal structures is related with enhanced pain perception. 8, 42, 55-57 Our current results extend our previous work by showing no pre-operative differences on the 5th pain rating or percent change of CPM for patients who improved and who do not improved their level of pain intensity after 6 months from surgery, even after controlling for pain intensity, and pain duration. However these results should be considered with some balance, as the reported effect sizes were large enough to potentially indicate patients with “higher” levels of pre-operative sensitization were more likely to experience a post-operative “normalization”. Collectively the results of our previous and current study indicated that elevated sensitization (to thermal stimuli) is expected prior to surgery when compared to healthy controls.21 Further determination of risk of continued post-operative pain may be difficult in this patient population because there were not obvious differences detected with QST for patients that improved and patients that did not.
In contrast, this measure of central sensitization may play a different role in determining risk of higher post-operative disability, where there is potential pre-operative differences observed for the 5th pain rating of SHPR for patients who improved and who do not improved their level of disability. We speculate this could be an indication that measures of central sensitization may have a stronger association with disability, as opposed to pain intensity. Indeed we have observed a similar pattern in patients with chronic low back pain where temporal summation was associated with disability, but not pain intensity.58 We do acknowledge that this explanation is speculative and future studies with larger sample sizes providing better statistical power and healthy control groups to reference sensitization status are needed to confirm or refute these findings about the relevance of baseline QST for predicting pain and disability outcomes.
Yarnitsky et al. 18 explored the predictive ability of CPM, indicating the potential for CPM to predict the development of chronic post-operative pain in patients having thoracotomy. In addition, Kosek et al. 42 found baseline differences on CPM between patients with painful osteoarthritis of the hip and healthy controls, and CPM normalized in arthritis patients after their pain was successfully treated. The present study shows no influence of CPM or pain threshold on pain and disability reports 6 months after shoulder surgery. However, if we consider the magnitude of our results, we observed a change in thermal pain sensitivity via SHPR, following the same pattern as has been described for CPM by Kosek et al.42 These two measures of central pain modulatory mechanisms are believed to reflect different components of central pain processing, however, future studies will be necessary to better understand the interaction between “excitatory” and “inhibitory” measures of experimental pain sensitivity. Our findings further suggest that if SHPR is a measure of facilitatory processes, then these processes might be more sensitive to immediate changes in clinical pain severity among patients with shoulder pain when compared to inhibitory processes (measured by CPM).
Multiple case-control studies imply that chronic pain is associated with changes in central nervous system (CNS) processing of pain-related information, 7-9, 40, 59 however the question of causality remains unanswered in such research designs. Even though a prospective study without a control group does not specifically elucidate the question of causality, our design addressed an important temporal issue. The current study21 extends our previous results by reporting that favorable changes in facilitatory capacity was predictive of less pain and disability at 6-months. Specifically, the SHPR decrease between baseline assessment and 3 months after surgery indicates that changes in CNS processing of pain 3 months after surgery were a precursor of 6 months postsurgical outcome. This implies that the 5th pain rating of SHPR may be more sensitive to changes than CPM in this particular setting with these methods, and a potential treatment monitoring target for this patient population. This finding held true even after controlling for variables that prior studies have shown to be related to postsurgical pain such as preoperative pain duration, baseline clinical pain, age, sex, pain catastrophizing and depression.
Psychological characteristics have been extensively examined in relation to pain perception and clinical outcomes, and have become an accepted factor in the development of chronic pain. 22, 60, 61 Our results showed that the hierarchical model explaining 6 month postoperative pain and disability with baseline psychological factors and the change score of 5th pain rating (Tables 4 and 5), show that the change score of our SHPR was the strongest predictor while baseline psychological factors were not significant. Therefore, our findings suggest that psychophysical assessment specifically of facilitatory pain sensitivity measures and measures of psychological distress are not redundant and they measure different pain constructs from psychological distress, supporting previous findings 20, 58. Some limitations of this study will need to be addressed by future research. First, CPM, SHPR, and heat pain threshold were the only QST measures employed in this study; future studies should include additional measures, such as pressure pain threshold, to have a more comprehensive QST assessment. Second, this study lacked a comparison group; therefore it is impossible to establish cause and effect of the relationship. In addition, even though an unequal group sizes are expected when explaining a low incidence outcome (such as chronic postoperative pain), a study with larger sample size or surgical model with higher incidence of postoperative chronic pain might be a better model to study changes on central pain processing associated with development of continued postoperative pain because it would allow better power for group comparisons.
We are suggesting that results from this study are related to differences in pain processing. However, we acknowledge that a viable alternative alternate explanation is that the difference we observed in 5th pulse change (our proxy measure of sensitization) scores could be from regression to the mean. This regression to the mean effect would have to be specific to thermal suprathreshold pain sensitivity because we did not find significant differences in modulatory capacity per our CPM protocol. To correctly address concerns related to regression to the mean future studies should not compare patients with high sensitivity to those with low sensitivity because there is always likely to be baseline differences to contend with. Instead, future longitudinal studies should incorporate experimental designs by randomly assigning groups of patients with baseline levels of high sensitization to different treatments and determine if the change scores differ. This type of study would likely require a larger sample size, but the enhanced rigor would remove concerns related to regression to mean.
Lastly, even though previous analysis from our group did not find a significant effect for taking pain medication on quantitative sensory testing,62 future studies should account for this potential confounding factor.
In summary, the findings from the present study imply that a 3 month change in the 5th pain rating of SHPR was associated with 6 month postoperative pain intensity and disability. In addition, categorical analyses support our findings and highlight the clinical relevance by showing that the 5th pain rating differentially changed over time, such that the improved group significantly decreased over time. This change in 5th pain rating of SHPR in explaining postoperative pain intensity and disability ratings was independent of psychological factors.
This finding provides evidence that facilitatory changes in the central modulatory system might be a unique factor in the transition to continued postoperative pain intensity and disability. These data also provide potential monitoring targets to distinguish between those that are likely to develop chronic pain syndromes and those that are not. However, because the assessment of central modulatory system requires expensive and special equipment, future studies may explore more clinically feasible quantitative sensory testing methods (e.g. mechanical) in surgical populations to predict postoperative pain intensity and disability63.
Acknowledgements
The authors wish to thank Warren Greenfield III for his assistance with clinical participant screening and recruitment. Dr. Deenesh T. Sahajpal provided subjects from his pre-operative clinic. The authors also wish to thank Roy Coronado, Corey Simon, and Lindsay Kindler for assistance with testing. This study was supported by grant #AR055899 from NIAMS/NIH.
Footnotes
Conflict of interest statement
The authors have no financial relationships that might lead to a conflict of interest
LIST OF REFERENCES
- 1.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 2.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichs-Rocker A,K, Schulz I, Jarvinen, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain. 2009;13:719–30. doi: 10.1016/j.ejpain.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723, 44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel A, Pogatzki-Zahn E. [Predictors of chronic pain following surgery. What do we know?] Schmerz. 2010;24:517–31. doi: 10.1007/s00482-010-0932-0. quiz 532-3. [DOI] [PubMed] [Google Scholar]
- 7.Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Med. 2001;2:208–15. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- 8.Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 9.Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26:782–9. doi: 10.1111/j.1468-2982.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 10.Leffler AS, Kosek E, Lerndal T, et al. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain. 2002;6:161–76. doi: 10.1053/eujp.2001.0313. [DOI] [PubMed] [Google Scholar]
- 11.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: implications for clinical management. Anesth Analg. 2004;99:510–20. doi: 10.1213/01.ANE.0000133383.17666.3A. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305–27. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- 13.Le Bars D, Villanueva L, Bouhassira D, et al. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter. 1992:55–65. [PubMed] [Google Scholar]
- 14.Staud R, Craggs JG, Robinson ME, et al. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granot M, Lowenstein L, Yarnitsky D, et al. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65:437–43. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Ness TJ, Weigent DA, et al. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106:427–37. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2007 doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Graven-Nielsen T, Wodehouse T, Langford RM, et al. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–16. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 20.Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–40. doi: 10.1016/j.jpain.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia C, Kindler LL, Fillingim RB, et al. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13:81–9. doi: 10.1016/j.jpain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 24.de Souza JB, Potvin S, Goffaux P, et al. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain. 2009;25:123–7. doi: 10.1097/AJP.0b013e318183cfa4. [DOI] [PubMed] [Google Scholar]
- 25.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21:439–45. doi: 10.1097/01.ajp.0000135236.12705.2d. [DOI] [PubMed] [Google Scholar]
- 26.Granot M, Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther. 2005;31:285–302. doi: 10.1080/00926230590950208. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan MJ, Stanish W, Sullivan ME, et al. Differential predictors of pain and disability in patients with whiplash injuries. Pain Res Manag. 2002;7:68–74. doi: 10.1155/2002/176378. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 29.Jensen MP, Turner LR, Turner JA, et al. The use of multiple-item scales for pain intensity measurement in chronic pain patients. Pain. 1996;67:35–40. doi: 10.1016/0304-3959(96)03078-3. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 31.Beaton DE, Katz JN, Fossel AH, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–46. [PubMed] [Google Scholar]
- 32.Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7:565–72. doi: 10.1016/s1058-2746(98)90002-7. [DOI] [PubMed] [Google Scholar]
- 33.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDermid JC, Drosdowech D, Faber K. Responsiveness of self-report scales in patients recovering from rotator cuff surgery. J Shoulder Elbow Surg. 2006;15:407–14. doi: 10.1016/j.jse.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–17. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 36.Fillingim RB, Maixner W, Kincaid S, et al. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–7. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 37.Lautenbacher S, Kunz M, Burkhardt S. The effects of DNIC-type inhibition on temporal summation compared to single pulse processing: does sex matter? Pain. 2008;140:429–35. doi: 10.1016/j.pain.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007;8:514–20. doi: 10.1111/j.1526-4637.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 39.Lautenbacher S, Kunz M, Strate P, et al. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–8. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Staud R, Robinson ME, Vierck CJ, Jr., et al. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–74. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 41.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–71. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 42.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 43.Staud R, Robinson ME, Vierck CJ, Jr., et al. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 44.Price DD, McHaffie JG. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. Pain. 1988;34:245–52. doi: 10.1016/0304-3959(88)90119-4. [DOI] [PubMed] [Google Scholar]
- 45.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 46.Michael ES, Burns JW. Catastrophizing and pain sensitivity among chronic pain patients: moderating effects of sensory and affect focus. Ann Behav Med. 2004;27:185–94. doi: 10.1207/s15324796abm2703_6. [DOI] [PubMed] [Google Scholar]
- 47.Greenland S, Kleinbaum DG. Correcting for misclassification in two-way tables and matched-pair studies. Int J Epidemiol. 1983;12:93–7. doi: 10.1093/ije/12.1.93. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 49.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11:109–18. doi: 10.1016/j.jpain.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Farrar JT, Young JP, Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 51.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J Clin Epidemiol. 2004;57:1008–18. doi: 10.1016/j.jclinepi.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Coronado RA, Kindler LL, Valencia C, et al. Thermal and pressure pain sensitivity in patients with unilateral shoulder pain: comparison of involved and uninvolved sides. J Orthop Sports Phys Ther. 2011;41:165–73. doi: 10.2519/jospt.2011.3416. [DOI] [PubMed] [Google Scholar]
- 54.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14:339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Wilder-Smith OH, Tassonyi E, Arendt-Nielsen L. Preoperative back pain is associated with diverse manifestations of central neuroplasticity. Pain. 2002;97:189–94. doi: 10.1016/S0304-3959(01)00430-4. [DOI] [PubMed] [Google Scholar]
- 56.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 57.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–96. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 58.George SZ, Wittmer VT, Fillingim RB, et al. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil. 2006;16:95–108. doi: 10.1007/s10926-005-9007-y. [DOI] [PubMed] [Google Scholar]
- 59.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13:12–7. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 60.George SZ, Fritz JM, Childs JD. Investigation of elevated fear-avoidance beliefs for patients with low back pain: a secondary analysis involving patients enrolled in physical therapy clinical trials. J Orthop Sports Phys Ther. 2008;38:50–8. doi: 10.2519/jospt.2008.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lethem J, Slade PD, Troup JD, et al. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behav Res Ther. 1983;21:401–8. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 62.George SZ, Hirsh AT. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J Pain. 2009;10:293–9. doi: 10.1016/j.jpain.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staud R, Weyl EE, Price DD, et al. Mechanical and heat hyperalgesia highly predict clinical pain intensity in patients with chronic musculoskeletal pain syndromes. J Pain. 2012;13:725–35. doi: 10.1016/j.jpain.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


