Abstract
Objective
A maximal aerobic capacity below the 20th percentile is associated with an increased risk of all-cause mortality.1 Adult burn survivors have a lower aerobic capacity compared to non-burned adults when evaluated 38±23 days post-injury.2 However, it is unknown if burn survivors with well-healed skin grafts (i.e., multiple years post injury), also have low aerobic capacity. This project tested the hypothesis that aerobic fitness, as measured by maximal aerobic capacity (VO2max), is reduced in well-healed adult burn survivors when compared to normative values from non-burned individuals.
Methods
Twenty-five burn survivors (36 ± 12 years old; 13 females) with well-healed split thickness grafts (median: 16 years post-injury, range: 1 to 51 years) covering at least 17% of their body surface area (mean: 40±16%; range: 17 to 75%) performed a graded cycle ergometry exercise test to volitional fatigue. Expired gases and minute ventilation were measured via a metabolic cart for the determination of VO2max. Each subject’s VO2max was compared with sex- and age-matched normative values from population data published by the American College of Sports Medicine (ACSM), the American Heart Association (AHA), and recent epidemiological data.3
Results
Subjects had a VO2max of 29.4 ± 10.1 ml O2/kg body mass/min (median: 27.5; range: 15.9 to 53.3). Using ACSM normative values, mean VO2max of the subjects was in the lower 24th percentile (median: 10th percentile). 88% of the subjects had a VO2max below AHA age-adjusted normative values. Similarly 20 of the 25 subjects had a VO2max in the lower 25% percentile of recent epidemiological data.
Conclusions
Relative to non-grafted subjects, 80–88% of the evaluated skin graft subjects had a very low aerobic capacity. Based upon these findings, adult burn survivors are disproportionally unfit relative to the general U.S. population, and this puts them at an increased risk of all-cause mortality.1
Keywords: skin graft, maximal oxygen uptake, burn recovery, rehabilitation
INTRODUCTION
Each year ~1.4 million people in the United States sustain burn injuries.4 Due to medical advances, survival rates of individuals with severe burns have dramatically increased. The psychological and physical recovery process from severe burns is multifactorial and can take months to years.5
Physical inactivity leads to poor cardiovascular fitness and poor fitness is highly correlated with increased all-cause mortality.6 Physical and psychological impairments, similar to those associated with burn recovery, can lead to sustained periods of physical inactivity.7 Thus, an individual with burn injuries can suffer from a decrease in cardiovascular fitness during their recovery. Relatively little is known about the cardiovascular fitness of adults that previously sustained severe burn injuries. De Lateur et al.2 examined maximal aerobic capacity (VO2max, the gold standard of cardiovascular fitness) in adults with 19 ± 16 % of their total body surface area (TBSA) burned 38 ± 23 days (range 9–122 days) post-injury. Although VO2max was low (21.7 ± 7.0 ml/kg/min) when compared to age-matched norms, the relatively short time period between injury and testing makes it difficult to speculate on the cause of poor cardiovascular fitness. Willis et al.8 observed reductions in VO2max in adults 5.1 ± 1.8 years post-injury, however the small sample size (n = 8) and limited range of injury severity (i.e., only 1 subject had >40% TBSA grafted) limits the interpretation of those findings. Given this paucity of data regarding cardiovascular fitness in adults with well-healed skin grafts, the current study extends previous findings by examining adults ~15 years post injury (range: 1 to 51 years) with a wide range of % TBSA grafted (i.e., 17–75% TBSA). We hypothesized that cardiovascular fitness, quantified by VO2max, is lower in adults with well-healed burn injuries compared to age- and sex-matched normative population values. Further, we hypothesize that the extent of the compromised cardiovascular fitness is independent of years post-injury and % TBSA grafted.
METHODS
Twenty-five burn survivors (36 ± 12 years old; 13 females) with well-healed split thickness grafts covering at least 17% of their body surface area (mean: 40±16%; range: 17 to 75%) participated in the study. Subjects must have been at least 1 year post-injury, while no upper range post-injury was imposed resulting in a median post-injury of ~16 years with a range of 1 to 51 years. Subjects were excluded if they had cardiovascular, metabolic or neurological diseases and thus subjects were generally healthy. The rule of Nine’s9 was used to calculate the area of skin covered by split-thickness grafts. While individuals with at least 15% of their total body surface area (TBSA) covered with grafts were eligible for participation, subject recruitment focused on inclusion of individuals across a wide range of TBSA grafted. Subjects refrained from alcohol and exercise 24 h, food 4 h, and caffeine 12 h before testing. Written informed consent was obtained from all subjects before participating in this study. Study procedures and the informed consent were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
The gold standard for measuring aerobic capacity is a VO2max test.10, 11 The employed VO2max protocol consisted of cycling on an electronically-braked ergometer (Lode Excalibur Sport, Lode B.V., Groningen, NL) with the power output starting at 25 or 50 W and progressively increasing 25 W every 2 min until volitional fatigue. Oxygen uptake and related gas exchange measures were obtained by open-circuit indirect calorimetry using a PARVO Medics TrueOne 2400 Metabolic Measurement System (Parvo Medics, Inc., Salt Lake City, UT) calibrated prior to use. Heart rate (HR) and rating of perceived exertion (RPE) were measured at rest, every 2 min, and at the end of the VO2max test. HR was measured from ECG and a Polar® Vantage XL heart rate monitor (Polar Electro, Inc. Woodbury, NY, model 145900). RPE was measured by the Borg 15-point category scale using standardized instructions.12 For some subjects (n = 13), a capillary finger-stick blood sample was obtained three min after completion of the test for determination of blood lactate concentration. Peak oxygen uptake was objectively identified based upon a heart rate within 10 bpm of age predicted maximum (220-age), an RPE score of 19–20, and/or a respiratory exchange ratio of >1.0.11
Interpretation of Data
Data are presented as mean ± SD. Subjects were grouped into one of three classifications according to the %TBSA grafted: 17–35%, 40–55%, and >60%. Differences between groups were analyzed with a one-way analysis of variance (ANOVA). Alpha was set at 0.05 and Bonferroni post-hoc tests were conducted if a significant main effect occurred. The relationship between VO2max and %TBSA and years since injury were examined using a Pearson-Product Moment correlation.
The obtained VO2max data were compared against three published normative datasets, each of which is based upon observations from thousands of healthy and presumably non-burned individuals. Using population normative data published by the American Heart Association,10 each subject was classified as having a VO2max above or below their sex- and age-matched norm. Similarly, each subject’s VO2max percentile was identified using age- and sex-matched normative values published by the American College of Sports Medicine.13 Lastly, the number of subjects in each quartile of fitness (i.e., low to high fitness) were identified using epidemiological data3 in which fitness, as quantified with a VO2max test, was correlated to cardiovascular risk factors.
RESULTS
Subject demographics are presented in Table 1. The median and range of % TBSA grafted was 34% and 17–75%, respectively. The median time since the burn injury was 15.7 yrs. Due to the study design, stratification of subjects into groups resulted in differences between % TBSA grafted in each group (17–35%, 40–55%, and >60%; P < 0.001). There were no differences between groups in length of time post-injury (P = 0.295), age (P = 0.488), height (P = 0.437), or body mass (P = 0.277).
Table 1.
Participant characteristics.
| % TBSA Grafted Classification | n | % TBSA Grafted | Length Post-injury1 (yrs) | Age (yrs) | Height (cm) | Body Mass (kg) |
|---|---|---|---|---|---|---|
| 17–35% | 13 (6 females) |
27.2* ± 5.8 | 21.1 ± 16.0 | 38 ± 13 | 168 ± 15 | 81.5 ± 17.2 |
| 40–55% | 7 (3 females) |
43.6* ± 4.7 | 15.9 ± 10.2 | 34 ± 13 | 175 ± 11 | 85.5 ± 17.7 |
| >60% | 5 (4 females) |
65.7* ± 8.0 | 9.9 ± 8.3 | 31 ± 9 | 168 ± 3 | 70.6 ± 3.2 |
|
| ||||||
| Mean±SD for all subjects | 25 (n = 13) |
39.5 ± 16.2 | 17.3 ± 13.5 | 36 ± 12 | 170 ± 13 | 80.4 ± 16.0 |
TBSA, Total Body Surface Area
Significant differences between classifications (P < 0.05)
All subjects achieved a VO2max based upon values obtained at the end of the incremental test to exhaustion (i.e., HRmax, RPE, RER, and blood lactate; Table 2). There were no differences between groups in absolute VO2max (i.e., oxygen uptake in L/min; P = 0.885), relative VO2max (oxygen uptake in ml/kg body mass/min; P = 0.260), HRmax (P = 0.990), RPE (P = 0.945), and RER (P = 0.393), while lactate approached significance (P = 0.052).
Table 2.
Values at the end of the incremental bike test to exhaustion
| % TBSA Grafted Classification | VO2max (l·min−1) | VO2max (mL·kg−1·min−1) | HRmax (bpm) | RPE (units) | RER | Lactate (mmol·L−1) |
|---|---|---|---|---|---|---|
| 20–35% | 2.34 ± 0.87 | 28.9 ± 9.1 | 180 ± 17 | 19 ± 1 | 1.16 ± 0.1 | 9.5 ± 2.1 |
| 40–55% | 2.26 ± 0.86 | 25.9 ± 5.6 | 180 ± 14 | 19 ± 1 | 1.09 ± 0.07 | 6.6 ± 0.9 |
| >60% | 2.36 ± 0.89 | 35.6 ± 15.7 | 179 ± 8 | 19 ± 1 | 1.11 ± 0.17 | 11.1 ± 1.6 |
|
| ||||||
| Mean±SD for all subjects | 2.36 ± 0.89 | 29.4 ± 10.1 | 180 ± 14 | 19 ± 1 | 1.13 ± 0.11 | 9.1 ± 2.3 |
N=25 for all variables except lactate where N=13
There were no differences between groups for any of these variables.
Table 3 shows VO2max responses of the evaluated subjects when compared to normative datasets. When referenced against AHA normative data,10 88% of the subjects had a VO2max below the age/sex-matched normative values. When VO2max of the subjects was classified into population percentiles, based upon normative data from the American College of Sports Medicine (ACSM),13 the median percentile for VO2max was the lowest 10th percentile (range 10th–90th percentile; mean 24±25 percentile). Notably, 76% of the subjects were in the lowest 20th percentile for VO2max when compared against this dataset.13 Similar to those observations, using relatively recent epidemiological data,3 80% of the subjects had a VO2max that was in the lowest quartile. VO2max was not correlated with years since injury (r = 0.003; P = 0.987; Figure 1) or % body surface area grafted (r = 0.190; P = 0.363; Figure 2).
Table 3.
Classification of VO2max values relative to three population normative datasets.
| % TBSA Grafted Classification | AHAa N (% of group) | ACSMb mean ± SD (median) percentiles | Aspenesc N in each quartile of VO2max | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Below Norm | Above Norm | 0–25% | 25–50% | 50–75% | 75–100% | ||
| 20–35% | 12 (92%) | 1 (8%) | 22 ± 21 (10th) | 10 | 2 | 0 | 1 |
| 40–55% | 7 (100%) | 0 | 14 ± 5 (10 th) | 7 | 0 | 0 | 0 |
| >60% | 3 (60%) | 2 (40%) | 44 ± 42 (20 th) | 3 | 0 | 0 | 2 |
|
| |||||||
| Mean±SD for all subjects | 22 (88%) | 3 (12%) | 24 ± 25 (10th) | 20 | 2 | 0 | 3 |
– From: Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740
– From: American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
– From: Aspenes ST, Nilsen TI, Skaug EA, et al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43:1465–1473.
Figure 1.
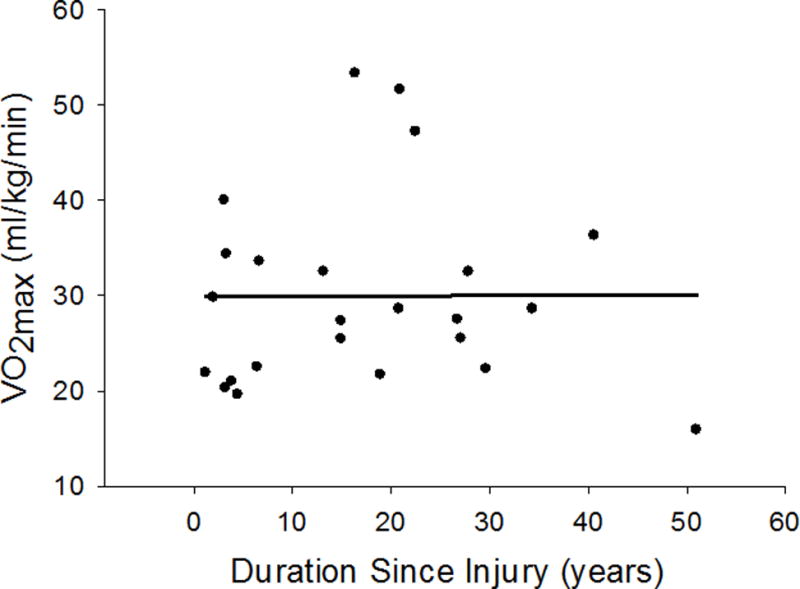
Maximal oxygen uptake (VO2max) was not correlated to duration since injury (r = 0.003; P = 0.987).
Figure 2.
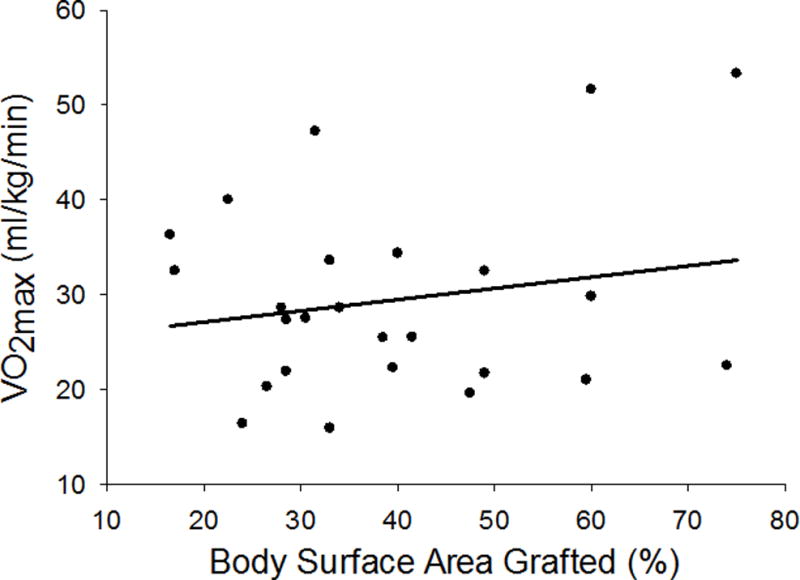
Maximal oxygen uptake (VO2max) was not correlated to body surface area grafted (%) (r = 0.190; P = 0.363)
DISCUSSION
The purpose of this study was to examine the aerobic capacity, as indexed from VO2max, of individuals with well-healed skin grafts (i.e., at least 1 year following the initial burn injury) covering 17–75% of their total body surface area. Using American Heart Association norms,10 we show that 88% of the evaluated subjects were below age- and sex-match normative values. With respect to population norms published by the American College of Sports Medicine,13 the VO2max from subjects in the current investigation averaged in the ~25% percentile, although the data were not normally distributed and thus a median of the lowest 10th percentile (range 10th–90th percentile) is more reflective of the evaluated subjects. This is further supported by data from Aspenes et al,3 in which 80% of the evaluated subjects fall in the lowest fitness quartile when compared to values from over 4,000 non-grafted individuals. Therefore, the primary finding of this study is that 80% or more of the evaluated skin grafted individuals have an aerobic capacity that is disproportionally lower than age-matched non-grafted individuals.
Since the obtained values were compared to normative VO2max datasets from thousands of individuals, it is important that a true maximum (or peak) oxygen uptake was achieved in the evaluated subjects. To that end, there is strong evidence that these subjects obtained a VO2max. For example, at test termination the subjects had heart rates at or near age-predicated max, had RPE values of 19 or 20, had RER values >1.0, and the 13 subjects assessed had lactate concentrations that were near or above 7 mmol/L (Table 2). Using these standard thresholds for evidence that subjects achieved a true VO2max allows us to make comparisons to the aforementioned normative datasets.11
The observed findings are consistent with prior findings suggesting that adults with skin grafts have a low VO2max.2, 8 We extend those findings in three distinct and important ways: 1) we examined individuals across a longer duration post-injury (i.e., 17.3 ± 13.5 years), relative to the duration post-injury by de Lateur et al. 2 (38 ± 23 days) and Willis et al.8 (5.1 ± 1.8 years); 2) approximately half of the examined individuals had skin grafts covering 40% or more of their TBSA, in contrast to Willis et al.8 who examined only 1 subject with greater than 40% TBSA burned; and 3) the overall number of subjects in the present investigation (25 subjects) is appreciably greater than the 8 subjects evaluated by Willis et al.,8 thereby allowing for correlative analyses between VO2max and demographic data.
The mechanism(s) by which burn survivors have such low aerobic capacities remains unclear. Acute burn injury results in a catabolic state in which resting metabolic rate is elevated for a period of time directly correlated to amount of TBSA burned.14 In the most severe cases (>75% TBSA burned), increased resting metabolic rate may persist for 1 year or more.14, 15 This acute hypermetabolic state can lead to substantial skeletal muscle loss.16 A lower skeletal muscle mass, relative to pre-burn injury, leads to a reduction in the amount of aerobically active muscle mass. This will decrease maximal oxygen extraction across that muscle, and according to the Fick equation [VO2max = Cardiac Outputmax * (arterial-venous oxygen contentmax)], VO2max will be reduced. Since the subjects in the present study were at least 1 year post-injury, it is unknown if in the subsequent years after the injury these subjects were able to rebuild the muscle loss that occurred during this hypermetabolic state. Given a lack of correlation between VO2max and duration since injury (Figure 1), and if reduced muscle mass is contributing to reduced VO2max, these data suggest that restitution of muscle mass was unlikely to occur despite many years post-injury. That said, we cannot distinguish whether a sustained loss of muscle mass, if in fact that occurred in the observed subjects, is due to an inability to increase muscle mass or simply a lack of physical training.
Decreased physical activity independently will reduce physical fitness (i.e. VO2max).17 Every 1% TBSA burned results in approximately 1 day of hospitalization.18 The cardiovascular detraining that occurs with bed rest alone can lead to a 15% reduction in VO2max in as little as 10 days.17 Therefore, it is likely that hospitalization associated with a burn injury causes an acute reduction in cardiovascular fitness secondary to bed rest deconditioning. This is particularly evident in the low VO2max values (21.7 ± 7.0 ml/kg/min) reported by de Lateur et al. in subjects 37.4 ± 23.3 days post-injury.2 Thus, upon leaving the hospital, skin grafted individuals’ VO2max is profoundly reduced. If that level of aerobic fitness is not returned to a pre-injured state, then the aerobic capacity of severely burned individuals is “reset” downward. This event, along with well-known age-related decreases in aerobic fitness19 would result in skin graft patients having persistently low VO2max values even decades post injury (Figure 1).
In the present dataset VO2max was not correlated to the duration post injury (Figure 1) or the percent of TBSA grafted (Figure 2). These observations suggest that the decrements in aerobic capacity years following an injury are unrelated to the extent of the injury and accompanying TBSA grafted or the period of time following the injury. Regarding the former, greater TBSA burned results in longer hospitalization and recovery times,18 and thus it would be reasonable to hypothesize a greater loss of aerobic fitness.17 On the contrary, one could hypothesize that the longer the duration post-injury the more time one would have to improve aerobic fitness following hospitalization, although this apparently is not the case with the present dataset. It is possible that the lack of relationships between VO2max and TBSA grafted, as well as years following the injury, is substantially influenced by level of physical activity after full recovery. Similarly, fitness level prior to the injury may also play a role in an individual’s ability to maintain or improve fitness during recovery. Unfortunately, an assessment of the level of physical activity before the injury, and from the time of recovery until we assessed the subject, was not obtained. Thus, we cannot identify if in the present dataset the few subjects showing normal (and even elevated) VO2max values had a higher level of physical activity before their injury or since their injury, relative to their lower fit counterparts. It is interesting to note that there were three subjects with VO2max values in the 80–90th percentiles of age- and sex-matched normative values. Based upon this very small sample size, it is possible that subjects who are more physically active prior to their injury (i.e., greater VO2max), and/or are more physically active after their injury, have elevated VO2max values relative to less active burned individuals. Prospective research will be required to address those questions.
A key question is whether low aerobic fitness years following the injury is based upon physiological mechanisms (i.e., an inability to improve VO2max for physiological reasons, inclusive of the aforementioned muscle atrophy) and/or perhaps psychological barriers associated with burn injury that lead to reduced desire to participate in physical activity. For example, years following a burn injury, fatigue is an “almost universal complaint” as a major barrier preventing such individuals from returning to work and performing activities of daily living.20 59% of burned individuals report problems with fatigue an average of 17 years post-injury.21 Other perceived or real barriers to physical activity may include impaired temperature regulation, hypertrophic scars and contractures that may limit range of motion, hyperpigmentation, psychosocial barriers, decreased cutaneous sensation, and a lower quality of life.7, 22–28
Even if these barriers can be overcome, the exercise training response in burned adults is relatively unknown. Grisbrook et al. showed similar improvements in VO2max (compared to non-burned subject) when previously burned adults participated in a 12-week aerobic/resistance training program.29 However, these results should be viewed in the context of a small sample size (n = 9), the absence of a difference in VO2max between groups at baseline, and peak heart rates that are appreciably less than age-predicted maximums (pre-training peak heart rates ~166 bpm for ~39 year olds). However in burned children (~10 years old), there is growing evidence that 12 weeks of aerobic/resistance training significantly improves lean body mass, muscle strength, and VO2max compared to burned children that do not undergo training.30, 31 Future studies are warranted to investigate the extent to which a large group of severely burned adults, across a wide range of TBSA, have the capacity to improve aerobic fitness with exercise training.
By design, the present study did not include a non-burned control group from which VO2max could be compared relative to the burned group. To eliminate selection bias we chose to use normative data representative of large population sets (upwards to thousands of individuals from three unique datasets), an approach that is routinely used in clinical exercise and pulmonary function testing.32–36 Therefore the employed comparisons are more representative of the non-burned population, relative to if we assessed 25 non-burned individuals. Lastly, by making comparisons with several data sets (AHA, ACSM, etc.) we show that the results are consistent independent of the epidemiological dataset used.
Regardless of the cause, a lower physical fitness compared to age-matched normative values puts skin grafted individuals at an increased cardiovascular risk. Every 5 ml/kg/min decrement in VO2max corresponds to a ~56% higher prevalence of a cardiovascular risk factor,3 and a maximal aerobic capacity below the 20th percentile (of which 76% of the investigated cohort resides) is associated with an increased risk of death from all causes.1 Importantly, improving aerobic fitness decreases one’s risk of all-cause mortality,1 with the strongest reduction in death being from a cardiovascular event.6 With the number of burn survivors escalating due to improved medical treatment, it is important that future studies investigate the barriers to physical activity and subsequent methods to improve physical fitness, which in turn can improve mortality and morbidity in skin graft patients years following the injury.
In conclusion, the present findings clearly show that a substantial percentage of skin graft patients have a disproportionally low VO2max many years after their injury relative to non-burned individuals. The reasons for this lower aerobic fitness are unknown, although it may be the result of reduced physical activity and perhaps associated physiological and/or psychological barriers. Alternatively, comparable levels of physical activity in skin grafted individuals may not result in the same improvements in cardiovascular fitness relative to non-burned individuals. Regardless of the mechanism(s), the present data show that individuals with well-healed skin grafts have lower cardiovascular fitness many years after injury which will increase their risk of all cause mortality.
Acknowledgments
We thank the subjects for their participation. We also thank Kim Hubing, Jena Langlois, and Gary Purdue for their assistance. This work was supported by the National Institutes of General Medical Sciences Grant GM068865.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no conflict of interest to declare.
References
- 1.Blair SN, Kohl HW, 3rd, Barlow CE, S PR, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. Jama. 1995;273:1093–8. [PubMed] [Google Scholar]
- 2.de Lateur BJ, Magyar-Russell G, Bresnick MG, et al. Augmented exercise in the treatment of deconditioning from major burn injury. Archives of physical medicine and rehabilitation. 2007;88:S18–23. doi: 10.1016/j.apmr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Aspenes ST, Nilsen TI, Skaug EA, et al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43:1465–1473. doi: 10.1249/MSS.0b013e31820ca81c. [DOI] [PubMed] [Google Scholar]
- 4.American burn association national burn repository. Chicago, IL: American Burn Association; 2007. [Google Scholar]
- 5.Blakeney PE, Rosenberg L, Rosenberg M, Faber AW. Psychosocial care of persons with severe burns. Burns. 2008;34:433–440. doi: 10.1016/j.burns.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Blair SN, Kohl HW, 3rd, S PR, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 7.van Baar ME, Essink-Bot ML, Oen IM, Dokter J, Boxma H, van Beeck EF. Functional outcome after burns: A review. Burns. 2006;32:1–9. doi: 10.1016/j.burns.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Willis CE, Grisbrook TL, Elliott CM, Wood FM, Wallman KE, Reid SL. Pulmonary function, exercise capacity and physical activity participation in adults following burn. Burns. 2011;37:1326–1333. doi: 10.1016/j.burns.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 9.SAKSON JA. Simplified chart for estimating burn areas. Am J Surg. 1959;98:693–694. doi: 10.1016/0002-9610(59)90492-1. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: A statement for healthcare professionals from the american heart association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 11.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: Review and commentary. Med Sci Sports Exerc J1 – msse. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 12.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 13.American College of Sports Medicine’s Guidlines for Exercise Testing and Prescription. 7. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 14.Milner EA, Cioffi WG, Mason AD, McManus WF, Pruitt BA., Jr A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994;37:167–170. doi: 10.1097/00005373-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe RR. Review: Acute versus chronic response to burn injury. Circ Shock. 1981;8:105–115. [PubMed] [Google Scholar]
- 17.Convertino V, Hung J, Goldwater D, DeBusk RF. Cardiovascular responses to exercise in middle-aged men after 10 days of bedrest. Circulation. 1982;65:134–140. doi: 10.1161/01.cir.65.1.134. [DOI] [PubMed] [Google Scholar]
- 18.Curreri PW, Luterman A, Braun DW, Jr, Shires GT. Burn injury. analysis of survival and hospitalization time for 937 patients. Ann Surg. 1980;192:472–478. doi: 10.1097/00000658-198010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astrand I, Astrand PO, Hallback I, Kilbom A. Reduction in maximal oxygen uptake with age. J Appl Physiol. 1973;35:649–654. doi: 10.1152/jappl.1973.35.5.649. [DOI] [PubMed] [Google Scholar]
- 20.Helm P, Herndon DN, Delateur B. Restoration of function. J Burn Care Res. 2007;28:611–614. doi: 10.1097/BCR.0B013E318093E4CA. [DOI] [PubMed] [Google Scholar]
- 21.Holavanahalli RK, Kowalske KJ, Helm PA. Long-term neuro musculoskeletal outcomes in patients surviving severe burns. 2009;30:S109. [Google Scholar]
- 22.Baker CP, Russell WJ, Meyer W, 3rd, Blakeney P. Physical and psychologic rehabilitation outcomes for young adults burned as children. Arch Phys Med Rehabil. 2007;88:S57–64. doi: 10.1016/j.apmr.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Simchon C, Tsur H, Keren G, Epstein Y, Shapiro Y. Heat tolerance in patients with extensive healed burns. Plast Reconstr Surg. 1981;67:499–504. doi: 10.1097/00006534-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Dyster-Aas J, Kildal M, Willebrand M. Return to work and health-related quality of life after burn injury. J Rehabil Med. 2007;39:49–55. doi: 10.2340/16501977-0005. [DOI] [PubMed] [Google Scholar]
- 25.Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: The skin. J Burn Care Res. 2010;31:631–639. doi: 10.1097/BCR.0b013e3181e4ca62. [DOI] [PubMed] [Google Scholar]
- 26.Klein MB, Lezotte DC, Heltshe S, et al. Functional and psychosocial outcomes of older adults after burn injury: Results from a multicenter database of severe burn injury. J Burn Care Res. 2011;32:66–78. doi: 10.1097/BCR.0b013e318203336a. [DOI] [PubMed] [Google Scholar]
- 27.Roskind JL, Petrofsky J, Lind AR, Paletta FX. Quantitation of thermoregulatory impairment in patients with healed burns. Annals of plastic surgery. 1978;1:172–6. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53:1019–22. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]
- 29.Grisbrook TL, Wallman KE, Elliott CM, Wood FM, Edgar DW, Reid SL. The effect of exercise training on pulmonary function and aerobic capacity in adults with burn. Burns. 2012;38:607–613. doi: 10.1016/j.burns.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 31.Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. J Burn Care Rehabil. 2002;23:288–93. doi: 10.1097/00004630-200207000-00013. discussion 287. [DOI] [PubMed] [Google Scholar]
- 32.Quanjer PH, Hall JL, Stanojevic S, Cole TJ, Stocks J. Age- and height-based prediction bias in spirometry reference equations. 2012;40:190–197. doi: 10.1183/09031936.00161011. [DOI] [PubMed] [Google Scholar]
- 33.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coates AL, Graham BL, McFadden RG, et al. Spirometry in primary care. Can Respir J. 2013;20:13–21. doi: 10.1155/2013/615281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegewald MJ, Crapo RO. Pulmonary function testing. In: Mason RJ, Broaddus VC, Martin TR, et al., editors. Textbook of Respiratory Medicine. Philadelphia, PA: Saunders Elsvevier; 2010. pp. 522–553. [Google Scholar]
- 36.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Lippincott Williams & Wilkins; 2010. Normal values; pp. 160–182. [Google Scholar]


