Abstract
In this study we investigated the development of the hypothalamic–pituitary–adrenal (HPA) axis in 21 group-living rhesus monkeys infants that were physically abused by their mothers in the first few months of life and in 21 nonabused controls. Cortisol and adrenocorticotropin hormone (ACTH) responses to a corticotropin-releasing hormone (CRH) challenge were assessed at 6-month intervals during the subjects’ first 3 years of life. Abused infants exhibited greater cortisol responses to CRH than controls across the 3 years. Abused infants also exhibited blunted ACTH secretion in response to CRH, especially at 6 months of age. Although there were no significant sex differences in abuse experienced early in life, females showed a greater cortisol response to CRH than males at all ages. There were no significant sex differences in the ACTH response to CRH, or significant interactions between sex and abuse in the ACTH or cortisol response. Our findings suggest that early parental maltreatment results in greater adrenocortical, and possibly also pituitary, responsiveness to challenges later in life. These long-term alterations in neuroendocrine function may be one the mechanisms through which infant abuse results in later psychopathologies. Our study also suggests that there are developmental sex differences in adrenal function that occur irrespective of early stressful experience. The results of this study can enhance our understanding of the long-term effects of child maltreatment as well as our knowledge of the development of the HPA axis in human and nonhuman primates.
Child maltreatment is a major determinant of adult psychopathologies such as clinical depression, anxiety and panic disorders, and posttraumatic stress disorders (Cicchetti & Cohen, 2006). One of the neuroendocrine systems most likely to be affected by maltreatment is the hypothalamic–pituitary–adrenal (HPA) axis, which plays a major role in the response to stress and regulation of emotions (Glaser, 2000; Nemeroff, 2004; Sanchez, 2006; Teicher et al., 2003). Some studies have suggested that maltreated children and adolescents show elevated basal cortisol levels and HPA hyperreactivity to challenges (e.g., Bugental, Martorell, & Barraza, 2003; Carrion et al., 2002; Hart, Gunnar, & Cicchetti, 1995, 1996; Kaufman, 1991; Kaufman et al., 1997; Tarullo & Gunnar, 2006), whereas adults with a history of childhood maltreatment tend to show basal hypofunctionality of the HPA axis and sensitized responses to psychological stress (Heim et al., 2000; Heim, Newport, Bonsall, Miller, & Nemeroff, 2001; Roy, 2002). Both maltreated children/adolescents and adults, however, often also suffer from clinical depression or posttraumatic stress disorder (e.g., Yehuda, Halligan, & Grossman, 2001), which complicates the interpretation of their hormonal profiles.
Our understanding of the neuroendocrine consequences of child maltreatment is constrained by our limited knowledge of the development of the HPA axis in general. Although recent studies have provided data on salivary cortisol concentrations in children of different ages (e.g., Gunnar & Donzella, 2002; Gunnar & Vazquez, 2006), few or no human studies have collected longitudinal information on HPA axis function and with measures other than salivary cortisol. In addition, because there are sex differences both in the probability of being victims of parental maltreatment and in the risk of developing certain psychopathologies later in life (e.g., Zahn-Waxler, Shirtcliff, & Marceau, 2008), knowledge of the normal development of the HPA axis in males and females is necessary to understand the mechanisms through which early maltreatment results in later psychopathology. Developmental sex differences in HPA axis function, however, are generally poorly understood. Research with an animal model of child maltreatment can allow us to conduct longitudinal studies of the effects of maltreatment on experimental assessments of HPA axis function and also illustrate the normative patterns of neuroendocrine development in males and females from birth to adulthood.
Child maltreatment is not a uniquely human phenomenon. Among rhesus macaques and other cercopithecine monkeys living in large captive groups, 5–10% of all infants born in a given year are physically abused by their mothers (Maestripieri & Carroll, 1998; Maestripieri, Wallen & Carroll, 1997). In rhesus macaques, abusive mothers may drag their infants by their tail or leg, or throw them in the air. Abuse bouts last only a few seconds and the rest of the time abusive mothers show competent patterns of maternal behavior. Unlike humans, in which child physical maltreatment is often accompanied by neglect, in rhesus macaques abuse and neglect do not occur together, and are displayed by individuals with different characteristics (e.g., neglectful mothers tend to be very young or very old, whereas abuse occurs regardless of the mother’s age). Abuse is most frequent in the first month of infant life and rare or nonexistent after the third month, when infants are more independent from their mothers (Maestripieri, 1998). Rhesus mothers can give birth once a year, and abusive mothers generally maltreat all of their infants with similar rates and patterns of behavior (Maestripieri, Tomaszycki, & Carroll, 1999). The contributions of infant behavior to the occurrence of abuse are negligible, whereas abusive behavior appears to be a stable maternal trait that is transmitted across generations, from mothers to daughters. As a result, it is concentrated in particular families and absent in others (Maestripieri & Carroll, 1998). Cross-fostering experiments demonstrated that early experience plays an important role in the intergenerational transmission of infant abuse (Maestripieri, 2005). Experience may include both social learning and long-term alterations of neurobiological systems regulating emotions and behavior. For example, cross-fostered and noncross-fostered infants reared by highly rejecting and abusive mothers have lower cerebrospinal fluid (CSF) concentrations of the serotonin metabolite 5-hydroxyindole-acetic acid (5-HIAA) in their first 3 years of life than females reared by control mothers (Maestripieri, Higley, Lindell, Newman, McCormack, & Sanchez, 2006; Maestripieri, Mc-Cormack, Lindell, Higley, & Sanchez, 2006). Approximately half of the females abused early in life exhibit abusive parenting with their first-born offspring (Maestripieri, 2005), and those who do so have lower CSF concentrations of 5-HIAA than those who do not (Maestripieri, Higley, et al., 2006; Maestripieri, Lindell, & Higley, 2007). Although behavioral data suggest that abusive mothers are highly anxious and hyperresponsive to stress, probably as a result of their early experience (Maestripieri, 1994; McCormack, Sanchez, Bardi, & Maestripieri, 2006; Troisi & D’Amato, 1991), no study to date has investigated the effects of early infant abuse on the development in HPA function in rhesus monkeys or other primates.
In this study, we investigated the development of HPA axis function in abused and nonabused rhesus monkeys during the first 3 years of life. HPA function was assessed at 6-month intervals by measuring cortisol and adrenocorticotropin hormone (ACTH) responses to administration of exogenous corticotropin-releasing hormone (CRH). In previous studies, maltreated children showed blunted ACTH secretion in response to the CRH challenge, whereas cortisol secretion was normal or elevated (De Bellis et al., 1994; Kaufman et al., 1993; but see Kaufman et al., 1997). In this study, we also investigated sex differences in hormonal responses to the challenge. Such sex differences could result from variation in the amount of abuse experienced early in life or could represent differences in neuroendocrine function between males and females, which occur regardless of early experience.
Method
Subjects
Subjects were 42 rhesus monkey infants living in large social groups at the Field Station of the Yerkes National Primate Research Center in Lawrenceville, Georgia. The groups were housed in 38×38 m outdoor compounds with indoor housing areas. They consisted of 20–50 adult females with their immature offspring and two to five unrelated adult males. All groups had a stable matrilineal structure and a linear dominance hierarchy. Female dominance ranks were assessed using data on unidirectional aggression and submission collected during previous studies.
Twenty-one infants (9 males, 12 females) were reared by multiparous mothers with a history of abusive parenting, whereas 21 of them (9 males, 12 females) were reared by non-abusive controls. The abusive mothers used in this study had been observed in previous years and their abusive behavior had been documented (Maestripieri, 1998; Maestripieri et al., 1999). Only mothers whose frequency and severity of abuse did not jeopardize their infant’s life were used. These abusive mothers were typically consistent in the frequency and severity with which they abused offspring born in successive years (Maestripieri et al., 1999). Control mothers were multiparous females without a history of abusive parenting who had with similar characteristics (e.g., age, parity, dominance rank, and infant sex) as the abusive mothers, and who gave birth in the same time period and in the same social groups as the abusive mothers.
Procedures
Behavioral data collection
All 42 infants were studied longitudinally, in their own social groups, from birth to 36 months of age. Infants and their mothers were focally observed in 30-min sessions, 5 times per week during the first month of life, 2 times per week during the second month of life, and 1 time per week from the third month through the end of the study. All behavioral data were converted into mean hourly rates of behavior per month for the purposes of data analysis. The three observers who collected the data were tested for reliability prior to the beginning of data collection. Analysis of the behavioral data focused on hourly rates of maternal abuse. Infant abuse was operationally defined as (see Maestripieri, 1998; Troisi & D’Amato, 1991): dragging: the mother drags her infant by its tail or leg while walking or running; crushing: the mother pushes her infant on the ground with both hands; rough grooming: the mother forces her infant onto the ground, and pulls out the infant’s hair with force causing distress calls; throwing: the mother throws her infant a short distance with one hand while standing or walking; hitting: the mother violently slaps her infant with one hand or arm; biting: common definition; stepping or sitting on: the mother steps on her infant with one foot or both feet, or sits on her infant; abusive carrying: the mother carries the infant with one arm away from her body, preventing the infant from clinging. Abuse events did not last more than a few seconds, and therefore only their frequency was recorded.
Training and capture procedures
Study subjects were trained for capture to accelerate this process and minimize arousal. Animals are trained to move on command from the outdoor corral into an indoor capture unit, from this to a transfer box, and from the box to a squeeze cage, where blood samples can be collected. Training is done using positive reinforcement and following guidelines and protocols approved by the Emory University IACUC. Infants less than 12 months of age are typically carried into the cage by their mothers and then separated from them. However, they learn the training procedure during their first year of life so that, in subsequent years, they can be captured without their mothers and with the same procedure used for the adults. Previous work has shown that infants who have experienced these procedures exhibit no alterations in normal development (Wilson, Gordon, & Collins, 1986). Once in the cage, subjects are anesthetized and their blood is collected within <10 min from the time the monkeys see the experimenters approach their outdoor compound. Under these conditions, elevations in plasma cortisol concentrations are minimal or nonexistent (e.g., Blank, Gordon, & Wilson, 1983). Circulating ACTH, however, rises more quickly than cortisol in response to environmental perturbations, making it difficult to obtain basal measures of plasma ACTH in group-living monkeys, even when they are trained for capture.
CRH challenge
When the subjects were 6, 12, 18, 24, 30, and 36 months old, their ACTH and cortisol responses to a CRH challenge were assessed at 6-month intervals. Subjects were immediately anesthetized after transfer to the squeeze cage with an intramuscular (i.m.) injection of Telazol (5 mg/kg). A basal blood sample was taken (0 min) by femoral venipuncture to assess prechallenge cortisol and ACTH plasma concentrations. An intravenous (i.v.) bolus of r/h CRH (50 μg/kg) or a vehicle solution (10 mM acetic acid/sterile 0.9% saline) was administered into the saphenous vein of the animal. Additional blood samples (1 ml) were taken from the femoral vein at 15, 30, and 60 min after the i.v. infusion, to analyze ACTH and/or cortisol responses to the treatment. Only one pharmacological challenge was done per week per animal, following a within-subject, repeated measures, counterbalanced design for drug order, and each animal serving as its own vehicle control to compare with the responses to the drug. A Telazol supplement (5 mg/kg, i.m.) was rarely needed to maintain animals sedated to complete testing, and this factor was considered in the data analysis. Upon recovery from anesthesia each subject was returned to its social group. These protocols have been previously validated in rhesus monkeys and humans, including studies in our own laboratory. The procedures were done in the early morning (at sunrise) according to published protocols for studies of HPA diurnal rhythms in macaques (Sanchez et al., 2005). Because the animals live under natural lighting conditions, sunrise times were obtained from charts published by the US Naval Observatory to use the daylight, and not the clock time, as a reference. This is important because of the acute stimulatory effect of awakening and light on cortisol secretion in the early morning (Scheer & Buijs, 1999). The study was done during the same season for all experimental groups, to control for cir-cannual differences in HPA axis activity. Experimental procedures were always performed prior to the animals being fed to avoid meal-induced HPA axis activation. Our choice to do the studies in the early morning, instead of the afternoon (when highest HPA responses would be expected because of reduced inhibition of the system), was based on the need to minimize arousal because animals in these groups are accustomed to routine procedures in the early morning. This choice is also supported by previous evidence that childhood maltreatment and orphanage rearing result in low early morning cortisol levels in children near what should be the peak of the circadian cycle (Gunnar & Fisher, 2006), and that adverse rearing experiences result in similar effects in developing rhesus monkeys (Boyce, Champoux, Suomi, & Gunnar, 1995; Sanchez et al., 2005). The response to the CRH challenge was assessed while the animals were under anesthesia to minimize the stress associated with repeated handling and blood sampling after the drug injection. Administration of saline solution was used to control for the possible effects of anesthesia and blood drawing procedures on the hormonal variables of interest. Although we analyzed the effects of sex and abuse on the prechallenge (basal) hormone concentrations, the investigation of the development of basal HPA axis activity in our subjects is beyond the scope of this paper. A comprehensive analysis of developmental changes in plasma cortisol levels under basal conditions and in response to stress in abused and nonabused infants (with a different set of data obtained while the subjects were not anesthetized) is presented elsewhere (Sanchez et al., unpublished data).
Hormonal assays
Blood samples were collected in prechilled polypropylene tubes containing EDTA and immediately placed on ice. Plasma was separated by centrifugation at 1000 × g for 10 min at 4°C and then aliquoted and stored at −80°C until assayed. Plasma concentrations of cortisol were assayed in duplicate 10-μl aliquots by radioimmunoassay using commercially available kits (Diagnostic Systems Lab, Webster, TX). The sensitivity of this assay was 1.25 μg/dl and intra- and interassay coefficients of variation in each assay were <10%. ACTH plasma levels were assayed in duplicate 200-μl aliquots by a two-site IRMA method using commercial kits (Nichols Institute Diagnostics, San Juan Capistrano, CA). The sensitivity of the assay was 1 pg/ml and inter- and intraassay coefficients of variation were <6%.
Data analyses
Sex differences and age effects on the frequency of infant abuse were analyzed with a mixed design analysis of variance (ANOVA), with sex being the fixed factor and age the repeated measure. Differences between abused and control infants, and between male and female infants, in their prechallenge hormone concentrations or in the hormonal responses to the challenge were analyzed with mixed design ANOVAs, with age as a repeated measure. We used 2 × 2 ANOVAs to assess possible interactions between sex and early experience on the hormonal responses to the challenge. The Pearson’s correlation coefficient was used for correlations. Whenever data were not normally distributed, analyses were conducted with log transformed data. All tests are two-tailed and p ≤.05 was considered statistically significant.
Results
Early maternal abuse
All infants reared by abusive mothers were abused by them, whereas none of the infants reared by the control mothers was abused. Infant abuse was most frequent in the first month of life and virtually disappeared after the third month. Abuse resulted in infant distress behavior and superficial scratches or bruises. No abused subject in this study suffered injuries that required the intervention of a veterinarian. Figure 1 illustrates the mean rates of abuse per hour experienced by male and female infants in the first 3 months of life. A repeated measures ANOVA revealed a significant effect of age, F (2, 38) =4.49, p =.01, but no significant sex difference in abuse, F (1, 38) = 1.38, p = .25, or a significant interaction between age and sex, F (2, 38) = 0.02, p = .97.
Figure 1.
Mean (+SE) number of abuse episodes per hour experienced by male and female abused infants in the first, second, and third month of life.
Effects of sex and abuse on cortisol responses to the CRH challenge
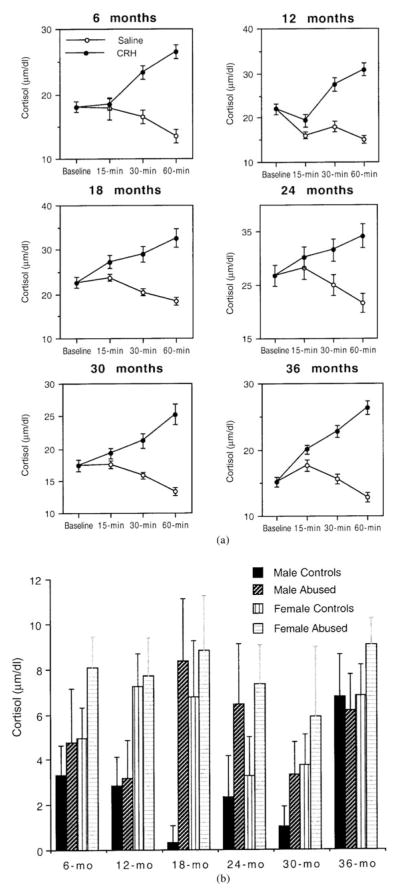
Figure 2a illustrates the plasma cortisol concentrations measured in the baseline sample and in the samples obtained 15, 30, and 60 min after the baseline sample at the ages of 6, 12, 18, 24, 30, and 36 months in all subjects and controls. There was a significant effect of age on baseline cortisol, F (5, 200) = 18.4, p <.0001, but no main effect of sex, F (1, 500) = 0.19, p = .66, or a significant interaction between age and sex, F (5, 200) = 0.51, p = .76. There was no significant main effect of abuse on basal cortisol, F (1, 200) = 0.35, p = .56, or a significant interaction between abuse and age, F (5, 200) = 0.88, p = .49. Neither sex nor abuse had significant effects on cortisol levels measured after the injection of saline solution across the six ages (all ANOVAs are nonsignificant).
Figure 2.
(a) Plasma cortisol concentrations in the baseline sample and in samples obtained 15, 30, and 60 min after the baseline in abused and control infants receiving CRH and saline solution at 6, 12, 18, 24, 30, and 36 months of life. (b) Plasma cortisol response to CRH (difference between 30-min post-CRH cortisol and baseline cortisol) in male and female abused and control infants at 6, 12, 18, 24, 30, and 36 months of life.
As depicted in Figure 2a, plasma cortisol concentrations increased following the administration of exogenous CRH, whereas they decreased following the injection of saline solution. The post-CRH increase and the postsaline decrease in cortisol were especially apparent in the samples obtained 30 and 60 min after the baseline sample. The cortisol concentrations measured 30 and 60 min after the baseline were highly positively correlated for both the post-CRH and the postsaline data (most coefficients of correlations were >.70, with probabilities of <.0001). All subsequent analyses of cortisol concentrations measured after 30 and 60 min produced similar results. Therefore, to avoid redundancy, only the results concerning the 30-min cortisol responses will be shown. Because sex and abuse did not affect baseline cortisol, the difference between post-CRH and baseline cortisol provides a good measure of the cortisol response to CRH without the need for controlling for differences in the baseline (if the difference between post-CRH cortisol and postsaline cortisol is used as a measure of cortisol response to CRH, the results of all analyses are very similar). However, because age significantly affected baseline cortisol, this variable was taken into consideration in subsequent analyses.
Sex had a significant main effect on the cortisol response to CRH, F (1, 200) = 6.14, p = .01, with females showing higher responses than males across the six ages (Fig. 2b). There were no significant effects of age, F (5, 200) = 1.75, p = .12, or significant interactions between sex and age: 30 min, F (5, 200) = 0.47, p = .79. Abuse had a significant main effect on the cortisol response to CRH as well, F (1, 200) = 5.71, p = .02, with abused infants showing higher responses than controls across the six ages (Fig. 2b). There were no significant interactions between abuse and age, F (5, 200) = 0.79, p = .55. Possible interactions between the effects of sex and abuse on the cortisol response were analyzed separately at each age with 2 × 2 factorial ANOVAs. There were no significant interactions between these variables at any age. As illustrated in Figure 2b, male controls had the lowest cortisol response to CRH, male abused subjects had comparable responses to those of female controls, and female abused subjects showed the highest cortisol responses to CRH across all ages. Among abused subjects, there was no significant correlation between the average hourly rates of abuse experienced in the first 3 months of life and the cortisol response to CRH at any of the six ages (all coefficients of correlation are nonsignificant). Subjects reared by mothers of high and low dominance rank did not differ significantly in their cortisol responses to CRH at any of the six ages (all t tests are nonsignificant).
Effects of sex and abuse on the ACTH response to the CRH challenge
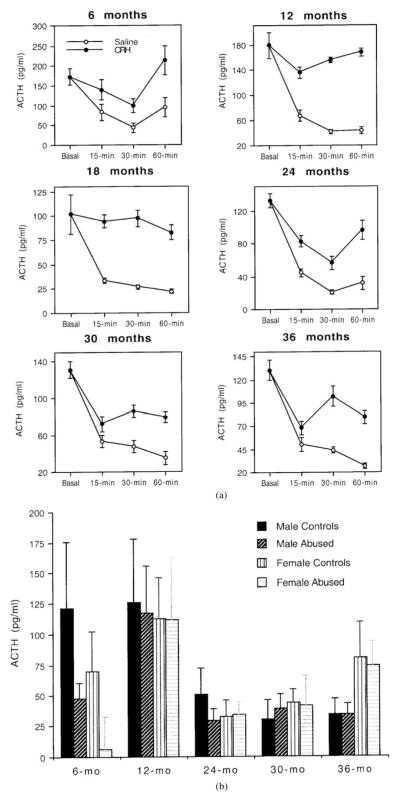
Figure 3a illustrates the plasma ACTH concentrations measured in the baseline sample and in the samples obtained 15, 30, and 60 min after the baseline sample at the ages of 6, 12, 18, 24, 30, and 36 months in all subjects and controls. Results at 18 months of age were obtained with less than half the subjects and controls because of technical problems with experimental procedures. Because of this, data at 18 months of age were excluded from subsequent analyses. At all ages, the plasma concentration of ACTH in the baseline sample was high because of the stress of capture. There was a significant effect of age on baseline ACTH, F (4, 160) = 3.36, p = .01, but no main effect of sex, F (1, 160) = 0.03, p = .86, or a significant interaction between age and sex, F (4, 160) = 1.35, p =.25. There was no significant main effect of abuse on basal ACTH, F (4, 160) = 2.38, p = .13, or a significant interaction between abuse and age, F (4, 160) = 1.60, p =.17. Neither sex nor abuse had significant effects on ACTH levels measured after the injection of saline solution across the five ages (repeated measures ANOVAs; all tests are nonsignificant).
Figure 3.
(a) Plasma ACTH concentrations in the baseline sample and in samples obtained 15, 30, and 60 min after the baseline in abused and control infants receiving CRH and saline solution at 6, 12, 18, 24, 30, and 36 months of life. (b) Plasma ACTH response to CRH (difference between 30-min post-CRH ACTH and baseline ACTH) in male and female abused and control infants at 6, 12, 24, 30, and 36 months of life. Data for 18 months were not available for analyses and are not shown in the figure.
As depicted in Figure 3b, plasma ACTH concentrations decreased markedly after the injection of saline solution, whereas they decreased less markedly or remained fairly stable following the administration of CRH. This pattern suggests that anesthesia decreased dramatically the high concentrations of ACTH resulting from capture stress, and that the administration of CRH delayed or suppressed this decrease of ACTH. The concentrations of ACTH in samples obtained 30 and 60 min were highly positively correlated, for both the post-CRH and the postsaline samples. Therefore, as with cortisol, we present only the results of analyses conducted with ACTH data obtained 30 min following the baseline. The difference between the ACTH concentrations measured at the baseline and those measured 30 min following CRH was considered the ACTH response to CRH (if the difference between post-CRH ACTH and postsaline ACTH is used as a measure of the ACTH response to CRH, all results are very similar).
There were no significant effects of sex or age on the ACTH response to CRH: sex, F (1, 160) = 0.07, p = .79; age, F (4, 160) = 2.21; p = .07, or a significant interaction between these variables, F (4, 160) = 0.76, p = .55. There was, however, a significant main effect of abuse on the ACTH response to CRH, F (1, 160) = 3.98, p = .05, as well as a significant interaction between abuse and age, F (4, 160) = 2.66, p = .03. Abused subjects showed a greater reduction in ACTH levels following CRH than controls. As illustrated in Figure 3b, this difference was particularly marked at 6 months of age (although there also seems to be a sex difference at this age, it is not significant), but minimal or nonexistent at subsequent ages. Among abused subjects, there was no significant correlation between the average hourly rates of abuse experienced in the first 3 months of life and the ACTH response to CRH at 6 months or later ages (all coefficients of correlation are nonsignificant). Subjects reared by mother of high and low dominance rank did not differ significantly in their ACTH responses to CRH at any of the six ages (all t tests are nonsignificant).
Discussion
Rhesus monkey infants reared by mothers with a history of abusive parenting were abused by them with a frequency of approximately 1.5 events per hour in their first month of life. Abuse was less frequent in the second month, and in most cases ended in the third month. Although the frequency of abuse appeared to be higher for female than for male infants, this difference was not statistically significant. A lack of significant difference in the frequencies of abuse experienced by male and female infants was also reported by previous studies, some of which used a much larger sample size (e.g., Maestripieri & Carroll, 1998; Maestripieri et al., 1997).
The administration of exogenous CRH resulted in an increase in plasma cortisol concentrations, and this cortisol response was greater among abused subjects than among controls, regardless of sex and age. Thus, a greater cortisol response to CRH in abused subjects was observed in both males and females and at all ages considered in this study, namely, when the subjects were 6, 12, 18, 24, 30, and 36 months old. There was also a significant difference between abused and nonabused subjects in the ACTH response to CRH, but this difference was mostly observed at 6 months of age. Our subjects showed elevated plasma ACTH in their prechallenge sample, presumably because of the capture procedure, and the CRH administration delayed or suppressed the ACTH decrease while the subjects were under anesthesia. Abused subjects showed a greater reduction in ACTH following CRH administration than controls did, suggesting that they showed blunted ACTH secretion post-CRH administration.
Taken together, these results suggest that the stressful (both physically and psychologically) experience of being abused early in life resulted in both short- and long-term alterations in HPA function. The greater cortisol responses to CRH observed in the abused subjects are consistent with the results of many animal and human studies showing that individuals who are exposed to traumatic stress early in life exhibit later HPA hyperreactivity to challenges (for a review, see Gunnar & Quevedo, 2007). Blunted ACTH responses to a CRH challenge have also been observed in abused children (De Bellis et al., 1994; Kaufman et al., 1993) as well as in adults suffering from clinical depression (e.g., Holsboer, Gerkin, Stalla, & Muller, 1987). Another study reported enhanced, rather than blunted, ACTH response to the CRH challenge in abused children, but this finding was limited to children living under conditions of chronic ongoing adversity (e.g., continued abuse and family violence, poverty, and lack of social support; Kaufman et al., 1997). The blunted ACTH response observed in the abused monkey infants may be due to rapid negative feedback glucocorticoid inhibition, which normally inhibits the secretion of ACTH when there is a rapid increase in circulating cortisol, or to a dysfunction of the mechanisms that regulate the anterior pituitary’s response to hypothalamic CRH.
In previous work conducted with cross-fostered subjects we demonstrated that hypofunction of the brain serotonergic system resulting from early exposure to abusive and rejecting maternal behavior may be one of the physiological mechanisms underlying the relation between early experience and later psychopathologies (Maestripieri, Higley, et al., 2006). Hyperresponsiveness of the HPA axis to exogenous stimulation may be another mechanism underlying the effects of early abuse on adult behavior and physiology. For example, high cortisol release in response to potentially risky situations involving infants(Maestripieri, 1993, 1994, 1995) may interfere with maternal behavior and trigger abuse.
In addition to documenting effects of abuse on the cortisol and ACTH responses to CRH, in the present study we also found sex differences in endocrine responsiveness. Specifically, female subjects, both abused and nonabused, exhibited higher cortisol responses to the CRH challenge than males did across the six ages, whereas there was no significant sex difference in the ACTH response. An early study of rhesus macaques reported higher cortisol responses to exogenous ACTH in females than in males (Bowman & Wolf, 1969) and several subsequent studies reported higher cortisol responses to various types of stressful challenges in females than in males (e.g., Clarke, 1993; Gust, Gordon, & Hambright, 1993; Meyer & Bowman 1972; Scallet, Suomi, & Bowman, 1981). In humans, there is some evidence that females have lower basal levels of ACTH and a higher ratio of cortisol to ACTH production rate than males, suggesting that the female adrenal cortex is more responsive to ACTH than its male counterpart (Born, Ditschumeit, Schreiber, Dodt, & Fehm, 1995; Roelfsema et al., 1993). Explanations for the previously reported sex differences in HPA axis function in human and nonhuman primates have implicated the influence of female gonadal hormones on HPA axis function (Scallet et al., 1981). The current study, however, shows that some sex differences are already present long before puberty.
Taken together, the results of this study contribute to our understanding of the long-term consequences of child maltreatment and the neuroendocrine mechanisms through which this early traumatic experience may result in later psychopathologies.
They also contribute to our understanding of the normal development of the HPA axis, including sex differences in adrenocortical responsiveness. Further research should address the exact mechanisms through which infant abuse alters ACTH and cortisol secretion in response to challenges, as well as the functional implications of sex differences in neuroendocrine function.
Acknowledgments
This research was supported by NIH Grants R01-MH62577 and K02-MH63097 (to D.M.), R21-MH01005 (to M.M.S.), and RR-00165 (to the Yerkes Center). Support was also provided by a NARSAD Young Investigator Award (to M.M.S.). The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. We thank Paul Plotsky for support in the initial stages of this project.
Footnotes
The authors declare no conflicting financial or other competing interests.
References
- Blank MS, Gordon TP, Wilson ME. Effects of venipuncture on serum levels of prolactin, growth hormone, and cortisol in outdoor compound-housed female rhesus monkeys. Acta Endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Born J, Ditschumeit X, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary–adrenocortical responsiveness in humans. European Journal of Endocrinology. 1995;132:705–711. doi: 10.1530/eje.0.1320705. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Wolf RC. Plasma 17-hydroxycorticosteroid response to ACTH in M. mulatta: Dose, age, weight, and sex. Proceedings of the Society for Experimental Biology and Medicine. 1969;130:61–64. doi: 10.3181/00379727-130-33488. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: Reactivity to peer interactions and altered circadian activity. Developmental Psychobiology. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones & Behavior. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Carrion V, Weems C, Ray R, Glaser B, Hessl D, Reiss A. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Cohen DJ, editors. Developmental psychopathology. New York: Wiley; 2006. [Google Scholar]
- Clarke AS. Social rearing effects on HPA activity over early development and in response to stress in rhesus monkeys. Developmental Psychobiology. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, et al. Hypothalamic–pituitary–adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology & Metabolism. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain: A review. Journal of Child Psychology and Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA. The early experience, stress, and prevention network: Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Developmental neuroscience. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Gust DA, Gordon TP, Hambright K. Response to removal from and return to a social group in adult male rhesus monkeys. Physiology and Behavior. 1993;53:599–602. doi: 10.1016/0031-9384(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Altered neuroendocrine activity in maltreated children related to symptoms of depression. Development and Psychopathology. 1996;8:201–214. [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–180. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:292–297. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Gerkin A, Stalla G, Muller A. Blunted aldosterone and ACTH release after human RH administration in depressed patients. American Journal of Psychiatry. 1987;144:229–231. doi: 10.1176/ajp.144.2.229. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:257–265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Brent D, Birmaher B, Perel J, Dahl RE, Nelson B, et al. Measures of family adversity, clinical symptomatology, and cortisol secretion in a sample of preadolescent depressed children. Paper presented at the Annual Meeting of the Society for Research in Child and Adolescent Psychopathology; Santa Fe, NM. 1993. [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993;95:19–31. [Google Scholar]
- Maestripieri D. Infant abuse associated with psychosocial stress in a group-living pigtail macaque (Macaca nemestrina) mother. American Journal of Primatology. 1994;32:41–49. doi: 10.1002/ajp.1350320105. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Assessment of danger to themselves and their infants by rhesus macaque (Macaca mulatta) mothers. Journal of Comparative Psychology. 1995;109:416–420. doi: 10.1037/0735-7036.109.4.416. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Animal Behaviour. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Science of the United States of America. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in rhesus monkeys. Psychological Science. 1998;9:143–145. [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuseruns infamilies of group-living pigtail macaques. Child Abuse and Neglect. 1997;21:465–471. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack K, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behavioral Neuroscience. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus monkeys and its underlying mechanisms. Developmental Psychobiology. 2007;49:165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behavior and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behavioural and Brain Research. 2006;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Developmental Psychobiology. 1999;34:29–35. [PubMed] [Google Scholar]
- McCormack KM, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Bowman RE. Rearing experience, stress, and adrenocorticosteroids in the rhesus monkey. Physiology and Behavior. 1972;8:339–343. doi: 10.1016/0031-9384(72)90382-4. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- Roelfsema F, van den Berg G, Frolich M, Veldhus JD, van Eijk A, Buurman MM, Etman BHB. Sex-dependent alteration in cortisol response to endogenous adrenocorticotropin. Journal of Clinical Endocrinology and Metabolism. 1993;77:234–240. doi: 10.1210/jcem.77.1.8392084. [DOI] [PubMed] [Google Scholar]
- Roy A. Urinary free cortisol and childhood trauma in cocaine dependent adults. Journal of Psychiatry Research. 2002;36:173–177. doi: 10.1016/s0022-3956(02)00002-x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in non-human primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Suomi SJ, Bowman RE. Sex differences in adrenocortical response to controlled agonistic encounters in rhesus monkeys. Physiology and Behavior. 1981;26:385–390. doi: 10.1016/0031-9384(81)90163-3. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. Journal of Clinical Endocrinology and Metabolism. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Troisi A, D’Amato FR. Anxiety in the pathogenesis of primate infant abuse: A pharmacological study. Psychopharmacology. 1991;103:571–572. doi: 10.1007/BF02244261. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development and Psychopathology. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]