Abstract
Background
In order to investigate the role of non-surgical treatment for early-stage esophageal cancer, we compared the outcomes of local therapy to esophagectomy using a large, national database.
Methods
Five-year cancer-specific and overall survival of patients with T1N0M0 squamous cell or adenocarcinoma of the mid or distal esophagus treated with either surgery or local therapy with ablative and/or excision techniques in the SEER cancer registry from 1998–2008 were compared using the Kaplan-Meier approach and multivariable and propensity score adjusted Cox proportional-hazard and competing risk models.
Results
Of 1458 patients with T1N0 esophageal cancer, 1204 (83%) had surgery and 254 (17%) had local therapy only. The use of local therapy increased significantly from 8.1% in 1998 to 24.1% in 2008 (p<0.001). The 5-year overall survival after local excisional therapy and surgery was not significantly different (55.5% vs 64.1% respectively, p=0.07); 5-year cancer-specific survival also did not differ (81.7% vs 75.8%, p=0.10). However, after propensity-score adjustment, cancer-specific survival was better for patients undergoing local therapy compared to surgery (HR: 0.46, 95% CI: 0.27–0.77, p=0.003), while overall survival remained similar.
Conclusion
The use of local therapy for T1N0 esophageal cancers increased significantly from 1998 to 2008. Compared to esophagectomy, patients treated with local therapy had similar overall survival but improved cancer specific survival, indicating a higher chance of dying from other causes. Further studies are needed to confirm the oncologic efficacy of local therapy when used in patients whose lifespans are not limited by conditions other than esophageal cancer.
Introduction
The prognosis for patients treated for intra- and submucosal esophageal cancers is significantly better than the prognosis for all other patients found to have esophageal cancer, even those also found in other relatively early-stage disease [1]. Historically, esophagectomy has been demonstrated to be oncologically efficacious; however, despite improvement over time, surgery is still associated with considerable morbidity and mortality [2–8]. Local treatments with modalities such as endoscopic mucosal resection, radiofrequency ablation, cryotherapy, and photodynamic therapy have shown potential for providing effective cancer treatment with much less treatment-related morbidity [9–20]. However, most reports related to these therapies involve relatively small clinical trials or single-institution retrospective reviews with limited long term follow up. We sought to investigate treatment trends of local therapy use for T1N0 esophageal cancer using the population-based, national Surveillance Epidemiology and End Results (SEER) cancer registry. To evaluate the efficacy of local therapy compared to esophagectomy, we also sought to test the hypothesis that patients with stage T1N0M0 esophageal cancer in the SEER database from 1998–2008 who underwent esophagectomy had improved survival compared to patients that had local therapy.
Methods
Approval was obtained from the Duke University Institutional Review Board prior to conducting this retrospective cohort analysis using SEER data for patients from 1998 to 2008. SEER*Stat 7.0.5 was used to extract patients 18 years or older with cancer of the mid or lower esophagus. Patients were primarily identified through the “SEER Site Recode” using the term “esophagus”. The variable “Histologic Type ICD-O-3″ (International classifications of Diseases for Oncology, 3rd edition) was used to restrict the study cohort to patients with either squamous cell cancer (codes 8050-8089) or adenocarcinomas (codes 8140-8389). To restrict the cohort to patients with T1N0M0 tumors, the tumor-node-metastasis (TNM) stage was either directly extracted from the SEER database or manually recoded using available SEER variables. The 6th edition of the AJCC Cancer Staging Manual served as the basis for this recoding [21]. Patients with unknown or other TNM stages were excluded from the analysis.
The primary outcome was 5-year cancer specific (CSS) and overall survival (OS), measured in months. Patients alive at the last available follow-up date in SEER were right censored at this date in the survival analysis. The following additional patient characteristics were extracted from the dataset: age, gender, race (White, Black, other/unknown), marital status (married, other/unknown), and cause of death (alive, esophagus, other cause of death). In addition, data on tumor grade (well/moderate, poor/undifferentiated, unknown), tumor location (mid or distal esophagus), and histology (adenocarcinoma, squamous cell) were collected. Based on treatment information available in SEER, we defined two distinct treatment groups: esophagectomy and local therapy. All other patients were excluded from the analysis.
Detailed information on the depth of invasion of T1 tumors was recorded starting in 2004, which allowed further stratification of T1 tumors into those that did not invade the submucosa (T1a) and those that invaded the submucosa (T1b), according to the newer, 7th edition of the AJCC Cancer Staging System [22]. Because the risk of lymph node metastases increases to 26% when the submucosa is involved, local therapy with endoscopic mucosal resection as curative intent has been proposed as indicated for T1 tumors that do not invade the submucosa [9, 23, 24]. Therefore, subgroup survival analyses were performed for patients with T1a tumors.
Statistical analysis
Comparisons of patient characteristics among the two treatment groups were performed using chi-square test for categorical (frequency, percentages) and two-sample, unpaired t-test for continuous variables (mean, standard deviations). To assess early versus late treatment among patients undergoing local therapy, patients were grouped in two time-periods; early from 1998–2003 and late from 2004–2008. To compare treatment trends over time between patients undergoing esophagectomy and patients undergoing local therapy, multivariable adjusted logistic regression models were calculated while year of operation was the main predictor. Adjustment was performed for gender, age at diagnosis, race, marital status, tumor grade, tumor localization, radiation therapy use, and histology.
Cancer specific survival (CSS) was defined by a cause of death from esophageal etiology while patients dying from another cause were treated as competing risk and patients alive were right censored. Meanwhile, overall survival (OS) included all deaths from any cause in the follow-up period while patients alive were right censored. Because staging in patients with local tumor destruction cannot be assessed with pathological examination, those patients were excluded from the survival analysis so clinical over-staging or under-staging in these patients would not bias survival results. To compare CSS and OS among the treatment groups, survival curves were initially constructed according to the Kaplan-Meier approach and compared using the log-rank test. Subsequently, unadjusted, multivariable, and propensity-score adjusted Cox proportional hazard models for OS and competing-risks regression models for CSS were calculated. Results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Adjustment in the survival analyses was performed for the following covariates: gender, age, race, marital status, tumor grade, tumor location, histology, and year of diagnosis (five groups). The propensity score was calculated based on a logistic regression model where esophagectomy and local therapy were representing the outcome while the following characteristics were used as covariates in the propensity score calculation: gender, age, race, marital status, tumor grade (unknowns were included as additional category), tumor location, histology, and year of diagnosis (five groups). In the propensity score adjusted survival models, the propensity score representing receipt of esophagectomy or local therapy was added as an additional potential confounder. To account for immortal time bias in regard of receipt of esophagectomy and radiation therapy after diagnosis, we performed two sets of landmark studies in the survival analyses by left truncating patients who survived less than 3 or 6 months [25]. These additional analyses allow to further decrease selection bias by excluding patients with short-term adverse perioperative mortality or for life limiting comorbidities.
All statistical analyses were performed using STATA/SE version 11.2 (Stata Corporation, College Station, TX, USA) and R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria), the significance level alpha was set at 0.05 and two-sided p-values were calculated for all analyses.
Results
A total of 1458 patients with T1N0M0 esophageal cancer of the mid and lower esophagus were identified in the SEER cancer registry during the study period from 1998 to 2008: Of these, 1204 (83%) were treated with surgery and 254 (17%) had local therapy only. Detailed patient and tumor characteristics stratified by treatment group are presented in table 1. Compared to patients undergoing surgery, patients treated with local therapy were significantly older, more likely to be female, more likely to have a mid esophageal cancer, and less likely to also have received radiation therapy. Table 2 shows the specific types of local therapy utilized. A modality that involved local tumor excision was most commonly used.
Table 1.
Patient characteristics
| Esophagectomy (n=1204) | Local Therapy (n=254) | p-value | |
|---|---|---|---|
|
| |||
| Age (mean, SD), years | 64.4 (10.0) | 73.4 (9.7) | <0.001 |
|
| |||
| Female | 190 (15.8%) | 60 (23.6%) | 0.003 |
|
| |||
| Race | |||
| White | 1,111 (92.3%) | 240 (94.5%) | 0.17 |
| Black | 45 (3.7%) | 10 (3.9%) | |
| Other/Unknown | 48 (4.0%) | 4 (1.6%) | |
|
| |||
| Marital Status | |||
| Married | 850 (70.6%) | 164 (64.6%) | 0.06 |
| Other/Unknown | 354 (29.4%) | 90 (35.4%) | |
|
| |||
| Tumor location | |||
| Mid esophagus | 199 (16.5%) | 56 (22.1%) | 0.04 |
| Lower esophagus | 1,005 (83.5%) | 198 (78.0%) | |
|
| |||
| Tumor grade | |||
| G1/2 (well/moderate) | 649 (53.9%) | 105 (41.3%) | <0.001 |
| G3/4 (poor/undifferentiated) | 342 (28.4%) | 41 (16.1%) | |
| Unknown | 213 (17.7%) | 108 (42.5%) | |
|
| |||
| Histology | |||
| Squamous cell carcinoma | 220 (18.3%) | 38 (15.0%) | 0.21 |
| Adenocarcinoma | 984 (81.7%) | 216 (85.0%) | |
|
| |||
| Radiation therapy | |||
| No radiotherapy | 957 (79.5%) | 217 (85.4%) | 0.02 |
| Beam radiotherapy | 242 (20.1%) | 34 (13.4%) | |
| Unknown | 5 (0.4%) | 3 (1.2%) | |
|
| |||
| Cause of death | |||
| Alive | 831 (69.0%) | 179 (70.5%) | <0.001 |
| Esophagus | 228 (18.9%) | 27 (10.6%) | |
| Other cause of death | 145 (12.0%) | 48 (18.9%) | |
|
| |||
| Time period | |||
| Early (1998–2003) | 487 (40.4%) | 48 (18.9%) | <0.001 |
| Late (2004–2008) | 717 (59.6%) | 206 (81.1%) | |
Table 2.
Distribution of Local Therapies
| Procedure | Overall; n (%) | Early (1998–2003); n (%) | Late (2004–2008); n (%) |
|---|---|---|---|
|
| |||
| Local tumor destruction | |||
| Photodynamic therapy | 22 (8.7) | 8 (16.7) | 14 (6.8) |
| Electrocautery | 2 (0.8) | 0 | 2 (1.0) |
| Cryosurgery | 4 (1.6) | 0 | 4 (1.9) |
| Laser | 8 (3.2) | 2 (4.2) | 6 (2.9) |
| NOS | 2 (0.8) | 0 | 2 (1.0) |
|
| |||
| Local tumor excision | |||
| Polypectomy | 23 (9.1) | 3 (6.3) | 20 (9.7) |
| Excisional biopsy | 96 (37.8) | 19 (39.6) | 77 (37.4) |
| Laser excision | 2 (0.8) | 0 | 2 (1.0) |
| NOS | 51 (20.1) | 6 (12.5) | 45 (21.8) |
|
| |||
| Combined local tumor destruction and excision | 44 (17.3) | 10 (20.8) | 34 (16.5) |
There was a significant increase in local treatment use from 8.1% in 1998 to 24.1% in 2008 (Figure 1). This trend held true after multivariable adjustment with an odds ratio of 1.18 per year (CI: 1.11–1.25, p<0.001). No difference was found for patient and tumor characteristics comparing patients undergoing local therapy during the early and late time period (data not shown).
Figure 1. Change of utilization of esophagectomy and local therapy from 1998 to 2008.
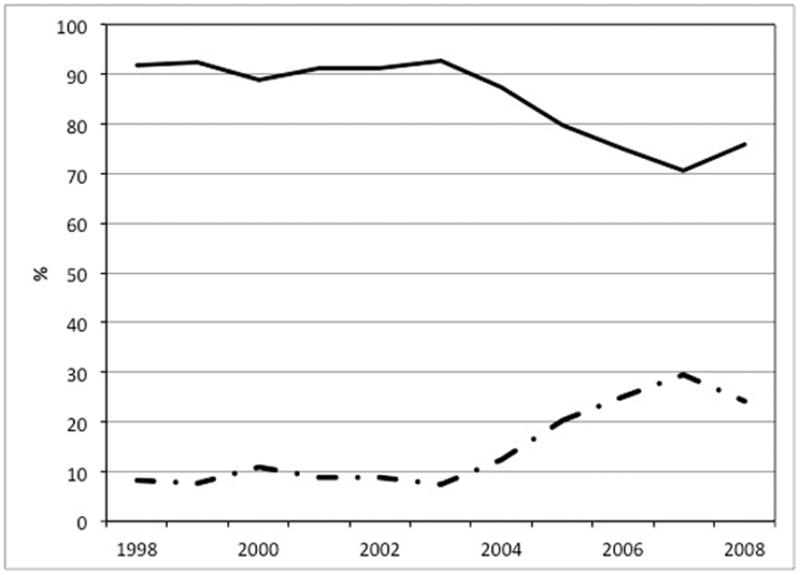
Straight line: esophagectomy. Discontinuous line: local therapy. Multivariable adjusted p for trend < 0.001 (OR per year: 1.18, 95% CI: 1.11–1.25)
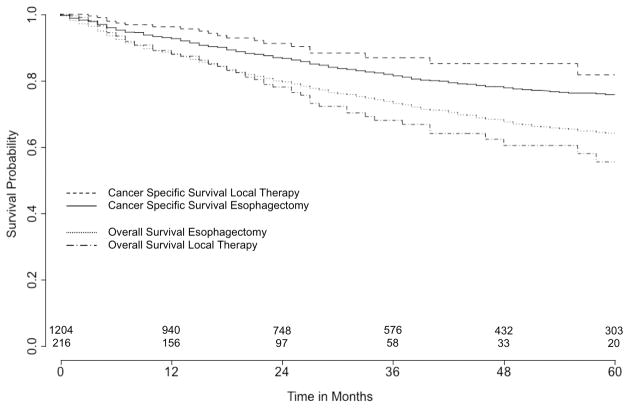
The results of survival analysis comparing esophagectomy with local excisional therapy are shown in table 3 and figure 2. Median follow-up was 34 months for surgery and 21 months for local excisional therapy (p<0.001), while mean follow-up was 40.5 months for surgery and 27.3 months for local excisional therapy (p<0.001). For all patients, 5-year OS was 63.2 % (95% CI: 60.0–66.3) and 5-year CSS was 76.5% (CI: 73.5–79.2). 5-year OS after local excisional therapy and surgery was not significantly different [55.5% (CI: 44.0–65.6) vs 64.1% (CI: 60.7–67.3), p=0.07] as was 5-year CSS [81.7% (CI: 70.2–89.1) vs 75.8% (CI: 72.6–78.7), p=0.10]. However, although local excisional therapy did not predict any statistically significant difference in overall survival compared to surgery in both multivariable (HR: 0.85, CI: 0.63–1.15, p=0.29) and propensity score (HR: 0.88, CI: 0.64–1.20, p=0.42) adjustment, local excisional therapy did predict improved CSS compared to surgery in both multivariable (HR: 0.46, CI: 0.28–0.76, p=0.002) and propensity score adjusted survival analyses (HR: 0.46, CI: 0.27–0.77, p=0.003).
Table 3.
Cancer specific and overall survival for patients undergoing esophagectomy and local therapy
| 5-year survival (95% CI) | p-value | Unadjusted HR (95% CI) | p-value | Multivariable adjusted HR (95% CI) | p-value | Propensity score adjusted HR (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cancer specific survival
| ||||||||
| Overall Treatment: | 76.5 (73.5–79.2) | |||||||
| Esophagectomy | 75.8 (72.6–78.7) | Ref. | Ref. | Ref. | ||||
| Local Therapy* | 81.7 (70.2–89.1) | 0.10 | 0.62 (0.40–0.98) | 0.04 | 0.46 (0.28–0.76) | 0.002 | 0.46 (0.27–0.77) | 0.003 |
|
| ||||||||
| Overall survival
| ||||||||
| Overall Treatment: | 63.2 (60.0–66.3) | |||||||
| Esophagectomy | 64.1 (60.7–67.3) | Ref. | Ref. | Ref. | ||||
| Local Therapy* | 55.5 (44.0–65.6) | 0.07 | 1.28 (0.98–1.68) | 0.07 | 0.85 (0.63–1.15) | 0.29 | 0.88 (0.64–1.20) | 0.42 |
Patients with local destruction only were excluded from this analysis.
Figure 2. 5-year cancer specific and overall survival comparing esophagectomy and local therapy.
Number of patients at risk at time 0 (esophagectomy: n=1,204; local therapy: n=254). Log rank test for cancer specific survival: p=0.10, for overall survival: p=0.07.
The short-term OS after esophagectomy was similar to local excisional therapy at 1 month [esophagectomy 98.6% (CI: 97.7–99.1) vs local excisional therapy 99.5% (CI: 96.7–99.9)], 3 months [esophagectomy 96.9% (CI: 95.7–97.7) vs local excisional therapy 98.0% (CI: 94.9–99.3)] and 6 months [esophagectomy 92.9% (CI: 91.2–94.2) vs local excisional therapy 93.4% (CI: 88.9–96.1)], p>0.26 for all comparisons. To address potential immortal bias in the survival analyses and to compare long-term outcomes of esophagectomy and local excisional therapy when short-term, presumably treatment and comorbidity-related, mortality is excluded, landmark studies for CSS and OS were performed by applying 3 and 6 month left truncation to survival times (Table 4). These landmark studies showed similar findings to the non-landmark survival analysis. Patients treated with local therapy continued to have better CSS in both the three and six month truncated studies, while OS was not different between the local excisional therapy and esophagectomy patients.
Table 4.
Landmark analysis with left truncation for 3 and for 6 months - cancer specific and overall survival
| Left truncation for 3 months | Left truncation for 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Multivariable adjusted HR (95% CI) | p-value | Propensity score adjusted HR (95% CI) | p-value | Multivariable adjusted HR (95% CI) | p-value | Propensity score adjusted HR (95% CI) | p-value | |
|
| ||||||||
|
Cancer specific survival
| ||||||||
| Esophagectomy Local Therapy* | Ref. 0.53 (0.32–0.89) | 0.02 | Ref. 0.53 (0.32–0.90) | 0.02 | Ref. 0.52 (0.30–0.91) | 0.02 | Ref. 0.53 (0.30–0.94) | 0.03 |
|
| ||||||||
|
Overall survival
| ||||||||
| Espohagectomy Local Therapy* | Ref. 0.90 (0.66–1.23) | 0.52 | Ref. 0.94 (0.68–1.30) | 0.69 | Ref. 0.94 (0.67–1.32) | 0.73 | Ref. 0.99 (0.70–1.41) | 0.96 |
Patients with local destruction only were excluded from this analysis.
Subgroup analysis for patients with T1a tumor stage
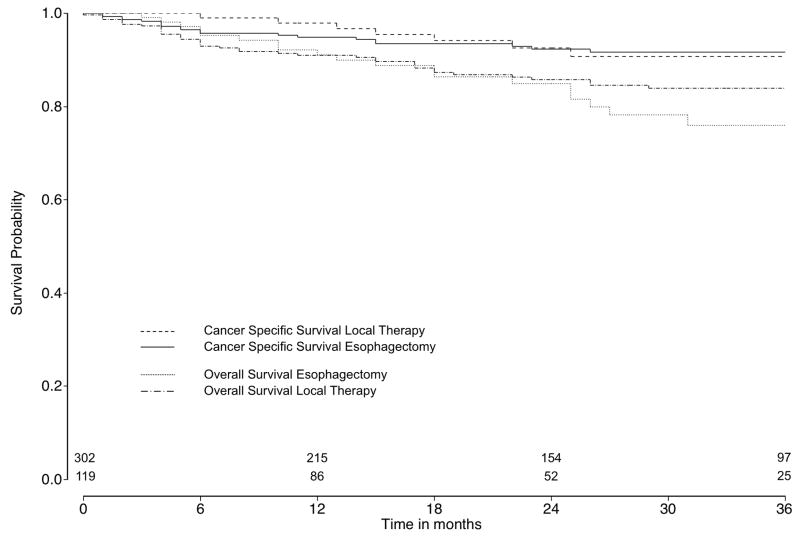
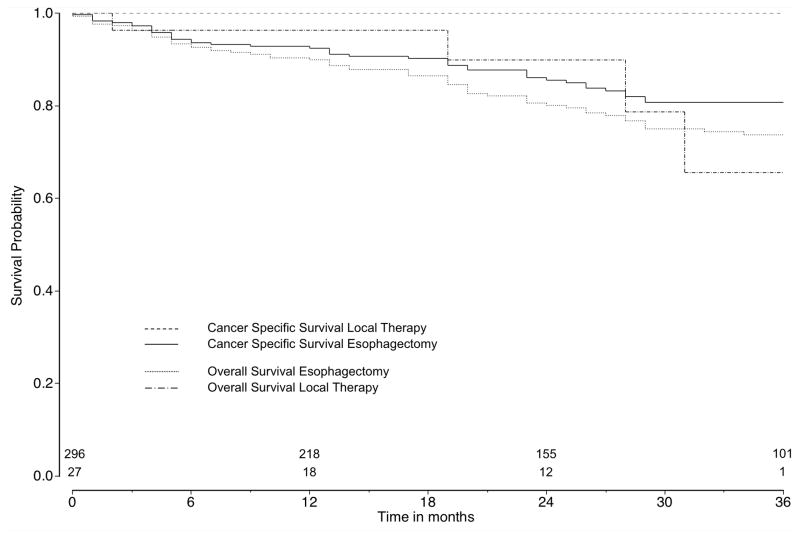
Among patients diagnosed from 2004 to 2008, information about T1a and T1b tumor stage was available for 764 patients (82.8%). Of those, 436 (57.1%) patients had a T1a esophageal cancer while 302 (69.3%) patients underwent esophagectomy and 134 (30.7%) patients local therapy. Among patients with T1b tumor stage (n=328, 42.9%), 296 (90.2%) patients underwent surgery and 32 (9.8%) patients local therapy. Among T1a and T1b patients, 15 (3.4%) and 5 (1.5%) patients underwent local tumor destruction, respectively, which were excluded from survival analysis. For patients with T1a tumors, survival for esophagectomy (2-year CSS: 92.3%, CI: 88.1–95.1; 2-year OS: 86.1, CI: 81.0–89.8) and local excisional therapy (2-year CSS: 92.6%, CI: 84.0–96.6; 2-year OS: 84.9, CI: 75.7–90.8) did not differ, even after multivariable (HR for CSS: 0.55, CI: 0.25–1.21, p=0.14; HR for OS: 1.01, CI: 0.57–1.81, p=0.96) and propensity score adjustment (HR for CSS: 0.56, CI: 0.27–1.19, p=0.13; HR for OS: 1.02, CI: 0.56–1.85, p=0.94) (Figure 3a). Kaplan-Meier survival curves for patients with T1b tumors are shown in Figure 3b, although similar comparative survival analyses were not performed due to the small numbers (n=27) of T1b tumors treated with local excisional therapy.
Figure 3.
Figure 3a. Cancer-specific and overall survival comparing esophagectomy and local therapy in T1a tumor subgroups
Number of patients at risk at time 0 (esophagectomy: n=302; local therapy: n=119). Log rank test for cancer specific survival: p=0.93, for overall survival: p=0.31.
Figure 3b. Cancer-specific and overall survival comparing esophagectomy and local therapy in T1b tumor subgroups
Number of patients at risk at time 0 (esophagectomy: n=296; local therapy: n=27). Log rank test for cancer specific survival: p=0.07, for overall survival: p=0.80.
Discussion
In this study using the SEER database, which is the largest United States population based cancer registry, we found that local therapy was increasingly utilized for the treatment of T1N0M0 esophageal cancer over the time period 1998–2008, with a concomitant decrease in the use of esophagectomy. Overall survival after local therapy was similar to overall survival after esophagectomy, including short-term follow-up of 1 month, 3 months, and 6 months after diagnosis. However, patients treated with local therapy had better cancer-specific survival. These results are encouraging and support the promise of utilizing local therapy as an effective oncologic treatment in patients with early-stage esophageal cancer. Considering the recovery and potential morbidity associated with the alternative treatment of esophagectomy, local therapy is a very attractive therapeutic option. However, some caution is needed before generalizing these results as support that all patients with superficial esophageal cancer should be treated with local therapy. The fact that patients treated with local therapy have similar overall survival but better cancer-specific survival than patients undergoing esophageal resection demonstrates that patients treated with local therapy are dying from causes other than esophageal cancer at a much higher rate than patients treated with esophagectomy. Although data recorded by SEER does not allow evaluation of comorbidities other than demonstrating that patients treated with local therapy were significantly older than the patients who underwent esophagectomy, many patients selected to receive local therapy likely had significant other medical conditions that were more immediately life-threatening than early-stage esophageal cancer and also may have made them medically ineligible for surgery. This possibility is supported by the observation that one month, three month, and six month survival after local therapy appears quite similar to that seen after esophagectomy, despite local therapies being generally thought to have less procedure-related mortality. This possibility is also further supported by the landmark analysis. Given that the results of the survival comparison between treatment modalities did not change in the landmark analysis with both three and six month truncation, the effects on survival on what would be expected to be increased peri-treatment mortality in the esophagectomy group appear to be offset from presumably non-procedure related mortality in the local therapy group.
Therefore, although results after local therapy appear similar to esophagectomy in this study, continued investigation is necessary before being able to conclude that oncologic outcomes are truly similar between the two approaches. The patients treated with local therapy in this study may have been dying of other causes before residual or recurrent esophageal cancer could occur or lead to mortality. Studies of local therapy with longer follow-up and younger patients who are not likely to have short-term deaths from other medical conditions are needed to truly demonstrate that local therapy does not have a higher rate of disease recurrence compared to esophagectomy. Further observational studies and perhaps even a multi-institutional randomized controlled trial are recommended to confirm the oncologic efficacy of local therapy. Until then, patients treated with local therapy should have close surveillance to evaluate for treatment failures and disease recurrence.
Despite the high mortality typically associated with esophagectomy, overall survival after esophageal cancer diagnosis at 1 month, 3 months, and 6 months after esophagectomy in this study was 98.6%, 96.9%, and 92.9%. These corresponding mortality rates of 1.4%, 3.1%, and 7.1% are generally better than those seen in multi-institutional studies or registries, and more inline with results reported by specialized centers [3–5,8]. Data from this study does not allow investigation into the reason for this lower than expected mortality. A possible explanation for this finding is that esophagectomy for early stage disease when there is not bulky tumor that increases the difficulty of resection and where patients are less likely to have been given induction therapy have significantly better results than when surgery is performed for more advanced tumors. Even if mortality is not significantly different in this study, it is important to acknowledge that esophagectomy likely has significantly higher peri-treatment morbidity than local therapy. In addition, esophagectomy may be associated with lifelong alterations in eating habits. To some patients, these potential short-term and long-term impacts on lifestyle may be worth the trade-off of potentially slightly higher disease recurrence rates.
Given that our data suggests that local treatment is at least not inferior to surgery, the use of local therapy may continue to increase for several reasons. First, the techniques of both local resection and local tissue destruction may improve and allow more patients to be adequately treated with these modalities. Second, the increasing percentage of older patients in society may result in more patients being found to have early-stage esophageal cancer but also considered marginal candidates for esophageal resection. Third, the encouraging results associated with local therapy may lead to its increased use in younger and healthier patients, who also would be considered good surgical candidates. However, the costs associated with long-term surveillance must be considered when local therapy is progressively used for younger and healthier patients.
Advantages to the use of SEER data for this analysis include its population based nature, with volume sufficient to enable subgroup analysis. However, SEER does also have some inherent limitations. First, data regarding chemotherapy administration are lacking. However, chemotherapy is not recommended for this stage of esophageal cancer [24], and so this limitation is not likely significant in this study. However, the exact influence of chemotherapy in this patient cohort cannot be evaluated. Second, as described above, there is a lack of data regarding patient co-morbidities that allow specific evaluation on the importance of co-morbidities on both treatment selection and survival. While propensity score adjusted analysis does account for the conditional probability of getting either surgery or local therapy, its power for controlling this selection bias is limited to the covariates available in the dataset.
In addition, because SEER does not contain detailed enough information about tumor stage to classify all patients to T1a and T1b tumors, we were limited to the broader tumor stage of T1 tumors in the main analysis. Patients with T1b tumors are recognized as being inappropriate candidates for local therapy as a curative intent, so the likely inclusion of at least some patients who had T1b tumors in the main analysis could bias the results seen with local therapy [9,23,24]. However, no survival benefit for either treatment was found in the time-limited subgroup analysis of patients with T1a tumors, further supporting the promise of local therapy as an equivalent oncologic treatment to esophagectomy. Also, early two-year cancer-specific survival for esophagectomy and local excisional therapy for patients with T1a tumors in the later years of the study (2004–2008) was not different. This finding suggests that local therapies may have been used more often in the later time period for patients who did not have significant co-morbidities that put them at risk of early death due to causes other than esophageal cancer. Further, the small number of patients with T1b tumors undergoing local excisional therapy in the subgroup analysis limits evaluation of the impact of tumor depth on the main analysis. Selection bias in treatment of T1b tumors may at least partially explain the difference seen for CSS for T1a tumors versus T1 tumors overall. Part of the selection bias may have arisen from the preferential use of local therapy for T1b tumors that were felt to be lower risk of recurring based on the more specific depth of submucosal invasion (sm1, sm2, sm3), which is not recorded in SEER. Given that local therapy is considered inadequate for T1b tumors due to high risk of lymph node involvement, the use of local therapy rather than surgery in these patients also may have been because the patients were not adequate surgical candidates due to high risk of death due to other significant co-morbid conditions. Indeed, none of the patients treated with local therapy for T1b tumors in the SEER dataset died for esophageal cancer reasons despite an overall 3-year survival of less than 75% (Figure 3b), further supporting selection bias of local therapy for T1b tumors in patients who were likely to have short-term death from something other than esophageal cancer. However, future studies including larger groups stratified for T1a and T1b including information on comorbidities with 5-year follow-up are warranted.
In conclusion, the use of local therapy for early stage superficial esophageal cancers in the SEER database has increased significantly over the time period from 1998 to 2008. Overall survival after esophagectomy and local therapy was similar, though patients treated with local therapy had shorter overall follow-up and were more likely to die from causes other than esophageal cancer. The ability to generalize the results found in this study to other patients who may not have comorbidities associated with significant short-term mortality is not clear. Further studies most likely including multi-institutional prospective randomized controlled trials as well as observational studies with longer follow-up are needed to determine if local therapy can be utilized with similar long-term oncologic outcomes to esophagectomy in all patients.
Acknowledgments
This work was in part supported by the NIH funded Cardiothoracic Surgery Trials Network (M.F.B).
References
- 1.Rice TW, Rusch VW, Ishwaran H, Blackstone EH Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116(16):3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 2.Chang LC, Oelschlager BK, Quiroga E, Parra JD, Mulligan M, Wood DE, Pellegrini CA. Long-term outcome of esophagectomy for high-grade dysplasia or cancer found during surveillance for Barrett’s esophagus. J Gastrointest Surg. 2006;10:341–6. doi: 10.1016/j.gassur.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–222. doi: 10.1016/s0003-4975(02)04368-0. [DOI] [PubMed] [Google Scholar]
- 4.Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85:424–429. doi: 10.1016/j.athoracsur.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–1120. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 6.Connors RC, Reuben BC, Neumayer LA, Bull DA. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg. 2007;205:735–740. doi: 10.1016/j.jamcollsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Dimick JB, Wainess RM, Upchurch GR, Jr, Iannettoni MD, Orringer MB. National trends in outcomes for esophageal resection. Ann Thorac Surg. 2005;79:212–216. doi: 10.1016/j.athoracsur.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Ra J, Paulson EC, Kucharczuk J, Armstrong K, Wirtalla C, Rapaport-Kelz R, et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol. 2008;15:1577–1584. doi: 10.1245/s10434-008-9867-4. [DOI] [PubMed] [Google Scholar]
- 9.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–8. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 10.Galey KM, Wilshire CL, Watson TJ, Schneider MD, Kaul V, Jones CE, Litle VR, Ullah A, Peters JH. Endoscopic management of early esophageal neoplasia: an emerging standard. J Gastrointest Surg. 2011;15:1728–35. doi: 10.1007/s11605-011-1618-3. [DOI] [PubMed] [Google Scholar]
- 11.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, Stolte M, Ell C. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 12.Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, Lutzke LS, Borkenhagen LS, Wang KK. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815–23. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC, Waxman I. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 14.Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–9. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 15.Larghi A, Lightdale CJ, Ross AS, Fedi P, Hart J, Rotterdam H, Noffsinger A, Memeo L, Bhagat G, Waxman I. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086–91. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 16.Sibille A, Lambert R, Souquet JC, Sabben G, Descos F. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108:337–44. doi: 10.1016/0016-5085(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 17.Corti L, Skarlatos J, Boso C, Cardin F, Kosma L, Koukourakis MI, Giatromanolaki A, Norberto L, Shaffer M, Beroukas K. Outcome of patients receiving photodynamic therapy for early esophageal cancer. Int J Radiat Oncol Biol Phys. 2000;47:419–24. doi: 10.1016/s0360-3016(00)00450-8. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Matono S, Nagano T, Murata K, Sueyoshi S, Yamana H, Shirouzu K, Fujita H. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc. 2011;73:1–6. doi: 10.1016/j.gie.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Fujita H, Sueyoshi S, Yamana H, Shinozaki K, Toh U, Tanaka Y, Mine T, Kubota M, Shirouzu K, Toyonaga A, Harada H, Ban S, Watanabe M, Toda Y, Tabuchi E, Hayabuchi N, Inutsuka H. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J Surg. 2001;25:424–31. doi: 10.1007/s002680020053. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein AJ, Wisnivesky JP, Litle VR. Effect of local therapy for the treatment of superficial esophageal cancer in non-operative candidates. Dis Esophagus. 2008;21:673–8. doi: 10.1111/j.1442-2050.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging manual. 6. Springer-Verlag; 2003. [Google Scholar]
- 22.Rice TW, Blackstone EH, Rybicki LA, Adelstein DJ, Murthy SC, DeCamp MM, Goldblum JR. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103–13. doi: 10.1067/mtc.2003.170. [DOI] [PubMed] [Google Scholar]
- 23.Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432–9. [PubMed] [Google Scholar]
- 24.Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, Fuchs CS, Gerdes H, Hayman JA, Hazard L, Ilson DH, Kleinberg LR, McAleer MF, Meropol NJ, Mulcahy MF, Orringer MB, Osarogiagbon RU, Posey JA, Sasson AR, Scott WJ, Shibata S, Strong VE, Swisher SG, Washington MK, Willett C, Wood DE, Wright CD, Yang G NCCN Esophageal Cancer Panel. Esophageal cancer. J Natl Compr Canc Netw. 2008;6:818–49. [PubMed] [Google Scholar]
- 25.Park HS, Gross CP, Makarov DV, Yu JB. Immortal Time Bias: A Frequently Unrecognized Threat to Validity in the Evaluation of Postoperative Radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–73. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]