Abstract
Background
Resection without adjuvant therapy results in a low recurrence rate for patients with stage I (T1/2 N0) rectal cancer, in the range of 4% to 16% at 5 years. There are limited data, however, regarding clinical or pathologic prognostic markers for recurrence in this population.
Objective
To assess clinical and pathologic factors associated with local recurrence and overall survival in patients with early stage rectal cancer after resection.
Design
Retrospective study.
Setting
This study was conducted at two tertiary care centers in Boston, MA.
Patients
From 2000 to 2008, 175 patients with stage I rectal cancer treated with local or total mesorectal excision without adjuvant therapy were identified.
Main Outcome Measures
Time to local recurrence after resection and overall survival were evaluated for all patients with complete follow up data. Perioperative data were reviewed to identify staging method, preoperative carcinoembryonic antigen, type of surgery, tumor size, number of lymph nodes resected, histological grade, circumferential resection margin, perineural invasion, lymphovascular invasion, and tumor ulceration. Data were analyzed using a Cox proportional hazards regression model.
Results
Of the eligible cohort, 137 patients had complete follow up data for analysis of time to local recurrence, and 23 (16.8%) recurred locally. Among these 23 patients, the median time to recurrence was 1.1 years (0.1-7.8). On multivariate analysis, male gender, current alcohol use, and tumor ulceration were associated with heightened risk of local recurrence. Of the original cohort, 173 patients had complete follow up for overall survival analysis. Among these patients, the median overall survival was 12 years. On multivariable analysis, age at diagnosis >65 years and T2 pathologic stage were associated with decreased survival.
Conclusions
For patients with stage I rectal cancer treated with resection alone, these results provide important prognostic information and may help identify those who could benefit from additional therapy.
Keywords: rectal cancer, recurrence, survival, prognosis
Introduction
More than 40,000 individuals are diagnosed with rectal cancer in the United States each year with a mortality rate near 40 percent.1 Surgery with or without chemoradiation therapy (CRT) is the primary treatment for these patients. Both local recurrence and distant metastasis are a major concern in rectal cancer, and each is associated with substantial morbidity and mortality.2 Advancements in surgical technique and neoadjuvant therapies, however, have resulted in reduced local and distant recurrence, with subsequent improvement in overall survival over the past decade.1,3 Tumor stage is the most important prognostic factor determining treatment strategy and outcomes. Preoperative CRT for locally advanced rectal cancer (T3/4 or node-positive) results in improved local control and disease-free survival.4-6 In these patients, neoadjuvant CRT often downstages the tumor, with a decrease in the size and depth of invasion and possible lymph node sterilization.
Approximately 25% of rectal cancer patients, however, present with stage I disease (i.e. T1/2 and N0).2 The standard of care for these patients is surgery alone without pre- or postoperative CRT. The local recurrence rate for these patients is low, in the range of 4 to 16%; adjuvant or neoadjuvant therapies have not resulted in an improvement in disease-free or overall survival that would outweigh the associated morbidity.5-7 Total mesorectal excision (TME) with abdominoperineal resection (APR) or sphincter-preserving low anterior resection (LAR) is the standard for radical resection of rectal cancer, and it is associated with improved local and distant recurrence rates for T1 and T2 tumors.8 Local excision of rectal cancer, on the other hand, remains controversial, particularly for T2 tumors. In a retrospective study comparing transanal local excision to radical resection without adjuvant CRT for T1 and T2 tumors, there was only a 4% local recurrence rate (0% for T1N0 tumors and 6% for T2N0 tumors) among patients treated with radical resection. The estimated five-year local recurrence rate for local excision patients was 28% (18% for T1N0 tumors and 47% for T2N0 tumors).8 However, the results from a recent prospective, multi-institutional study showed a local recurrence rate as low as 8% with a 10-year overall survival at 84% for T1N0 tumors treated with local excision alone.9 Thus, many institutions continue to manage early stage rectal cancer, especially T1N0 tumors, with transanal local excision without CRT due to improved morbidity and mortality, patient satisfaction, and reduced cost.10-12 Nonetheless, a 5-year mortality of 16 to 23% has been consistently reported for patients with stage I rectal cancer, regardless of surgical approach.13-16
Previous retrospective studies have identified specific pathologic characteristics, including lymphovascular invasion (LVI), positive circumferential resection margin (CRM), or absence of lymphocytic infiltration, as markers of worse survival for advanced staged rectal cancer.17-19 Nevertheless, few studies have assessed how such factors influence local recurrence and survival in early stage disease. This retrospective analysis sought to identify clinical and pathologic features that are prognostic for local recurrence and overall survival of early stage rectal cancer treated with surgery alone.
Materials and Methods
Study Population
Patients with histologically confirmed stage T1/2 N0 rectal adenocarcinoma who underwent resection without neoadjuvant or adjuvant CRT were eligible for this IRB-approved study. All patients underwent LAR, APR, or transanal local excision at the Brigham and Women's Hospital or Massachusetts General Hospital between 2000 and 2008. Patients with apparent metastases on preoperative computed tomography (CT) or positron emission tomography (PET) were excluded.
Clinical and Pathologic Evaluation
Data for each patient who underwent curative resection were retrospectively reviewed. Each of the following characteristics was collected for all patients: age, gender, race, smoking history, alcohol use, family history of gastrointestinal malignancies, clinical presentation, date of diagnosis, method of clinical staging, tumor distance from the anal verge, extent of circumferential involvement, preoperative CEA level, type of surgery (LAR, APR, local excision), and interval of time between diagnosis and surgery. Pathology reports were reviewed for TNM staging, tumor size, histological grade, CRM, number of lymph nodes resected, and the presence of lymphovascular invasion (LVI), perineural invasion (PNI), large vessel invasion, or ulceration. For patients with local or distant recurrence following surgery, medical records were reviewed to assess clinical presentation preceding diagnosis, location of recurrence, histological grade, and time interval from resection to recurrence. The date and status at last follow-up visit was recorded.
The tumor distance from the anal verge was defined as the distance from the caudal tumor edge to the anal verge, which was assessed by rigid sigmoidoscopy, colonoscopy, flexible sigmoidoscopy, MRI, and/or digital examination. Pretreatment clinical staging was performed using a combination of physical examination, CT imaging, magnetic resonance imaging (MRI) with or without endorectal coil, and/or endorectal ultrasound. The clinical and pathologic TNM stages were determined according to the American Joint Committee on Cancer TNM staging system (7th edition), and the histological grade of adenocarcinoma was described according to the World Health Organization classification.
Statistical Analysis
Descriptive statistics were used to describe patient characteristics and surgical/pathologic characteristics at study entry. Differences in the distribution of patient characteristics by gender were evaluated using Fisher's exact test and Wilcoxon rank sum test. The method of Kaplan and Meier was used to characterize time to local recurrence and overall survival. The Cox proportional hazards model was used to evaluate the associations between the factors of interest and time to local recurrence as well as overall survival. The variables with p < 0.1 in the univariable analysis were added to a multivariable model with p < 0.05 as the criterion to select variable. Analyses were conducted using SAS version 9.2.
Results
Study Population
Between 2000 and 2008, 175 patients were surgically treated for early stage rectal cancer at the Brigham and Women's Hospital and Massachusetts General Hospital. The median age was 65 years (24-89), and there were 95 men (54.3%) and 80 women (45.7%). Most patients were Caucasian (n=157, 90.8%). Most patients presented with rectal bleeding (n=106, 60.6%), followed by screening colonoscopy (n=60, 34.3%). Thirty-one patients (19.3%) had a first-degree relative with colorectal cancer. Patient characteristics are shown in Table 1.
Table 1. Patient Characteristics.
| Overall | LR Analysis | OS Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | n = 175 | % | n = 137 | % | n = 173 | % |
| Age at diagnosis, years | ||||||
| Median (range) | 65 (24-89) | 63 (24-85) | 65.5 (24-89) | |||
| ≤65 | 88 | 50.3% | 77 | 56.2% | 87 | 50.3% |
| >65 | 87 | 49.7% | 60 | 43.8% | 86 | 49.7% |
| Gender | ||||||
| Male | 95 | 54.3% | 72 | 52.6% | 95 | 54.9% |
| Female | 80 | 45.7% | 65 | 47.5% | 78 | 45.1% |
| Race | ||||||
| Caucasian | 157 | 90.8% | 123 | 90.4% | 156 | 91.2% |
| African American | 8 | 4.6% | 7 | 5.2% | 8 | 4.7% |
| Hispanic | 8 | 4.6% | 6 | 4.4% | 7 | 4.1% |
| Unknown | 2 | 1 | 2 | |||
| Smoking | ||||||
| Current | 13 | 7.8% | 10 | 7.5% | 13 | 7.9% |
| Former | 69 | 41.3% | 55 | 41.0% | 69 | 41.8% |
| Never | 85 | 50.9% | 69 | 51.5% | 83 | 50.3% |
| Unknown | 8 | 3 | 8 | |||
| Alcohol | ||||||
| Current | 84 | 50.3% | 71 | 53.0% | 83 | 50.3% |
| Former | 12 | 7.2% | 9 | 6.7% | 12 | 7.3% |
| Never | 71 | 42.5% | 54 | 40.3% | 70 | 42.4% |
| Unknown | 8 | 3 | 8 | |||
| Family History | ||||||
| None | 123 | 76.4% | 95 | 73.1% | 121 | 76.1% |
| Colorectal cancer | 31 | 19.3% | 29 | 22.3% | 31 | 19.5% |
| Gastric cancer | 4 | 2.5% | 4 | 3.1% | 4 | 2.5% |
| Esophageal cancer | 2 | 1.2% | 2 | 1.5% | 2 | 1.3% |
| Pancreatic cancer | 1 | 0.6% | 0 | 0.0% | 1 | 0.6% |
| Unknown | 14 | 7 | 14 | |||
| Clinical Presentation | ||||||
| Routine screening | 60 | 34.3% | 48 | 35.0% | 60 | 34.7% |
| Rectal bleeding | 106 | 60.6% | 83 | 60.6% | 104 | 60.1% |
| Stool changes | 12 | 6.9% | 9 | 6.6% | 12 | 6.9% |
| Constipation | 4 | 2.3% | 2 | 1.5% | 4 | 2.3% |
| Tenesmus | 4 | 2.3% | 3 | 2.2% | 4 | 2.3% |
| Abdominal distention | 2 | 1.1% | 1 | 0.7% | 2 | 1.2% |
| Incontinence | 1 | 0.6% | 1 | 0.7% | 1 | 0.6% |
The study included 102 patients (58.3%) with T1 tumors and 73 patients (41.7%) with T2 tumors. LAR was the most common surgical procedure, which was performed in 104 patients (59.4%), followed by local excision in 47 patients (26.9%) and APR in 21 patients (12.0%). Of the 47 patients who underwent local excision, the majority (n=37) received a traditional open transanal approach using an operative anoscope, while only 10 patients underwent transanal endoscopic microsurgery. There were no significant differences with regards to tumor level or margins obtained between the two approaches. Among the patients who underwent LAR, 60 (57.7%) had T1 and 44 (42.3%) had T2 tumors. Among those who underwent local excision, 37 (78.7%) had T1 and 10 (21.3%) had T2 tumors. Among those who underwent APR, 4 (19%) had T1 and 17 (81%) had T2 tumors. Three patients underwent total proctocolectomy because of familial adenomatous polyposis (n=1) or ulcerative colitis (n=2). The median tumor size for all patients was 2.2 cm (0.2-15.0 cm). The median CRM was 0.53 cm (0.2-1.1) for patients who underwent local excision, 1.06 cm (0.1-3.5) for APR, and 1.97 (0.1-6.5) for LAR. Among patients who underwent LAR or APR, there was a median of 12 lymph nodes (1-38) resected, and all were negative for metastasis. Surgical and pathologic characteristics are shown in Table 2. The median follow up time for all patients was 12 years.
Table 2. Surgical and Pathologic Characteristics.
| Overall | LR Analysis | OS Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | n = 175 | % | n = 137 | % | n = 173 | % |
| Surgical procedure | ||||||
| Low anterior resection | 104 | 59.4% | 83 | 60.6% | 103 | 59.5% |
| Abdominoperineal resection | 21 | 12.0% | 11 | 8.0% | 20 | 11.6% |
| Local excision | 47 | 26.9% | 42 | 30.7% | 47 | 27.2% |
| Total proctocolectomy | 3 | 1.7% | 1 | 0.7% | 3 | 1.7% |
| Preoperative CEA | ||||||
| Median (range) | 1.6 (0-16.8) | 1.45 (0.2-13.6) | 1.6 (0-13.6) | |||
| <5 | 64 | 90.1% | 53 | 91.4% | 63 | 91.3% |
| ≥5 | 7 | 9.9% | 5 | 8.6% | 6 | 8.7% |
| Unknown | 104 | 79 | 104 | |||
| Method of staging | ||||||
| Endoscopic US | 21 | 12.2% | 20 | 14.8% | 20 | 11.8% |
| MRI | 49 | 28.5% | 40 | 29.6% | 49 | 28.8% |
| None | 102 | 59.3% | 75 | 55.6% | 101 | 59.4% |
| Unknown | 3 | 2 | 3 | |||
| Pathologic stage | ||||||
| T1 | 102 | 58.3% | 84 | 61.3% | 101 | 58.4% |
| T2 | 73 | 41.7% | 53 | 38.7% | 72 | 41.6% |
| Differentiation | ||||||
| Low (well/moderate) | 157 | 90.2% | 122 | 89.7% | 155 | 90.1% |
| High (poor/mucinous) | 17 | 9.8% | 14 | 10.3% | 17 | 9.9% |
| Unknown | 1 | 1 | 1 | |||
| Lymphovascular Invasion | ||||||
| Yes | 24 | 14.0% | 21 | 15.6% | 24 | 14.2% |
| No | 147 | 86.0% | 114 | 84.4% | 145 | 85.8% |
| Unknown | 4 | 2 | 4 | |||
| Large Vessel Invasion | ||||||
| Yes | 4 | 3.4% | 3 | 3.1% | 4 | 3.4% |
| No | 115 | 96.6% | 93 | 96.9% | 113 | 96.6% |
| Unknown | 56 | 41 | 56 | |||
| Perineural Invasion | ||||||
| Yes | 3 | 2.3% | 2 | 1.9% | 3 | 2.3% |
| No | 128 | 97.7% | 101 | 98.1% | 126 | 97.7% |
| Unknown | 44 | 34 | 44 | |||
| Ulceration | ||||||
| Yes | 51 | 29.1% | 40 | 29.2% | 51 | 29.5% |
| No | 124 | 70.9% | 97 | 70.8% | 122 | 70.5% |
| Annularity | ||||||
| Yes | 27 | 18.0% | 25 | 20.7% | 27 | 18.1% |
| No | 123 | 82.0% | 96 | 79.3% | 122 | 81.9% |
| Unknown | 25 | 16 | 24 | |||
| Tumor size, cm | ||||||
| Median (range) | 2.2 (0.2-15.0) | 2.1 (0.2-15.0) | 2.2 (0.2-15.0) | |||
| <4.5 | 144 | 85.2% | 116 | 88.6% | 143 | 85.6% |
| ≥4.5 | 25 | 14.8% | 15 | 11.5% | 24 | 14.4% |
| Unknown | 6 | 6 | 6 | |||
| Circumferential resection margin, cm | ||||||
| Median (range) | 1.5 (0.1-6.5) | 1.35 (0.2-5.0) | 1.5 (0.1-6.5) | |||
| ≤0.2 | 6 | 5.7% | 2 | 2.6% | 6 | 5.8% |
| 0.21-1.0 | 36 | 34.3% | 29 | 37.2% | 35 | 34.0% |
| >1.0 | 63 | 60.0% | 47 | 60.3% | 62 | 60.2% |
| Unknown | 70 | 59 | 70 | |||
| Total number LN resected (TME) | ||||||
| Median (range) | 12 (1-38) | 12 (1-38) | 12 (1-38) | |||
| <12 | 56 | 44.4% | 43 | 46.2% | 56 | 45.2% |
| ≥12 | 70 | 55.6% | 50 | 53.8% | 68 | 54.8% |
| Unknown | 2 | 2 | 2 | |||
Local Recurrence
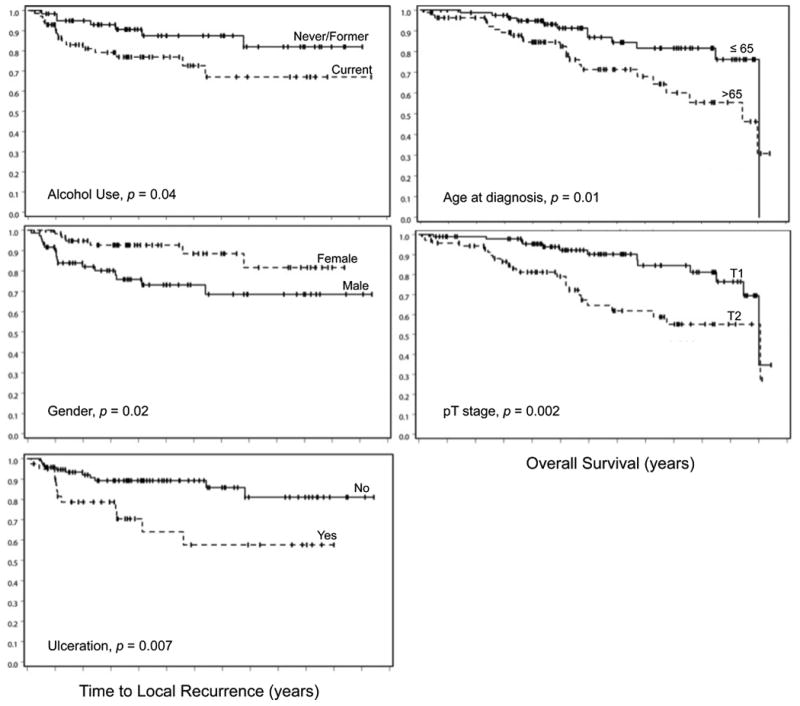
Time to local recurrence (TTLR) was used as a surrogate for absolute risk of LR due to variation in extent of postoperative follow up between patients. Complete clinical and pathologic follow up data were available for 137 patients in the analysis for time to local recurrence, and 23 (16.8%) recurred locally. Among these 23 patients, the median time to LR was 1.1 years (0.1-7.8). On univariable analysis, male gender (HR 2.9, p=0.02), current alcohol use (HR 2.4, p=0.04), lymphovascular invasion (HR 2.6, p=0.05), and tumor ulceration (HR 2.9, p=0.01) were associated with an increase risk of LR. Of note, tumor size, CRM, and histological grade were not related to local failure, though no patient had a positive CRM. On multivariable analysis, male gender (HR 3.3, p=0.02), current alcohol use (HR 3.4, p=0.01), and tumor ulceration (HR 4.2, p=0.001) were associated with reduced time to LR. Within the clinical data, race, smoking status, family history, and clinical presentation were not associated with local recurrence. Within the pathologic data, degree of circumferential involvement, preoperative CEA, PNI, and large vessel invasion were unrelated to the risk of local recurrence. The remaining results of univariable analysis and multivariable Cox proportional hazards analysis for time to LR are shown in Table 3. Analysis of TTLR was also completed for surgery performed (i.e. LAR, APR, or local excision) stratified by pathologic T1 or T2 stage. Results for this comparison are shown in Table 4, which reveal no significant difference in risk of local recurrence between patients undergoing radical resection or local excision for T1 or T2 tumors.
Table 3. Analysis of LR and OS.
| Time to Local Recurrence | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Characteristic | n = 137 | 25th %ile (yr) | HR | uv p | mv p | n = 173 | Median OS (yr) | No. deaths | HR | uv p | mv p |
| Age at diagnosis, years | |||||||||||
| ≤65 | 77 | 6.4 | 1.6 | 0.30 | 87 | 12.1 | 12 | 1.0 | 0.015 | 0.04 | |
| >65 | 60 | NR | 1.0 | 86 | 11.5 | 23 | 2.3 | ||||
|
| |||||||||||
| Gender | |||||||||||
| Male | 72 | 4.1 | 2.9 | 0.02 | 0.02 | 95 | 12.0 | 23 | 1.4 | 0.31 | |
| Female | 65 | NR | 1.0 | 78 | 12.1 | 13 | 1.0 | ||||
|
| |||||||||||
| Alcohol | |||||||||||
| Never/Former | 63 | NR | 1.0 | 0.04 | 0.01 | 82 | 12.0 | 22 | 1.0 | 0.15 | |
| Current | 71 | 5.6 | 2.4 | 83 | NR | 13 | 0.6 | ||||
| Unknown | 3 | - | - | 8 | - | 1 | |||||
|
| |||||||||||
| Type of surgery | |||||||||||
| LAR | 83 | 7.8 | 1.2 | 0.07 | 103 | 12.0 | 21 | 1.2 | 0.71 | ||
| APR/TP | 12 | NR | 0.0 | 23 | NR | 6 | 1.6 | ||||
| LE | 42 | 5.6 | 1.0 | 47 | NR | 9 | 1.0 | ||||
|
| |||||||||||
| Method of staging | |||||||||||
| EUS | 20 | NR | 0.2 | 0.13 | 20 | NR | 2 | 0.4 | 0.42 | ||
| MRI | 40 | NR | 0.5 | 49 | NR | 8 | 0.9 | ||||
| None | 75 | 5.6 | 1.0 | 101 | 12.0 | 26 | 1.0 | ||||
| Unknown | 2 | - | 3 | - | 0 | - | |||||
|
| |||||||||||
| T stage | |||||||||||
| T1 | 84 | NR | 1.0 | 0.19 | 101 | 12.0 | 13 | 1.0 | 0.002 | 0.008 | |
| T2 | 53 | 6.4 | 1.7 | 72 | 12.1 | 23 | 2.9 | ||||
|
| |||||||||||
| Differentiation | |||||||||||
| Low (well/moderate) | 122 | NR | 1.0 | 0.16 | 155 | 12.0 | 32 | 1.0 | 0.36 | ||
| High (poor/mucinous) | 14 | 1.1 | 2.3 | 17 | NR | 4 | 1.7 | ||||
| Unknown | 1 | - | - | 1 | - | 0 | - | ||||
|
| |||||||||||
| LVI | |||||||||||
| No | 114 | NR | 1.0 | 0.05 | NS | 145 | 12.0 | 30 | 1.0 | 0.52 | |
| Yes | 21 | 2.4 | 2.6 | 24 | NR | 6 | 1.4 | ||||
| Unknown | 2 | - | - | 4 | - | 0 | - | ||||
|
| |||||||||||
| Ulceration | |||||||||||
| No | 97 | NR | 1.0 | 0.01 | 0.001 | 122 | 12.0 | 24 | 1.0 | 0.41 | |
| Yes | 40 | 3.2 | 2.9 | 51 | 12.1 | 12 | 1.3 | ||||
|
| |||||||||||
| Tumor size, cm | |||||||||||
| <4.5 | 116 | NR | 1.0 | 0.31 | 143 | 12.0 | 27 | 1.0 | 0.04 | NS | |
| ≥4.5 | 15 | NR | 2.0 | 24 | 8.7 | 8 | 2.5 | ||||
| Unknown | 6 | - | - | 6 | - | 1 | - | ||||
|
| |||||||||||
| CRM, cm | |||||||||||
| ≤1.0 | 31 | NR | 1.9 | 0.38 | 41 | 12.0 | 10 | 1.2 | 0.67 | ||
| >1.0 | 47 | NR | 1.0 | 62 | 11.5 | 12 | 1.0 | ||||
| Unknown | 59 | - | - | 70 | - | 14 | - | ||||
|
| |||||||||||
| Number LN resected | |||||||||||
| <12 | 43 | 6.4 | 2.2 | 0.15 | 56 | 12.0 | 13 | 1.0 | 0.82 | ||
| ≥12 | 50 | NR | 1.0 | 68 | 11.5 | 14 | 1.1 | ||||
| Unknown | 2 | - | - | 49 | - | 9 | - | ||||
Table 4. TTLR and OS by surgical procedure stratified by pathologic stage.
| LR Analysis | OS Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| n | No of LR | HR | p | n | No of deaths | HR | p | ||
| T1 | LAR | 46 | 6 | 0.71 | 0.35 | 58 | 8 | 0.95 | 0.29 |
| APR | 4 | 0 | 0 | 6 | 0 | 0 | |||
| LE | 34 | 6 | 1 | 37 | 5 | 1 | |||
| T2 | LAR | 37 | 10 | 2.34 | 0.07 | 45 | 13 | 0.65 | 0.65 |
| APR | 8 | 0 | 0 | 17 | 6 | 0.94 | |||
| LE | 8 | 1 | 1 | 10 | 4 | 1 | |||
A Kaplan-Meir plot of TTLR for the entire cohort, as well as for patients stratified by surgical procedure, is shown in Figure 1. Kaplan-Meier plots of significant prognostic factors for TTLR on multivariable analysis are shown in Figure 2.
Figure 1. Kaplan-Meier pots of TTLR and OS for entire population (a) and by each surgical procedure (b).
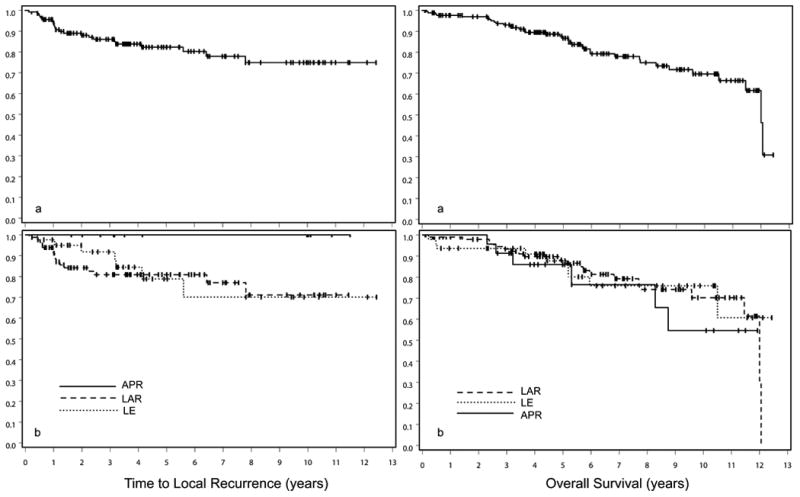
Figure 2. Kaplan-Meier plots of TTLR and OS for significant risk factors on multivariate analysis.
Patterns of Recurrence
Among the 23 patients that had a LR, 14 patients (61%) were diagnosed with routine postoperative surveillance, including scheduled colonoscopy and sigmoidoscopy, CT of abdomen and pelvis, and/or elevated CEA. Six patients presented with new onset rectal bleeding, two presented with constipation, and one presented with rectal or low back pain and pressure. Four patients (17.4%) had distant metastases at the time of local recurrence diagnosis (liver (n=2), adrenal gland (n=1), and periaortic lymph nodes (n=1)). Among the 19 patients with only local recurrence, the majority involved the LAR anastomosis, with other cases varying in location, as shown in Table 5. Notably, the rate of distant recurrence for patients who underwent local excision (i.e. 2 of 7) was greater than twice the rate for patients who underwent an LAR (i.e. 2 of 16).
Table 5. Patterns of Recurrence.
| Overall | LAR | APR | Local Excision | |
|---|---|---|---|---|
|
|
|
|
|
|
| Location | n = 23 | n = 16 | n = 0 | n = 7 |
| Local only | 19 | 14 | 0 | 5 |
| Single site | 15 | 11 | 0 | 4 |
| Multiple sites | 4 | 3 | 0 | 1 |
| Local and distant | 4 | 2 | 0 | 2 |
| Local | ||||
| Rectum | ||||
| Anastomosis | 13 | 13 | 0 | 0 |
| Resection site | 1 | 0 | 0 | 1 |
| > 10 cm | 1 | 0 | 0 | 1 |
| 5 - 10 cm | 4 | 1 | 0 | 3 |
| < 5 cm | 0 | 0 | 0 | 0 |
| Pelvis | ||||
| Presacral LN | 3 | 2 | 0 | 0 |
| Perirectal LN | 3 | 1 | 0 | 2 |
| Distant | ||||
| Liver | 2 | 2 | 0 | 0 |
| Adrenal gland | 1 | 0 | 0 | 1 |
| Periaortic LN | 1 | 0 | 0 | 1 |
Overall Survival
Of the 175 patients, 173 had complete clinical follow up data for analysis of overall survival. Among these patients, the median overall survival was 12 years. On univariable analysis, age at diagnosis greater than 65 years (HR 2.3, p=0.015), T2 pathologic stage (HR 2.9, p=0.002), and tumor size greater than 4.5 cm (HR 2.5, p=0.04) were associated with decreased survival. On multivariable analysis, age at diagnosis greater than 65 years (HR 2.1, p=0.04) and T2 pathologic stage (HR 2.5, p=0.008) were independent predictors of increased mortality. Within the clinical data, race, smoking status, family history, and clinical presentation were not associated with overall survival. Within the pathologic data, degree of circumferential involvement, preoperative CEA, PNI, and large vessel invasion were unrelated to mortality. The remaining results for OS are shown in Table 3. Univariable and multivariable analysis of OS was also completed for surgery performed (i.e. LAR, APR, or local excision) stratified by pathologic T1 or T2 stage. Results for this comparison are shown in Table 4, which demonstrate no significant difference in survival between patients undergoing radical resection or local excision for T1 or T2 tumors.
A Kaplan-Meir plot of OS for the entire cohort, as well as for patients stratified by surgical procedure, is shown in Figure 1. Kaplan-Meier plots of significant prognostic factors for OS on multivariable analysis are shown in Figure 2.
Discussion
There has been an improvement in the management of rectal cancer over the past two decades; total mesorectal excision with neoadjuvant and adjuvant therapies for stage II and III cancers have been associated with a decrease in the rate of LR and an increase in survival. Nevertheless, the use of adjuvant therapy is not recommended for stage I tumors since only a small percentage of these tumors recur after surgical resection alone. The Swedish Rectal Cancer Trial, however, was a prospective study that showed a significant reduction in local recurrence of stage I patients with preoperative pelvic radiation, from 12 to 4%.20 Although adjuvant therapy may clearly benefit some early stage patients, indiscriminate use is not recommended in this population due to overtreatment of the majority. In the current study, almost 17% of stage I rectal cancer patients developed local tumor recurrence despite curative surgery. Those patients treated with local excision had comparable outcomes to those treated with radical resection after several years follow up, as there was not a significant difference in time to local recurrence or OS between these groups. This finding may be attributable to the fact that most patients (nearly 80%) treated with local excision had T1 tumors. Based on eligibility criteria for this study, the patients treated with local excision alone had very favorable pathologic characteristics.
The primary aim of our analysis was to determine whether a high-risk group of patients could be identified using routine clinical and pathologic data. This in turn might identify patients with stage I cancers who could benefit from adjuvant therapies. Both univariable and multivariable analyses of these prognostic factors were performed for all eligible patients with stage I cancer treated with surgery alone at the affiliated institutions. Male gender, current alcohol consumption, and tumor ulceration were independent predictors of LR. Additionally, age greater than 65 at diagnosis and T2 pathologic stage were independently associated with decreased survival.
Male patients have been shown to have a worse prognosis than female patients in prior studies.2,21,22 It is known that wider lateral margins are more difficult to obtain in the male pelvis, which suggests that difference in outcome by gender might be related to the extent of surgical clearance.23 In our analysis, we found a significantly smaller CRM in men compared to women, as seen in Table 6. This finding supports the hypothesis that a worse prognosis in men might be related to a greater difficulty of obtaining adequate resection margins.21-23 No cases of tumor involvement of the resection margin were identified in this study. Male and female patients underwent a similar proportion of local excisions, LAR, and APR as well as extent of lymph node dissection. Male patients had a significantly higher proportion of alcohol users, but no other clinical characteristics differed between these groups, as shown in Table 6.
Table 6. Selected comparison between males and females.
| Male | Female | ||
|---|---|---|---|
|
|
|
||
| Characteristic | n = 95 | n = 80 | p |
| LAR | 54 | 50 | 0.54 |
| APR | 13 | 8 | 0.49 |
| Ulceration | 26 | 25 | 0.62 |
| Current alcohol use | 53 | 31 | 0.01 |
| Mean LN clearance | 12 (4-36) | 13 (1-38) | 0.36 |
| Mean CRM (cm) | 1.2 (0.1-4.2) | 1.6 (0.3-6.5) | 0.02 |
Though macroscopic tumor ulceration is generally considered an unfavorable finding for colorectal tumors, this study is one of few to show an increased in risk of local recurrence in patients with ulcerated tumors, both after radical surgery and local excision. A previous retrospective analysis showed worsened survival and LR rate with non-exophytic tumors (i.e. ulcerated, flat) compared to polypoid and sessile lesions.24 That study, however, found that tumor ulceration was not an independent risk factor for recurrence or mortality, but rather was associated with histological characteristics that have been shown to be predictors of recurrence and reduced survival, including poor differentiation, lymphovascular invasion, and depth of invasion. Our study now suggests that macroscopic tumor ulceration is an independent prognostic factor for local recurrence on multivariable analysis. This finding may allow preoperative risk stratification of patients. In this series, the sensitivity, specificity, and positive predictive value (PPV) of tumor ulceration for LR at one year were 50%, 71%, and 11%, respectively. The sensitivity, specificity, and PPV of ulceration for LR at two years were 53%, 73%, and 24%, respectively. Sensitivity, specificity, and PPV of ulceration for LR for each surgical procedure (i.e. LAR, APR, local excision) at one and two years are shown in Table 7.
Table 7. Sensitivity, specificity, and positive predictive value (PPV) of tumor ulceration for LR for each surgical procedure at 1 and 2 years.
| LAR | APR | Local Excision | ||
|---|---|---|---|---|
|
|
|
|
||
| 1 year | Sensitivity | 43% | - | 100% |
| Specificity | 78% | 58% | 62% | |
| PPV | 17% | - | 7% | |
|
| ||||
| 2 years | Sensitivity | 58% | - | 33% |
| Specificity | 87% | 55% | 55% | |
| PPV | 50% | - | 7% | |
This series is unique in showing that alcohol use is a significant prognostic factor for patients with rectal cancer after resection. Prior studies have indicated a modest increased risk of colorectal cancer incidence with alcohol consumption. The results of more recent reviews suggest that this association could be stronger with rectal compared to that of colon tumors.25 However, few studies, if any, detail the association between alcohol consumption and prognosis after diagnosis and treatment. Only one large analysis reported modestly increased rectal cancer mortality for regular versus rare drinkers for men (HR 1.33), while other studies have been largely null.26 To our knowledge, this analysis is the first to report an increased risk of local recurrence for regular alcohol consumers with early stage rectal cancer after resection. The biological basis for the observed increased risk, however, remains unclear. Evidence suggests that the effect of alcohol is modulated by polymorphisms in genes encoding dehydrogenases responsible for ethanol metabolism. It is hypothesized that there is a genotoxic effect of acetaldehyde that promotes carcinogenesis most pronounced in patients who carry specific alleles of aldehyde dehydrogenase.27
T2 pathologic stage was an independent prognostic factor for reduced survival in our patients. Previous studies have shown an increased risk of lymph node metastases among pT2 colorectal tumors (up to 19.7%) compared to pT1 lesions (5.6%), suggesting that undetected lymph node involvement may underlie the increase in cancer mortality.28,29 However, those studies demonstrating an increase in lymph node metastasis in pT2 tumors had a lower median number of lymph nodes examined than the number of nodes examined in the current series.28-30 Although none of our patients had lymph node metastases, we cannot exclude the possibility of micrometastasis within perirectal or pelvic lymph nodes that were not sampled. The number of patients with coexisting local and distant recurrence despite negative lymph nodes on initial resection is consistent with this possibility. Other common adverse pathologic variables, such as differentiation, LVI, PNI, and mucinous features, were not associated with reduced overall survival. This may be due to the low incidence of these adverse features in the eligible patients with intramural tumors, as well as the limited sample size of the study. Nonetheless, depth of invasion was a significant prognostic factor in our analysis, and it is possible that T2 rectal tumors with other poor prognostic features may require radical surgery (i.e. APR or LAR) and/or adjuvant therapies in order to maximize the likelihood of local control and survival. Interestingly, there was a trend towards a significantly greater risk of LR for patients with pT2 tumors treated with LAR alone (HR 2.34, p=0.07), as seen in Table 4, indicating that even radical resection alone may be a suboptimal approach in these patients with other adverse clinical or pathologic variables.
Age at diagnosis was also an independent predictor of increased mortality after resection. A majority of patients in this series died from causes not related to their tumor burden, including cardiopulmonary and neurologic pathology. There were also a small number of documented deaths in the postoperative period for patients treated with radical resection. Given the confounding variables of perioperative morbidity and other age related combordities, the basis of reduced overall survival in elderly patients treated for stage I rectal cancer is likely multifactorial.
This study has a number of limitations. First, there are potential biases inherent in any retrospective study. Only patients with complete clinical and pathologic follow up data were included in this analysis, which excluded 38 patients from the original cohort for the analysis of local recurrence risk. These patients lacked post-surgical endoscopic data, imaging, CEA measurement, or other clinical evaluation. This could potentially bias our results due to patients being lost to follow up or lacking stringent surveillance for those with low comorbidities. Second, there were a large number of patients who were referred to our institutions secondarily, possibly due to increased comorbidities or difficult surgical approach, potentially leading to selection bias that would overestimate the rate of local failure and mortality. Nevertheless, our study found a local recurrence rate of 16.8%, similar to that found in prior retrospective analyses. Finally, our series includes only 23 patients with local recurrence. Because of the small cohort and a retrospective design, these findings would need to be validated in a larger study. But given the number of patients with combined local and distant recurrence, it is possible that patients with unfavorable prognostic factors might benefit from adjuvant or neoadjuvant therapy to reduce the risk of both local and systemic relapse.
Conclusions
Through the evaluation of routine clinical and pathologic data for patients treated with curative surgery, this study defined a subpopulation of patients with early stage rectal cancer that are at increased risk of local tumor recurrence or mortality. If validated in a larger study, these findings could identify patients with early stage rectal cancer who might benefit from more aggressive therapy, such as the employment of adjuvant chemotherapy or radiation. Identification of prognostic factors could also allow for improved patient counseling and consideration of more intensive surveillance, as well as more aggressive surgery in those patients who would otherwise undergo transanal local excision.
As cancer research moves to the molecular level, it is possible that genetic analysis of tumors may identify markers that could improve prognostic accuracy beyond that provided by clinical factors. These studies have been carried out in non-irradiated advanced stage tumors.31 The identification of genetic abnormalities in stage I tumors, however, could be an important step toward understanding varying tumor behaviors and ultimately promoting individualized treatment.
Footnotes
Disclosures: Actual or potential conflicts of interest do not exist.
Financial Disclosures: None
References
- 1.Stamos MJ, Murrell Z. Management of early rectal T1 and T2 cancers. Clin Cancer Res. 2007;13:6885–6889. doi: 10.1158/1078-0432.CCR-07-1150. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg D, Paty PB, Picon AI, et al. Stage I Rectal Cancer: Identification of High-Risk Patients. J Am Coll Surg. 1998;186:574–580. doi: 10.1016/s1072-7515(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagetgaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–357. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer: a multicenter, randomized trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Huh JW, Chang HK, Hyeong RK, Young JK. Oncologic outcomes of pathologic stage I lower rectal cancer with or without preoperative chemoradiotherapy: Are they comparable? Surgery. 2011;150:980–984. doi: 10.1016/j.surg.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.You YN, Baxter NN, Steward A, Nelson H. Is the increasing rate of local excision for stage 1 rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–33. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellgren A, Sirivongs P, Rothenberge DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071. doi: 10.1007/BF02236551. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JA, Shibata D, Herndon JE, et al. Local excision of distal rectal cancer: an update of Cancer and Leukemia Group B 8984. Dis Colon Rectum. 2008;51:1185–1194. doi: 10.1007/s10350-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 10.You YN. Local excision: Is it an adequate substitute for radical resection in T1/T2 patients? Semin Radiat Oncol. 2011;21:178–184. doi: 10.1016/j.semradonc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Rutten HJ, den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. 2008;9:494–501. doi: 10.1016/S1470-2045(08)70129-3. [DOI] [PubMed] [Google Scholar]
- 12.De Graaf DJ, Doornebosch PG, Rollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol. 2009;35:1280–1285. doi: 10.1016/j.ejso.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Patel SC, Jovee JM, Langer B. Twenty-five years of experience with radical surgical treatment of carcinoma of the extraperitoneal rectum. Surgery. 1977;82:460–465. [PubMed] [Google Scholar]
- 14.Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::aid-cncr2820520731>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.McDermott FT, Hughes ES, Pihl E, et al. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg. 1985;72:34–37. doi: 10.1002/bjs.1800720115. [DOI] [PubMed] [Google Scholar]
- 16.Fandrich F, Schroder DW, Saliveros E. Longterm survival after curative resection for carcinoma of the rectum. J Am Coll Surg. 1994;178:271–276. [PubMed] [Google Scholar]
- 17.Bufalari A, Boselli C, Giustozzi G, Moggi L. Locally advanced rectal cancer: a multivariate analysis of out outcome risk factors. J Surg Oncol. 2000;74:2–10. doi: 10.1002/1096-9098(200005)74:1<2::aid-jso2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Jang HS, Kim JG, et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol. 2012;19:1213–1221. doi: 10.1245/s10434-011-2062-z. [DOI] [PubMed] [Google Scholar]
- 19.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147:366–371. doi: 10.1001/archsurg.2012.35. [DOI] [PubMed] [Google Scholar]
- 20.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 21.Griffin MR, Bergstralh EJ, Coffey RJ, et al. Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer. 1987;60:2318–2324. doi: 10.1002/1097-0142(19871101)60:9<2318::aid-cncr2820600934>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Isbister WH, Fraser J. Survival following resection for colorectal cancer: a New Zealand national study. Dis Colon Rectum. 1985;28:725–727. doi: 10.1007/BF02560285. [DOI] [PubMed] [Google Scholar]
- 23.Heald RJ, Chir M, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848–857. doi: 10.1007/BF02066981. [DOI] [PubMed] [Google Scholar]
- 24.Chambers WM, Khan U, Gagliano A. Tumour morphology as a predictor of outcome after local excision of rectal cancer. Br J Surg. 2004;91:457–459. doi: 10.1002/bjs.4504. [DOI] [PubMed] [Google Scholar]
- 25.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: A dose-response meta-analysis of published cohort studies. Int J Cancer. 2006;120:664–671. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 26.Ozaka K. Alcohol use and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8:81–88. [PubMed] [Google Scholar]
- 27.Tiemersma EW, Wark PA, Ocke MC, et al. Alcohol consumption, alcohol dehydrogenase 3 polymorphism, and colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2003;12:419–425. [PubMed] [Google Scholar]
- 28.Chok KS, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surgery. 2007;31:1485–1490. doi: 10.1007/s00268-007-9089-0. [DOI] [PubMed] [Google Scholar]
- 29.Sitzler PJ, Seow-Choen F, Ho Y, Leong AP. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum. 1997;40:1472–1476. doi: 10.1007/BF02070714. [DOI] [PubMed] [Google Scholar]
- 30.Rasheed S, Bowley DM, Aziz O, et al. Can depth of tumour invasion predict lymph node positivity in patients undergoing resection for early stage rectal cancer? A comparative study between T1 and T2 cancers. Colorectal Dis. 2008;10:231–238. doi: 10.1111/j.1463-1318.2007.01411.x. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Van't Veer LJ, Mikolajewska-Hanclich I, et al. PIK3CA mutations predict local recurrences in rectal cancer patients. Clin Cancer Res. 2009;15:6956–6962. doi: 10.1158/1078-0432.CCR-09-1165. [DOI] [PubMed] [Google Scholar]


