Abstract
Background
Neonates with hypoxic-ischemic encephalopathy (HIE) are at risk of cerebral blood flow dysregulation. Our objective was to describe the relationship between autoregulation and neurologic injury in HIE.
Methods
Neonates with HIE had autoregulation monitoring with the hemoglobin volume index (HVx) during therapeutic hypothermia, rewarming, and the first 6 h of normothermia. The 5-mmHg range of mean arterial blood pressure (MAP) with best vasoreactivity (MAPOPT) was identified. The percentage of time spent with MAP below MAPOPT and deviation in MAP from MAPOPT were measured. Neonates received brain MRIs 3–7 days after treatment. MRIs were coded as no, mild, or moderate/severe injury in five regions.
Results
HVx identified MAPOPT in 79% (19/24), 77% (17/22), and 86% (18/21) of neonates during hypothermia, rewarming, and normothermia, respectively. Neonates with moderate/severe injury in paracentral gyri, white matter, basal ganglia, and thalamus spent a greater proportion of time with MAP below MAPOPT during rewarming than neonates with no or mild injury. Neonates with moderate/severe injury in paracentral gyri, basal ganglia, and thalamus had greater MAP deviation below MAPOPT during rewarming than neonates without injury.
Conclusion
Maintaining MAP within or above MAPOPT may reduce the risk of neurologic injuries in neonatal HIE.
INTRODUCTION
Neonatal hypoxic ischemic encephalopathy (HIE) affects approximately 3 in 1000 births (1) and causes significant neurologic morbidity despite therapeutic hypothermia (2). Identifying modifiable factors and additional interventions may improve outcomes. The healthy brain maintains constant cerebral blood flow (CBF) across changes in blood pressure through cerebrovascular autoregulation. This physiologic mechanism functions within a specific hemodynamic range, and the term optimal mean arterial blood pressure (MAPOPT) refers to the range of MAP where cerebral vasoreactivity is most robust. That is, MAPOPT is the blood pressure range in which the cerebral vasculature has maximal pressure reactivity (3,4). Neonates with HIE may be at risk of CBF dysregulation with shifts in the limits of autoregulation, particularly with intracranial hypertension (5). The hemodynamic goals that conform to the limits of autoregulation are unknown in neonatal HIE. Moreover, traditional blood pressure goals based on gestational age have not been tested against neurologic outcomes in HIE.
Traditionally, autoregulation has been monitored with transcranial Doppler (TCD) or intracranial pressure (ICP) (6,7). However, continuous TCD monitoring requires expertise and equipment that are not widely available, and ICP is not routinely monitored in neonates. We developed a method to monitor cerebrovascular reactivity using near-infrared spectroscopy (NIRS): the hemoglobin volume index (HVx) (8). HVx represents the relationship between relative tissue hemoglobin (rTHb; a surrogate measure of cerebral blood volume (CBV) obtained by NIRS) and MAP. The rTHb is a trend of total hemoglobin measurements obtained by NIRS using light with a wavelength of 805 nm. Because the 805 nm wavelength is isobestic to both oxyhemoglobin and deoxyhemoglobin, rTHb is not affected by fluctuations in oxygen saturation. HVx is based on the premise that autoregulatory vasoconstriction and vasodilation induce changes in CBV that are proportional to changes in rTHb (8). In a neonatal swine model of HIE, HVx accurately identified the lower limit of autoregulation (9,10). We sought to translate our laboratory work to neonates with HIE.
The goal of this pilot study was to describe the relationship between autoregulation and neurologic injury on MRI in neonates with HIE who receive therapeutic hypothermia. First, we determined whether HVx would identify MAPOPT. Second, we investigated whether neonates who spent more time with blood pressure below MAPOPT and who had greater blood pressure deviation below MAPOPT would have more severe neurologic injury than neonates whose blood pressure remained within or above MAPOPT. Third, we assessed whether measurements based on HVx and MAPOPT would be more strongly associated with injury than regional cerebral oxygen saturation (rSO2) or hemodynamic goals based on gestational age.
RESULTS
Forty-four neonates with HIE were identified. Seven families did not consent to enroll, and one family did not speak English or Spanish. Eight neonates were not eligible, including five who did not have arterial cannulae, and three who died or had support withdrawn. Two neonates had intracranial hemorrhages or congenital heart disease, which precluded the use of hypothermia. Twenty-six neonates were enrolled in the study. Autoregulation monitoring could not be accomplished with one patient due to technical problems, and one neonate’s MRI had motion artifact. Thus, results were analyzed on 24 neonates (15 males, 9 females).
Of these 24 neonates, autoregulation monitoring was carried out during hypothermia in all patients, during rewarming in 22 patients, and during normothermia in 21 patients. Reasons for early cessation of monitoring included technical failure (1 patient), early removal of the arterial cannula (1 patient), and transfer to the pediatric ICU for extracorporeal membranous oxygenation (1 patient).
Patient Descriptions
The mean gestational age was 39.2±1.5 (standard deviation (SD)) weeks with birth weight 3353±596 g. Seventeen neonates (71%) were born by caesarean section, and eight (33%) required chest compressions after delivery. The umbilical cord gases had a mean pH of 6.98±0.13 (n=19) and base deficit of −13±3 (n=17). Blood gases obtained within 1±0.5 h of birth had a mean pH of 7.11±0.17 (n=24) and base deficit of −18±6 (n=21). Sixteen neonates (67%) had moderate encephalopathy, and eight (33%) had severe encephalopathy. Median Apgar scores were 2 (range: 0–7), 4 (range: 0–8), and 6 (range: 2–9) at 1, 5, and 10 min of life. Fourteen patients (58%) had seizures diagnosed clinically or electrographically, and all were treated with phenobarbital. Six neonates also received fosphenytoin, levetiracetam, or topiramate. Head ultrasounds were abnormal in 18 patients (75%): all 18 had cerebral edema, one had a germinal matrix hemorrhage, and another had cystic white matter changes. None of the neonates had an intraventricular hemorrhage. Eight neonates (33%) received opiate infusions; 16 (67%) received vasoactive infusions, including dopamine (16/24), dobutamine (15/24), epinephrine (1/24), and milrinone (2/24); and 22 (92%) had respiratory compromise. Eighteen neonates (75%) were mechanically ventilated with a mean oxygenation index of 3.7±3 at the beginning of the study. One neonate had a positive bacterial blood culture. The patients’ physiologic and laboratory variables are shown in Table 1. All enrolled neonates survived to NICU discharge.
Table 1.
Physiologic variables and laboratory measurements during the study period (n=24)
| Parameter | Hypothermia | Rewarming | Normothermia |
|---|---|---|---|
| Temperature (°C) | 33.5 (0.3) | 35.2 (0.4) | 36.9 (0.3) |
| Heart rate (bpm) | 108 (12) | 117 (13) | 135 (17) |
| MAP (mmHg) | 52 (5) | 49 (4) | 50 (4) |
| pH | 7.37 (0.04) | 7.37 (0.06) | 7.37 (0.05) |
| PaCO2 (mmHg) | 43 (6) | 48 (9) | 48 (7) |
| PaO2 (mmHg) | 115 (47) | 94 (29) | 105 (48) |
| Hemoglobin (g/dl) | 15.2 (1.2) | 13.7 (0.7)a | 13.4 (0.6)b |
| WBC (no./mm3) | 10,032 (3,156) | 8,315 (3,646)a | 9,566 (1,423)b |
| Sodium (mEq/l) | 138 (3) | 138 (3)a | 141 (3)b |
Data are shown as means with SD. Bpm, beats per minute; WBC, white blood count.
Laboratory measurements were taken in 9 patients.
Laboratory measurements were taken in 11 patients.
Neurologic Injury
Brain MRIs were obtained 3–7 days after treatment on day of life 9±3 (range: 4–14). Three neonates had no injuries in any region. Twenty-one had injury in at least one region, and four had moderate/severe injury in all regions. Moderate/severe injury was more common in the white matter than in paracentral gyri, basal ganglia, thalamus, or brainstem (Table 2). No infants had selective, unilateral brain injury.
Table 2.
Summary of anatomical and diffusion MRI findings
| Brain region | No. of Patients |
|---|---|
| Paracentral gyri | |
| No injury | 14 |
| Mild injury | 4 |
| Moderate/severe injury | 6 |
| White mattera | |
| No injury | 5 |
| Mild injury | 7 |
| Moderate/severe injury | 12 |
| Basal ganglia | |
| No injury | 11 |
| Mild injury | 7 |
| Moderate/severe injury | 6 |
| Thalamus | |
| No injury | 10 |
| Mild injury | 8 |
| Moderate/severe injury | 6 |
| Brainstem | |
| No injury | 12 |
| Mild injury | 7 |
| Moderate/severe injury | 5 |
Includes the posterior limb of the internal capsule.
Blood Pressure and Autoregulation
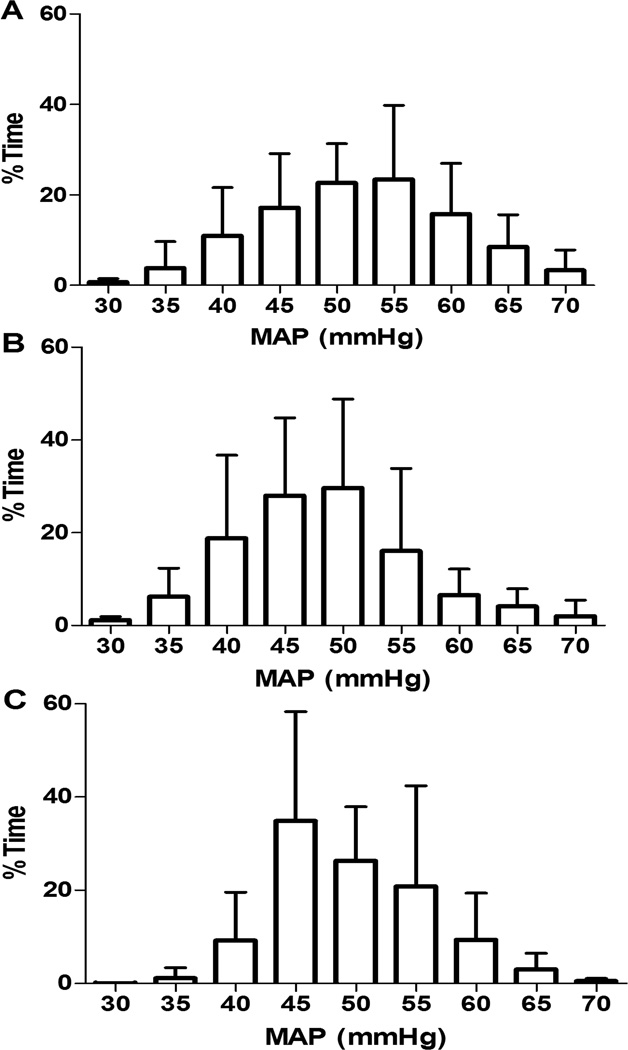
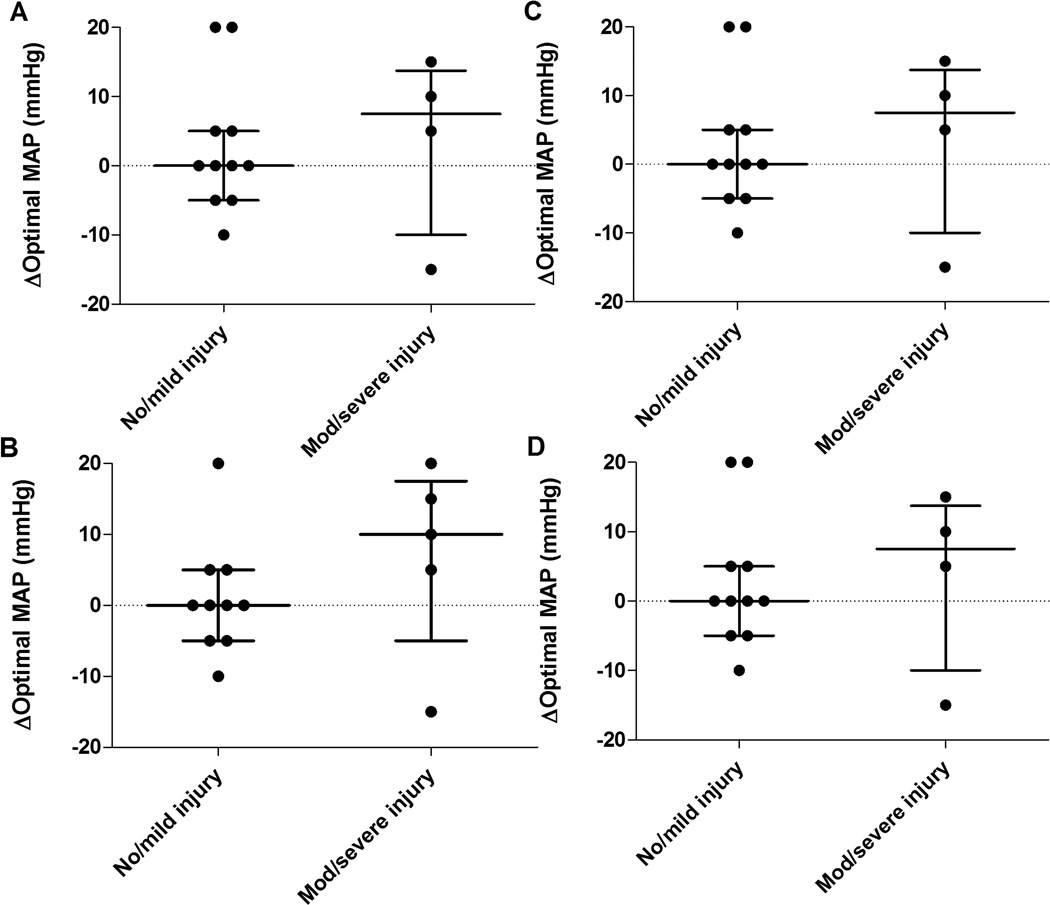
The median duration of HVx monitoring was 30.9 h (22.6, 42.6 (interquartile range, IQR); n=24), 6.5 h (5.4, 7.9; n=22), and 6 h (6, 6; n=21) during hypothermia, rewarming, and normothermia, respectively. Figure 1 illustrates the neonates’ range of MAP. MAPOPT was identified in 19/24 (79%), 17/22 (77%), and 18/21 (86%) neonates during hypothermia, rewarming, and normothermia. The median MAPOPT bin was 45 mmHg (45, 55 (IQR); n=19), 50 mmHg (45, 50; n=17), and 50 mmHg (45, 55; n=18) during hypothermia, rewarming, and normothermia. In some individual patients, the MAPOPT differed between time periods. Neonates with no or mild brain injuries had no or minimal change in MAPOPT as they progressed from hypothermia to rewarming in comparison to neonates with moderate/severe injuries (Figure 2).
Figure 1.
The percentage of time during (A) hypothermia (n=24), (B) rewarming (n=22), and (C) normothermia (n=21) that neonates spent at each mean arterial blood pressure (MAP). Data are shown as means with SDs.
Figure 2.
Fifteen neonates had an identifiable optimal mean arterial blood pressure (MAPOPT) during both hypothermia and rewarming. When progressing from hypothermia to rewarming, some individuals had a shift in MAPOPT. This shift is represented on the y-axis. For instance, a value of 5 indicates the MAPOPT increased by 5 mmHg as the patient moved from hypothermia to rewarming. A value of 0 represents no shift. Patients with no or mild injury in (A) paracentral gyri, (B) white matter, (C) basal ganglia, and (D) thalamus had no or minimal shift in MAPOPT when moving from hypothermia to rewarming. Data are shown as medians and interquartile ranges.
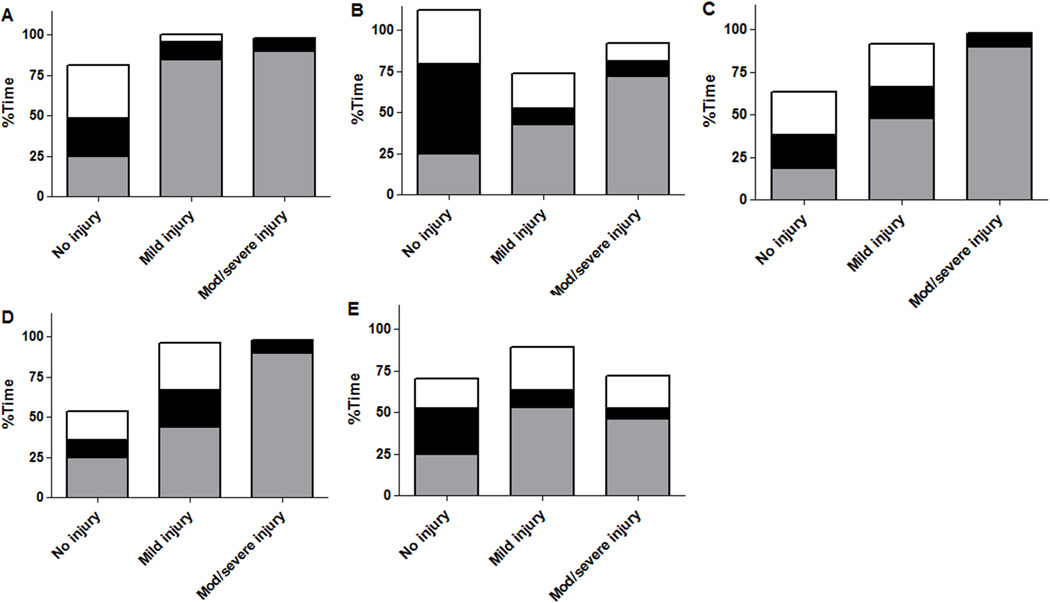
Patients with moderate/severe injuries in paracentral gyri, white matter, basal ganglia, thalamus, and brainstem spent a greater proportion of time with blood pressure below MAPOPT during rewarming than uninjured neonates. Injury severity in paracentral gyri, white matter, basal ganglia, and thalamus correlated with the percentage of time spent below MAPOPT during rewarming. Neonates with no or mild injuries in all brain regions spent a greater proportion of time with blood pressure within the MAPOPT bin than patients with moderate/severe injuries (Figure 3; Table 3).
Figure 3.
The percentage of time neonates (n=17) spent below, within, or above the optimal mean arterial blood pressure bin (MAPOPT) during rewarming in relation to injury in (A) paracentral gyri, (B) white matter, (C) basal ganglia, (D) thalamus, and (E) brainstem. Gray represents the percentage of time spent with blood pressure below MAPOPT. Black represents the percentage of time spent with blood pressure within MAPOPT. White represents the percentage of time spent with blood pressure above MAPOPT. Neonates with injuries in all regions spent more time with blood pressure below MAPOPT than patients without injury. The degree of injury in paracentral gyri, white matter, basal ganglia, and thalamus increased with greater time below MAPOPT. Neonates with no or mild injury spent a greater proportion of time with blood pressure within the MAPOPT bin than patients with moderate/severe injury. Data are displayed as medians.
Table 3.
Brain injury and percent of time spent in relation to optimal MAP during rewarming
| Brain region | Below optimal MAP (%, median, IQR) |
At optimal MAP (%, median, IQR) |
Above optimal MAP (%, median, IQR) |
|---|---|---|---|
| Paracentral gyri | |||
| No injury | 25 (9, 52) | 24 (5, 28) | 33 (22, 63) |
| Mild injury | 85 (41, 89) | 11 (9, 44) | 4 (2, 14) |
| Moderate/severe injury | 90 (2, 92) | 8 (2, 10) | 0 (0, 39) |
| White matter | |||
| No injury | 25 (13, 52) | 54 (13, 58) | 33 (18, 35) |
| Mild injury | 42 (5, 84) | 10 (3, 27) | 22 (8, 80) |
| Moderate/severe injury | 72 (2, 92) | 9 (2, 24) | 11 (0, 39) |
| Basal ganglia | |||
| No injury | 19 (9, 85) | 19 (9, 54) | 25 (4, 63) |
| Mild injury | 48(41, 54) | 18 (5, 26) | 26 (14, 35) |
| Moderate/severe injury | 90 (2, 92) | 8 (2, 10) | 0 (0, 39) |
| Thalamus | |||
| No injury | 25 (13, 25) | 11 (9, 11) | 18 (4, 18) |
| Mild injury | 44 (9, 44) | 24 (5, 24) | 29 (14, 29) |
| Moderate/severe injury | 90 (2, 90) | 8 (2, 8) | 0 (0, 0) |
| Brainstem | |||
| No injury | 25 (9, 85) | 28 (9, 54) | 18 (4, 63) |
| Mild injury | 53 (44, 84) | 11 (5, 24) | 26 (12, 35) |
| Moderate/severe injury | 46 (1, 94) | 6 (2, 34) | 20 (0, 69) |
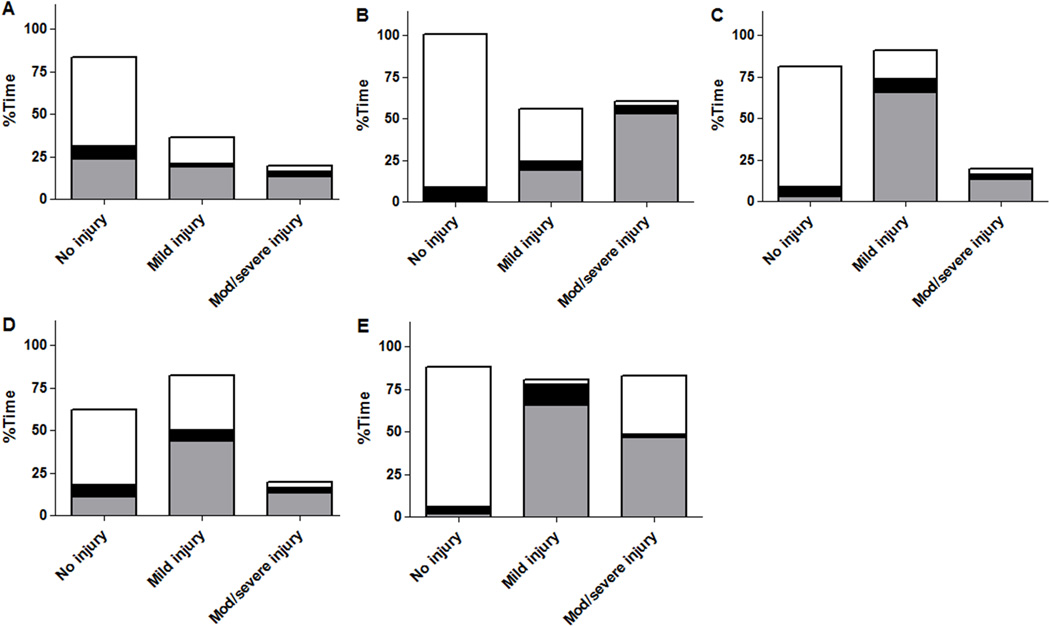
During normothermia, neonates with injuries in white matter and brainstem spent a greater proportion of time with blood pressure below MAPOPT than neonates without injuries in these regions. Injury severity in the white matter increased with more time below MAPOPT. Neonates with no or mild injuries in all regions spent a greater proportion of time with blood pressure above MAPOPT than neonates with moderate/severe injuries. Injuries in the paracentral gyri, white matter, basal ganglia, and thalamus were less severe in neonates who spent more time above MAPOPT (Figure 4; Table 4).
Figure 4.
The percentage of time neonates (n=18) spent below, within, or above the optimal mean arterial blood pressure bin (MAPOPT) during normothermia in relation to injury in (A) paracentral gyri, (B) white matter, (C) basal ganglia, (D) thalamus, and (E) brainstem. Gray represents the percentage of time spent with blood pressure below MAPOPT. Black represents the percentage of time spent with blood pressure within MAPOPT. White represents the percentage of time spent with blood pressure above MAPOPT. Patients with injury in white matter and brainstem, and patients with more severe injury in white matter spent more time with blood pressure below MAPOPT. Neonates with no or mild injury in all regions spent more time with blood pressure above MAPOPT than patients with moderate/severe injury. Injury severity was lower in paracentral gyri, white matter, basal ganglia, and thalamus with greater time spent above MAPOPT. Data are displayed as medians.
Table 4.
Brain injury and percent of time spent in relation to optimal MAP during normothermia
| Brain region | Below optimal MAP (%, median, IQR) |
At optimal MAP (%, median, IQR) |
Above optimal MAP (%, median, IQR) |
|---|---|---|---|
| Paracentral gyri | |||
| No injury | 24 (0, 93) | 8 (3, 14) | 52 (1, 92) |
| Mild injury | 19 (1, 99) | 2 (1, 65) | 16 (0, 98) |
| Moderate/severe injury | 13 (0, 93) | 3 (2, 33) | 4 (2, 67) |
| White matter | |||
| No injury | 1 (0, 87) | 8 (3, 10) | 92 (3, 97) |
| Mild injury | 19 (2, 94) | 5 (2, 23) | 32 (1, 84) |
| Moderate/severe injury | 53 (0, 93) | 5 (2, 33) | 2 (2, 67) |
| Basal ganglia | |||
| No injury | 3 (0, 94) | 5 (2, 24) | 73 (0, 97) |
| Mild injury | 66 (2, 93) | 8 (2, 14) | 17 (1, 84) |
| Moderate/severe injury | 13 (0, 93) | 3 (2, 33) | 4 (2, 67) |
| Thalamus | |||
| No injury | 11 (1, 94) | 7 (3, 24) | 44 (0, 92) |
| Mild injury | 44 (1, 93) | 7 (2, 14) | 32 (1, 98) |
| Moderate/severe injury | 13 (0, 93) | 3 (2, 33) | 4 (2, 67) |
| Brainstem | |||
| No injury | 2 (0, 57) | 4 (2, 16) | 82 (8, 97) |
| Mild injury | 66 (13, 93) | 12 (7, 23) | 3 (1, 32) |
| Moderate/severe injury | 47 (0, 95) | 2 (2, 18) | 34 (2, 82) |
Neurologic injury and the percentages of time spent with blood pressure below, within, or above MAPOPT were not consistently associated during hypothermia (Table 5). Furthermore, time spent below the MAP threshold of gestational age + 5 did not correlate with injury severity in any brain region (Table 6). Patients spent little time with MAP below their gestational age (data not shown).
Table 5.
Brain injury and percent of time spent in relation to optimal MAP during hypothermia
| Brain region | Below optimal MAP (%, median, IQR) |
At optimal MAP (%, median, IQR) |
Above optimal MAP (%, median, IQR) |
|---|---|---|---|
| Paracentral gyri | |||
| No injury | 11 (1, 43) | 12 (6, 19) | 78 (26, 90) |
| Mild injury | 47 (0, 96) | 2 (1, 3) | 50 (1, 99) |
| Moderate/severe injury | 1 (0, 16) | 27 (3, 27) | 73 (57, 96) |
| White matter | |||
| No injury | 55 (1, 64) | 12 (11, 19) | 26 (13, 87) |
| Mild injury | 5 (0, 41) | 4 (2, 12) | 90 (46, 98) |
| Moderate/severe injury | 1 (0, 16) | 27 (3, 27) | 73 (57, 96) |
| Basal ganglia | |||
| No injury | 5 (1, 55) | 6 (2, 12) | 87 (26, 90) |
| Mild injury | 41 (0, 43) | 14 (3, 24) | 7 (21, 97) |
| Moderate/severe injury | 1 (0, 16) | 27 (3, 27) | 73 (57, 96) |
| Thalamus | |||
| No injury | 11(1, 75) | 8 (3, 12) | 78 (14, 89) |
| Mild injury | 20 (0, 43) | 8 (2, 24) | 71 (21, 98) |
| Moderate/severe injury | 1 (0, 16) | 27 (3, 27) | 73 (57, 96) |
| Brainstem | |||
| No injury | 3 (0, 55) | 5 (2, 12) | 88 (26, 98) |
| Mild injury | 41 (22, 43) | 24 (14, 35) | 44 (21, 46) |
| Moderate/severe injury | 1 (0, 8) | 15 (3, 27) | 84 (65, 97) |
Table 6.
Brain injury and percentage of time spent with mean arterial blood pressure below the gestational age + 5
| Brain region | Hypothermia (%, median, IQR) |
Rewarming (%, median, IQR) |
Normothermia (%, median, IQR) |
|---|---|---|---|
| Paracentral gyri | |||
| No injury | 14 (3, 20) | 25 (9, 44) | 3 (0, 31) |
| Mild injury | 17 (8, 2) | 53 (3, 62) | 19 (2, 31) |
| Moderate/severe injury | 15 (3, 22) | 5 (2, 21) | 5 (2, 33) |
| White matter | |||
| No injury | 11 (8, 13) | 21 (15, 34) | 3 (0, 39) |
| Mild injury | 16 (3, 20) | 39 (2, 53) | 2 (0, 31) |
| Moderate/severe injury | 16 (3, 28) | 8 (2, 53) | 5 (2, 33) |
| Basal ganglia | |||
| No injury | 13 (2, 20) | 25 (9, 44) | 3 (0, 25) |
| Mild injury | 16 (3, 25) | 39 (3, 54) | 16 (1, 60) |
| Moderate/severe injury | 15 (3, 22) | 5 (2, 21) | 5 (2, 33) |
| Thalamus | |||
| No injury | 16 (5, 20) | 33 (6, 48) | 3 (0, 31) |
| Mild injury | 12 (3, 20) | 28 (6, 49) | 9 (2, 41) |
| Moderate/severe injury | 15 (3, 22) | 5 (2, 21) | 5 (2, 33) |
| Brainstem | |||
| No injury | 12 (2, 20) | 19 (3, 44) | 2 (0, 19) |
| Mild injury | 16 (8, 25) | 44 (17, 54) | 22 (1, 62) |
| Moderate/severe injury | 14 (3, 16) | 3 (2, 8) | 4 (2, 5) |
Brain injury was also associated with maximal deviation in blood pressure from MAPOPT during rewarming. Neonates with no, mild, or moderate/severe injury in paracentral gyri had median MAP deviations below MAPOPT of 10 mmHg (5, 10 (IQR)), 15 mmHg (15, 20), and 15 mmHg (5, 15), respectively. For neonates with no, mild, or moderate/severe injury in basal ganglia, the median MAP deviations below MAPOPT were 10 mmHg (5, 15), 12.5 mmHg (10, 15), and 15 mmHg (5, 15). Patients with no, mild, or moderate/severe injury in thalamus had median MAP deviations below MAPOPT of 10 mmHg (5, 15), 10 mmHg (10, 15), and 15 mmHg (5, 15).
Neonates with no or mild injury in all brain regions had greater blood pressure deviation above MAPOPT during rewarming than patients with moderate/severe injury. During normothermia, neonates with no or mild injury in paracentral gyri, white matter, basal ganglia, and thalamus also had greater blood pressure deviation above MAPOPT than patients with moderate/severe injury (data not shown). No complications were associated with the autoregulation monitoring.
Among neonates with an identified MAPOPT during hypothermia, rewarming, and normothermia, the rates of moderate/severe injury in paracentral gyri, basal ganglia, thalamus, and brainstem were similar or greater than the rates of moderate/severe injury in these regions in neonates without an identified MAPOPT. The proportions of neonates with moderate/severe white matter injury were similar between those with and without an identified MAPOPT during rewarming (data not shown.) Of neonates with an MAPOPT during hypothermia (n=19), 74% had moderate/severe white matter injury, whereas 100% of neonates without an MAPOPT during hypothermia (n=5) had moderate/severe white matter injury. During normothermia, the rate of moderate/severe white matter injury was 83% in neonates with an MAPOPT (n=18) and 100% in neonates without an MAPOPT (n=3).
Cerebral Oximetry
Patients with moderate/severe injuries had slightly higher rSO2 values than patients with no or mild injuries (Table 7). Phenobarbital was administered to 14 neonates during hypothermia, two neonates during rewarming, and five neonates during normothermia. Compared to those with no or mild injuries, a higher proportion of neonates with moderate/severe injuries received phenobarbital during hypothermia or rewarming and received a second anti-epileptic (fosphenytoin, levetiracetam, or topiramate) during hypothermia, rewarming, or normothermia. Neonates with moderate/severe injuries had median PaO2 values of 82–122 mmHg, and those with no or mild injuries had PaO2 levels of 39–155 mmHg during hypothermia, rewarming, and normothermia. PaCO2, arterial oxygen saturation, hemoglobin levels, the administration of opiate infusions, and the incidence of red blood cell transfusions were not associated with injury or rSO2 (data not shown).
Table 7.
Brain injury and absolute regional cerebral oxygen saturation (right and left averaged)
| Brain region | Hypothermia (%, median, IQR) |
Rewarming (%, median, IQR) |
Normothermia (%, median, IQR) |
|---|---|---|---|
| Paracentral gyri | |||
| No injury | 83 (78, 86) | 80 (79, 90) | 83 (71, 89) |
| Mild injury | 90 (85, 92) | 90 (78, 94) | 89 (78, 91) |
| Moderate/severe injury | 90 (86, 94) | 94 (90, 95) | 92 (89, 94) |
| White matter | |||
| No injury | 86 (81, 89) | 86 (79, 93) | 92 (76, 94) |
| Mild injury | 83 (73, 88) | 80 (78, 90) | 81 (67, 89) |
| Moderate/severe injury | 91 (86, 94) | 94 (87, 95) | 91 (87, 94) |
| Basal ganglia | |||
| No injury | 84 (79, 89) | 82 (79, 92) | 85 (72, 92) |
| Mild injury | 84 (73, 90) | 80(74, 90) | 81 (74, 89) |
| Moderate/severe injury | 90 (86, 94) | 94(90, 95) | 92 (89, 94) |
| Thalamus | |||
| No injury | 85 (79, 89) | 86 (79, 93) | 85 (78, 92) |
| Mild injury | 83 (73, 90) | 79 (76, 89) | 79 (70, 89) |
| Moderate/severe injury | 90 (86, 94) | 94 (90, 95) | 92 (89, 94) |
| Brainstem | |||
| No injury | 85 (80, 90) | 86 (79, 92) | 85 (78, 91) |
| Mild injury | 84 (73, 91) | 80 (74, 91) | 81 (74, 90) |
| Moderate/severe injury | 87 (86, 93) | 94 (90, 95) | 91 (89, 94) |
DISCUSSION
The results of this pilot study suggest that continuous autoregulation monitoring with HVx may identify blood pressures associated with reduced risk of neurologic injury in neonatal HIE. HVx successfully identified MAPOPT during therapeutic hypothermia, rewarming, and normothermia. Descriptive analyses identified an association between neurologic injury and blood pressure in relation to MAPOPT. By contrast, MAP goals based on the gestational age + 5 were not associated with brain injury. Greater severity of brain injury in neonates was associated with more time spent with blood pressure below MAPOPT during rewarming. Conversely, neonates with no or mild injury spent more time with blood pressure within or above MAPOPT. Moreover, patients with no injury or only mild injury had minimal shift in MAPOPT when moving from hypothermia to rewarming.
Because this was an observational study, we do not know if maintaining MAP within or above MAPOPT provides neuroprotection or is the result of better cardiovascular regulation in those with less injury. However, the data suggest that maintaining blood pressure within or above MAPOPT may be safer than maintaining blood pressure below MAPOPT. Clinicians use many techniques to maintain cerebral perfusion, including selecting a minimal tolerable MAP of gestational age + 5 or monitoring rSO2. Thresholds of MAP based on gestational age did not correlate with neurologic injury in this study. Absolute rSO2 values depend on cerebral metabolic rate, which varies with temperature, sedation, anti-epileptic treatment, oxygen saturation, and hematocrit. Because these variables change frequently in neonates with HIE, interpretation of rSO2 is challenging and is unlikely to define optimal cerebral perfusion pressure as a single measure. Alternatively, HVx is calculated from MAP and rTHb, a surrogate measure of CBV (8). HVx is less affected by cerebral oxygenation and primarily reflects changes in cerebral vasoconstriction/vasodilation during autoregulatory responses to changing perfusion pressure. We theorized that identifying MAPOPT with HVx would be a more reliable method of guiding hemodynamic management to support cerebral perfusion. Indeed, neonates who had no neurologic injury or only mild injury spent more time with blood pressures within or above MAPOPT than neonates with moderate/severe injuries.
The association between time spent within MAPOPT and less neurologic injury was strongest during the rewarming period for the paracentral gyri and white matter. NIRS measures superficial cortex, and cortical measurements of rTHb would most closely reflect vasoreactivity in the paracentral gyri. The white matter, posterior limb of the internal capsule (PLIC), basal ganglia, thalamus, and brainstem are also vulnerable to hypoxic injury. In neonatal HIE, early MRI evidence of basal ganglia and thalamic injury are associated with future motor impairments, and brainstem injury is associated with death (11). The association between basal ganglia and thalamic injury and MAPOPT suggests that cortical autoregulation measurements reflect vascoreactivity in deeper regions and that MAPOPT may be similar in all brain regions. Alternatively, injury to deep brain structures may cause cardiovascular instability and poor autoregulatory function measured in the cortex. Because brainstem injury may induce hemodynamic instability and impaired autoregulation could cause brainstem injury, we expected a complex association between autoregulation and brainstem injury.
Related findings have been reported in adults. In adults with traumatic brain injury, mortality increased as cerebral perfusion pressure (CPP) decreased below optimal CPP (CPPOPT) (4). Spending more time with CPP below CPPOPT was associated with severe disability, vegetative state, or death in adults with aneurysmal subarachnoid hemorrhage (12). Neonates with no or mild injury had greater maximal blood pressure deviation above MAPOPT than those with moderate/severe injury in our study. In contrast, a greater difference in median CPP above CPPOPT was associated with severe disability in adult traumatic brain injury (4). We evaluated the maximal deviation in MAP above MAPOPT, and neonates spent little time at higher blood pressures (Figure 1). Additional studies are needed to evaluate the effects of higher blood pressures in HIE.
In several patients, the MAPOPT value changed between the hypothermic and rewarming periods. Neonates with no or mild injury had no or minimal change in MAPOPT. The lower limit of autoregulation shifts to a higher CPP with intracranial hypertension (5). Theoretically, if ICP increased during rewarming in the more severely injured patients (13), a shift in the autoregulation curve could also shift MAPOPT. Variation in MAPOPT among patients and changes in MAPOPT within patients emphasize the importance of using continuous, real-time autoregulation monitoring to individualize hemodynamic goals.
We did not identify a correlation between neurologic injury and MAPOPT during hypothermia. Hypothermia may preserve the cerebral vasodilatory response to hypotension. In a swine model of neonatal HIE, hypothermia acutely decreased the lower limit of autoregulation (9). If the lower limit of autoregulation was decreased during hypothermia in our patients, the adverse effect of blood pressure below MAPOPT would be diminished.
Neonates with moderate/severe neurologic injuries had slightly higher rSO2. Compared to their counterparts, these patients more often received phenobarbital, which suppresses cerebral metabolism and increases rSO2. Whether more severe neurologic injury is consistently associated with higher rSO2 requires additional studies. Nonetheless, in situations with frequent changes in cerebral metabolism, we propose that autoregulation monitoring with HVx would be better than rSO2 alone to guide hemodynamic management. Additional studies are needed to evaluate this theory.
Metabolic acidosis (14), prostaglandins (15), and altered adenosine homeostasis (16,17) after hypoxia may affect CBF regulation (17–19). Autoregulation monitoring was initiated once study consent was obtained and after an arterial cannula was placed. It is possible that before monitoring was established, some neonates may have had severe metabolic derangements with altered autoregulatory function. Moreover, rewarming may increase lactate, adenosine, and prostaglandin production in injured regions of brain and limit myogenic reactivity and the range of autoregulation. Because the brain was not imaged early after rewarming, the timing of the injury and alterations in vasoreactivity cannot be linked on an individual basis.
An association between impaired autoregulation and mortality in neonatal HIE has been suggested previously. Using Xenon techniques 2–3 times over a 2-h period in neonates with asphyxia, Pryds et al. (20) reported an association between pressure-passive CBF and death. Our findings expand upon the relationship between autoregulation and neurologic outcomes. We obtained autoregulation measurements continuously over days and across a wide hemodynamic range. In individual patients, HVx identified MAPOPT and distinguished this MAP from levels with poorer autoregulation. Neonates with moderate/severe brain injury displayed an increase in MAPOPT when transitioning from hypothermia to rewarming despite similar blood pressure distributions between these periods, which suggests a rightward shift in the autoregulation curve. Deviation in blood pressure below MAPOPT was associated with worse neurologic injury. Therefore, we suggest that neonates with HIE and poor neurologic outcomes do not have completely impaired autoregulation. Rather, the blood pressure range within the confines of autoregulation may shift, necessitating an adjustment in hemodynamic management to maintain pressure-reactive CBF. This possibility emphasizes the importance of continuous autoregulation monitoring to individualize hemodynamic goals as injury evolves and therapeutic conditions change.
Our pilot study had limitations. First, monitoring duration differed among patients during hypothermia because monitoring was started after obtaining consent. The durations of monitoring during rewarming and normothermia were more consistently 6 h. We analyzed the data using the percentage of time of the monitoring period to account for the different absolute monitoring durations. Second, tests for reproducibility in MRI interpretation were not performed in this single-institution study. MRI analyses were qualitative, which is generally considered to be less sensitive than quantitative analyses. Specific MRI findings as a function of postnatal age were not evaluated. It is possible that neurologic injury on MRI reflected prenatal insults and that cardiovascular regulation was worse in these patients than in those with less injury. Third, MRIs were obtained within the first 2 weeks of life. Although early MRI evidence of brain injury correlates to poor motor outcomes or death (11), long-term outcome data were not available for our study. Fourth, the effects of vasoactive infusions or seizures on autoregulation were not examined. The impact of vasopressors on autoregulation in neonatal HIE is unclear, although phenylephrine did not affect autoregulation in a neonatal swine model of HIE (10). Finally, an alternative measure of CBF, such as TCD, was not used to validate HVx, because continuous Doppler over 3–4 days is not feasible in neonates. Nonetheless, HVx correlates with ICP-derived autoregulation measurements in patients (3), HVx identified the limits of autoregulation determined by laser-Doppler in a swine model of HIE (9,10), and HVx was validated against TCD in identifying MAPOPT during cardiopulmonary bypass (21).
In conclusion, blood pressure maintenance within or above MAPOPT was associated with decreased neurologic injuries in neonates with HIE. HVx monitoring could enable clinicians to target optimal hemodynamic ranges for individual patients to support autoregulation and prevent secondary brain injury. Future clinical studies are indicated to further evaluate the utility of HVx in neonatal HIE.
METHODS
This study was approved by the Johns Hopkins University Institutional Review Board, and written informed consent was obtained from the parents. Between September 2010–April 2012, neonates with HIE who were admitted to the Johns Hopkins Neonatal Intensive Care Unit (NICU) for therapeutic hypothermia were screened. To be eligible for the study, the patient’s parent had to speak English or Spanish (the languages available for the consent forms), and the attending neonatologist had to agree to enroll the neonate. Eligibility criteria included gestational age ≥35 weeks, birth weight ≥1800 g, initiation of cooling before 6 h of age, and an arterial blood pressure cannula. Criteria for HIE were based on the National Institute of Child Health and Human Development Neonatal Research Network’s clinical trial of hypothermia in neonatal HIE (22) and included a blood gas obtained from the umbilical cord or in the first hour of life with pH <7.15 or base deficit >10 mmol/l, and moderate to severe encephalopathy. If a blood gas was unavailable, an acute perinatal event, 10-min Apgar score <5 or assisted ventilation for ≥10 min after birth, and moderate to severe encephalopathy were required to diagnose HIE. Neonates without arterial blood pressure cannulae or who had congenital anomalies or coagulopathy with active bleeding that could make cooling unsafe were ineligible for the study.
Clinical Care
Clinical care was determined by the clinical team and per NICU protocol. Neonates received whole-body hypothermia with a cooling blanket (Mul-T-Blanket Hyper/Hypothermia Blanket and Mul-T-Pad Temperature Therapy Pad; Gaymar Medi-Therm III; Gaymar Industries Inc., Orchard Park, NY) to maintain a rectal temperature of 33.5±0.5°C for 72 h. Neonates were rewarmed over 6 h (goal 0.5°C/h) to normothermia (36.5°C). Hemodynamic goals were determined by the clinical team. Neonates who required vasoactive medications were given dopamine followed by dobutamine, epinephrine, or milrinone infusions as necessary. Sedation was provided with morphine, fentanyl, or hydromorphone infusions and boluses. Neonates received full montage electroencephalograms (EEG) during hypothermia and after rewarming, and continuous amplitude-integrated EEG monitoring (Brainz BRM3 Monitor or CFM Olympic Brainz Monitor, Natus Medical Inc., San Carlos, CA) during hypothermia, rewarming, and the first 6 h of normothermia. Phenobarbital was administered for electrographic or clinical seizures. Fosphenytoin, levetiracetam, or topiramate were added for persistent seizures. Head ultrasounds were obtained upon NICU admission and after rewarming. Clinicians could view the blood pressure and rSO2, but they were blinded to HVx. Clinical variables, including vital signs and laboratory measurements, were extracted from a replicated database of the electronic medical record. Clinical histories were obtained by chart reviews.
Autoregulation Monitoring
Bilateral, adhesive, neonatal cerebral oximetry probes (INVOS; Covidien, Boulder, CO) were placed on the patients’ foreheads. Arterial blood pressure from the patient monitor (GE Marquette, Garnerville, NY) and NIRS signals were synchronously sampled at 100 Hz and processed with an analog-to-digital converter (DT9804, Data Translation, Marlboro, MA) and bedside computer using ICM+ software (Cambridge Enterprises, Cambridge, UK) (8–10,23,24). Artifacts in the NIRS and MAP signals (e.g., arterial line flushes) were manually removed, and data comprising less than 1% of the recording period were excluded as an additional measure to remove artifacts (23).
HVx was calculated with a continuous, moving correlation coefficient between MAP and rTHb (a surrogate measure of CBV obtained by NIRS) (8–10). Consecutive, paired, 10-s averaged values from 300-s duration were incorporated into each calculation, utilizing 30 data points for each HVx calculation (24). HVx ranges from −1 to +1. Negative or near-zero HVx represents functional vasoreactivity (and therefore intact autoregulation) because MAP and CBV are either negatively correlated or are not correlated. When blood pressure decreases and vasoreactivity becomes impaired, HVx becomes positive and approaches +1 because MAP and CBV positively correlate (8–10). HVx values for the right and left sides were averaged and sorted into 5-mmHg bins of MAP to generate bar graphs. The MAPOPT in each time period (hypothermia, rewarming, and first 6 h of normothermia) was identified as the bin with the most negative HVx when the bar graph showed a trend of increasing index values as MAP deviated from this nadir (3) (Figure 5). Two physicians (JKL and MMG), who were blinded to the patient’s history and MRI results, independently interpreted the HVx bar graph. Both physicians had to agree on a patient’s MAPOPT to include the patient in the analysis of MAPOPT and neurologic injury.
Figure 5.
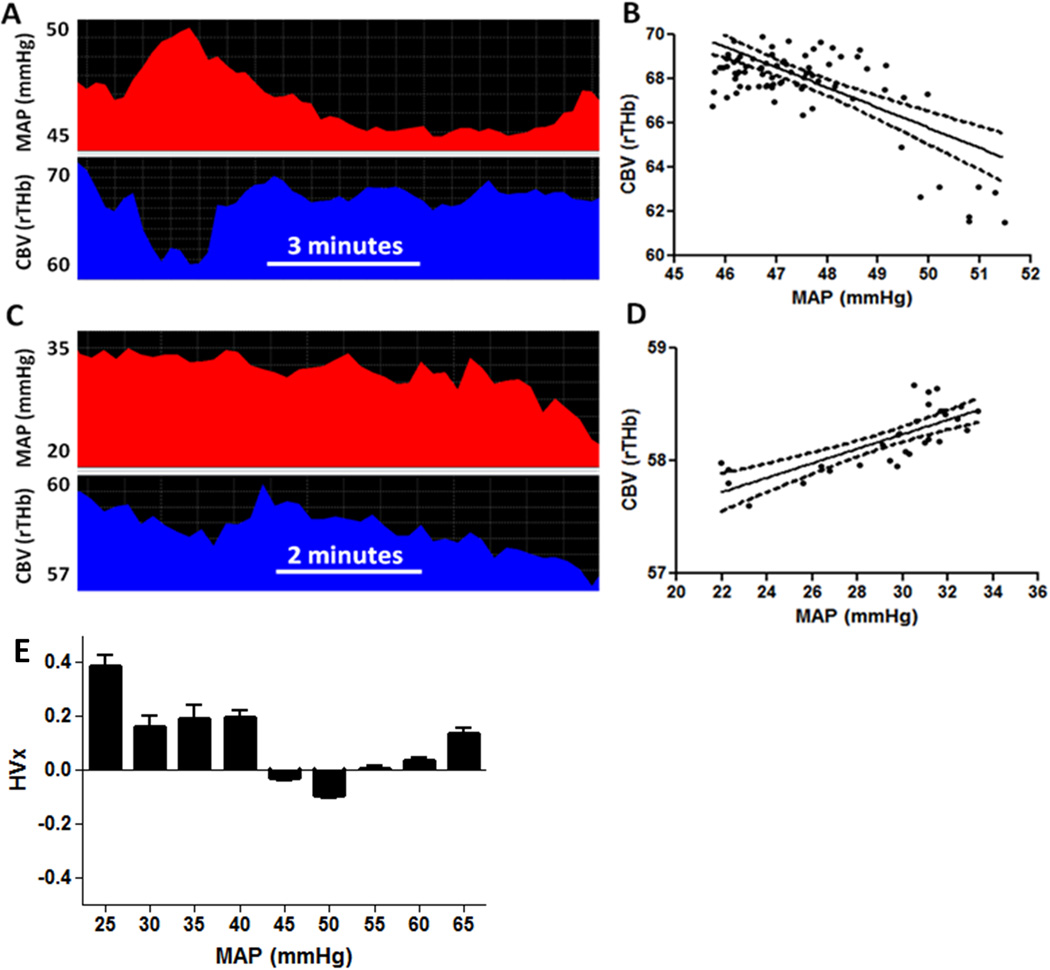
Hemoglobin volume index (HVx) calculation in a neonate with hypoxic-ischemic encephalopathy. (A, B) When mean arterial blood pressure (MAP) exceeded 45 mmHg, MAP negatively correlated with cerebral blood volume (CBV, or the relative total hemoglobin (rTHb) measured by near-infrared spectroscopy). This negative correlation yielded an HVx of −0.29, indicating pressure-reactive vasoreactivity with functional autoregulation. The linear regression line is illustrated (E(Y)=111.5−0.91X; 95% confidence interval for slope: −1.18, −0.66; p<0.0001). (C, D) When MAP was less than 35 mmHg, MAP and CBV positively correlated. This resulted in an HVx of 0.12, indicating pressure-passive vasoreactivity with impaired autoregulation. The linear regression line is illustrated (E(Y)=56.3+0.06X; 95% confidence interval for slope: 0.04, 0.08; p<0.0001). (E) Six hours of HVx monitoring. HVx was sorted into 5-mmHg bins of MAP. Optimal MAP (MAPOPT) was identified at the HVx nadir and represents the range of MAP with most robust vasoreactivity. This patient’s MAPOPT was 50 mmHg. Data in panels B and D are shown with linear regression lines and 95% confidence intervals. Data in panel E are shown as means with SDs.
MRI
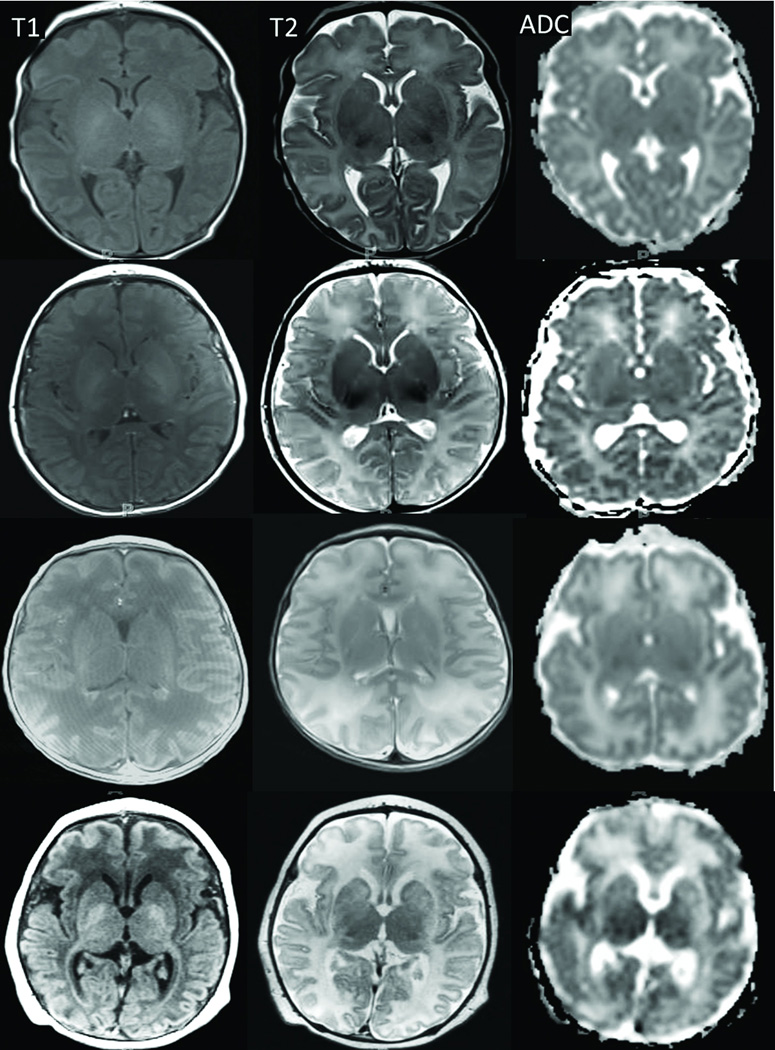
Neonates received brain MRIs 3–7 days after completion of hypothermia on a 1.5-Tesla Magnetom Avanto (Siemens AG, Erlangen, Germany). All neonates received sequences with T1-weighted (T1-W), T2-weighted (T2-W), and diffusion tensor imaging (DTI). Two pediatric neuroradiologists (AT and TH) with 5 and 15 years of dedicated pediatric neuroradiology experience evaluated the MRIs in consensus. Injury was graded as none, mild, moderate, or severe in paracentral gyri, white matter (including PLIC), basal ganglia, thalamus, and brainstem. These regions are associated with motor impairment or death in HIE (11). Qualitative evaluation for injury grading was based on the severity of signal alterations on T1-W, T2-W, and diffusion-weighted imaging (derived from the DTI data) (25). Increasing T1 and T2 signals in the cortex, basal ganglia, PLIC, and thalami represented more severe injury. Increased T2 signal and loss of gray-white matter differentiation identified greater white matter injury. Increased signal in the apparent diffusion coefficient (ADC) maps confirmed the presence of injury (Figure 6). The radiologists were blinded to the patients’ HVx, blood pressures, and clinical histories.
Figure 6.
Axial T1-weighted (first column) and T2-weighted (second column) images and ADC maps (third column) of four neonates with no (first row), mild (second row), moderate (third row), or severe (fourth row) injury. T1 and T2 signals increased in the cortex, basal ganglia, thalami, and posterior limb of the internal capsule with greater injury. With worsening white matter injury, the T2 signal increased and the gray-white matter differentiation became less distinct. ADC maps confirmed the injuries, particularly in the white matter, as signal increased with greater injury.
Statistical Analysis
Descriptive summary statistics were conducted with SAS v9.2 (SAS Institute Inc., Cary, NC), and graphs were generated with GraphPad Prism (v5.03, GraphPad Software Inc., La Jolla, CA). Data are reported as means with SD or medians with IQR when appropriate. Neurologic outcomes in each anatomic region were categorized as no, mild, or moderate/severe injury. Right and left rSO2 values were averaged to analyze the relationship between rSO2 and injury. Time was analyzed as the percentage of the autoregulation monitoring period obtained during hypothermia, rewarming, or normothermia.
ACKNOWLEDGMENTS
We are grateful to Claire Levine for her editorial assistance.
STATEMENT OF FINANCIAL SUPPORT: This project was supported by research grants from Covidien (Boulder, CO; to JKL), the Thomas Wilson Sanitarium for Children Research Foundation (Baltimore, MD; to JKL), the American Heart Association (Dallas, TX; to JKL), the International Anesthesia Research Society (San Francisco, CA; to JKL), National Institutes of Health (NIH) grants NS060703 (Bethesda, MD; to RCK) and HD070996 (to FJN).
REFERENCES
- 1.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zweifel C, Castellani G, Czosnyka M, et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma. 2010;27:1951–1958. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 4.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 5.Brady KM, Lee JK, Kibler KK, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108:1278–1283. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 6.Budohoski KP, Czosnyka M, de Riva N, et al. The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery. 2012;71:652–660. doi: 10.1227/NEU.0b013e318260feb1. [DOI] [PubMed] [Google Scholar]
- 7.Vavilala MS, Tontisirin N, Udomphorn Y, et al. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9:45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee JK, Kibler KK, Benni PB, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40:1820–1826. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Brady KM, Mytar JO, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39:2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JK, Yang ZJ, Wang B, et al. Noninvasive autoregulation monitoring in a swine model of pediatric cardiac arrest. Anesth Analg. 2012;114:825–836. doi: 10.1213/ANE.0b013e31824762d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Biarge M, Diez-Sebastian J, Kapellou O, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–2061. doi: 10.1212/WNL.0b013e31821f442d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasulo FA, Girardini A, Lavinio A, et al. Are optimal cerebral perfusion pressure and cerebrovascular autoregulation related to long-term outcome in patients with aneurysmal subarachnoid hemorrhage? J Neurosurg Anesthesiol. 2012;24:3–8. doi: 10.1097/ANA.0b013e318224030a. [DOI] [PubMed] [Google Scholar]
- 13.Iida K, Kurisu K, Arita K, Ohtani M. Hyperemia prior to acute brain swelling during rewarming of patients who have been treated with moderate hypothermia for severe head injuries. J Neurosurg. 2003;98:793–799. doi: 10.3171/jns.2003.98.4.0793. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, McDonald SA, et al. Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatr Crit Care Med. 2012;13:53–59. doi: 10.1097/PCC.0b013e31821926bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan X, Kavelaars A, Heijnen CJ, Groenendaal F, van Bel F. Pharmacological neuroprotection after perinatal hypoxic-ischemic brain injury. Curr Neuropharmacol. 2010;8:324–334. doi: 10.2174/157015910793358150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimentel VC, Pinheiro FV, De Bona KS, et al. Hypoxic-ischemic brain injury stimulates inflammatory response and enzymatic activities in the hippocampus of neonatal rats. Brain Res. 2011;1388:134–140. doi: 10.1016/j.brainres.2011.01.108. [DOI] [PubMed] [Google Scholar]
- 17.Kusano Y, Echeverry G, Miekisiak G, et al. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30:808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakalova RA, Matsuura T, Kanno I. Cyclooxygenase-pathway participates in the regulation of regional cerebral blood flow in response to neuronal activation under normo- and hypercapnia. Prostaglandins Leukot Essent Fatty Acids. 2002;67:379–388. doi: 10.1054/plef.2002.0445. [DOI] [PubMed] [Google Scholar]
- 19.Pollock JM, Deibler AR, Whitlow CT, et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am J Neuroradiol. 2009;30:378–385. doi: 10.3174/ajnr.A1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990;117:119–125. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 21.Easley RB, Kibler KK, Brady KM, et al. Continuous cerebrovascular reactivity monitoring and autoregulation monitoring identify similar lower limits of autoregulation in patients undergoing cardiopulmonary bypass. Neurol Res. 2013;35:344–354. doi: 10.1179/1743132812Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31:722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 24.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liauw L, van der Grond J, van den Berg-Huysmans AA, Palm-Meinders IH, van Buchem MA, van Wezel-Meijler G. Hypoxic-ischemic encephalopathy: diagnostic value of conventional MR imaging pulse sequences in term-born neonates. Radiology. 2008;247:204–212. doi: 10.1148/radiol.2471070812. [DOI] [PubMed] [Google Scholar]