Abstract
Objectives:
To better inform patients and physicians of the expected risk of adverse events, and to assist hospitals’ efforts to improve the outcomes of patients undergoing implantable cardioverter defibrillation (ICD) implantation, we developed and validated a risk model using data from the National Cardiovascular Data Registry’s (NCDR®) ICD Registry™.
Background:
ICDs prolong life in selected patients, but ICD implantation carries risk of periprocedural complications.
Methods:
We analyzed data from 240,632 ICD implantation procedures between 4/2010 and 12/2011 in the Registry. The population was divided into a derivation (70%) and a validation (30%) cohort. Multivariable logistic regression was used to identify factors associated with in-hospital adverse events (complications or mortality). A parsimonious risk score was developed based on beta estimates derived from the logistic model. Hierarchical models were then used to calculate risk-standardized complication rates to account for differences in case mix and procedural volume.
Results:
Overall 4388 (1.8%) of patients experienced at least one in-hospital complication or death. Thirteen factors were independently associated with an increased risk of adverse outcomes. Model performance was similar in the derivation and validation cohorts (C statistics = 0.724 and 0.719, respectively). The risk score characterized patients into low and high risk subgroups for adverse events (≤10 points, 0.3%; ≥30 points, 4.2%). The risk-standardized complication rates varied significantly across hospitals (median 1.77, IQR 1.54 to 2.14, 5th/95th percentiles 1.16/3.15).
Conclusions:
We developed a simple model that predicts risk for in-hospital adverse events among patients undergoing ICD placement. This can be used for shared decision making and to benchmark hospital performance.
Keywords: Implantable cardioverter defibrillator, registry, quality of care
INTRODUCTION
Randomized clinical trials have demonstrated that implantable cardioverter defibrillators (ICDs) prolong life in patients at high risk of sudden cardiac death (SCD) (1). Over the past decade, ICD implantation rates have increased. However, despite the proven benefits of these devices, their implantation is associated with a risk of procedure-related adverse events that are associated with prolonged hospital length of stay (2), higher cost (2), and decreased six-month survival (3). In order to collect information about characteristics and outcomes of patients undergoing ICD implantation in routine clinical practice, the ICD Registry was developed by the ACC NCDR in partnership with the Heart Rhythm Society (4). One goal of the Registry is to provide information about adverse events that can be used for quality improvement efforts (5).
Each participating hospital whose submission passes data quality thresholds receives quarterly feedback on quality metrics, including the incidence of adverse events at their hospital compared with average performance for all participating hospitals. To date these reports have not adjusted for differences in patient or procedural characteristics between participating hospitals. As a result, currently reported variations in crude event rates may in part reflect differences in case mix rather than hospital performance. To address this limitation, we developed a risk model for adverse events (complications or mortality) that uses ICD registry data to calculate hospitals’ risk standardized complication rates. Hospitals can use this information to identify whether their performance is above or below average, and target quality improvement efforts accordingly. In addition, the model provides a mechanism for providing individualized estimates of procedural risk that can be used to facilitate shared decision making and determine the appropriate level of care following the procedure.
METHODS
Data source
Details of the ICD Registry have been previously described (4). In brief, the Registry currently collects data on over 90% of ICDs placed in the U.S. (6). Information about patients undergoing ICD implantation is collected using standardized definitions and submitted by participating hospitals to the ICD Registry via a secure website. Submitted data then undergo quality checks and are returned to hospitals for cleaning and resubmission if they do not pass criteria for completeness (7). For the current study we used Version 2 data, which were collected beginning in 4/2010. Full elements are available on the NCDR website (5). For our study population, we included all patients undergoing a new ICD implantation or an ICD replacement from 4/2010 to 12/2011 with the exclusion of those undergoing epicardial lead placement, lead only procedures, and any procedure involving lead extraction.
Selection of candidate variables
To determine the optimal outcomes to include in the risk model, a working group was established (SA, JC, JD, MK, MM, EP, MR) who met on a bimonthly basis. The group reviewed all complications collected by the registry and excluded those whose occurrence was either exceedingly rare (venous obstruction 0.03%, conduction block 0.02%, peripheral embolus 0.01%, valve injury <0.01%, peripheral nerve injury <0.01%), or unlikely to be related to the procedure (drug reaction 0.05%). Based on the working group’s consensus, the final primary outcome (any adverse event) consisted of a composite measure of procedure-related complications including any of the following: cardiac arrest, cardiac perforation, coronary venous dissection, hemothorax, device-related infection, lead dislodgement, myocardial infarction, pericardial tamponade, pneumothorax, stroke/transient ischemic attack, urgent cardiac surgery, hematoma, or set screw problem. Given its importance, mortality was included in the composite endpoint with the understanding that it may not always have been directly attributable to the procedure.
For candidate covariates in risk model development, we initially screened all relevant demographic, clinical, and procedural characteristics available from the Registry. The working group determined that certain covariates would be excluded for the following reasons: potential collinearity with other covariates (diastolic blood pressure, ischemic heart disease), low frequency (post-transplant, syndrome with high risk of sudden cardiac death), clinical judgment of factors deemed unlikely to influence complication risk (PR duration, family history of sudden cardiac death), subjective reporting (life expectancy <1 year), as well as variables that could reflect potential disparities in care (race, insurance status). In order to assess the potential impact of excluding race from the risk model, we performed a sensitivity analysis that included race and found that it did not significantly change our results.
Statistical Analysis
We present continuous variables as means (± standard deviation) and categorical variables as percentages. For continuous variables, we examined their distribution and established clinically relevant cutpoints reflecting their association with adverse events. Bivariate comparisons of patients with and without the primary outcome were performed using the t-test (continuous variables) and the chi-square test (categorical variables).
Data were missing for <1% of variables except for cardiac arrest (1.5%), laboratory values (GFR 1.3%, potassium 1.4%, sodium 1.6%, BUN 1.8%, hemoglobin 2.3%), and LVEF (9.0%). For cardiac arrest, we assumed “not present” if missing. We imputed GFR to the gender-specific median consistent with prior NCDR models (8). Missing data for LVEF were imputed to the median and we added a dummy variable as missing indicator. Missing continuous lab values were imputed to the median.
We then randomly split our sample into derivation and validation cohorts (70% derivation/30% validation). In the derivation cohort, we developed a risk model using logistic regression with backwards selection of candidate variables. We generated odds ratios with 95% confidence intervals in order to determine the strength of association for covariates that remained significant. We then evaluated model discrimination in the derivation and validation cohorts by C statistics. For validation, we applied coefficients of the models from derivation cohort to the validation cohort assessing the predicted versus observed rate of adverse event within deciles of predicted adverse event risk. We also generated C statistics for primary versus secondary ICDs in the derivation cohort, and in three other clinically meaningful subgroups: (1) first-time ICD implants (patients without prior ICD or pacemaker); (2) prior ICD excluding those undergoing generator replacements; and (3) CRT-D devices.
For purposes of clinical application, we developed a parsimonious risk score that could be used to calculate patient risk at the bedside. To develop the score, we removed variables from the full model until the adjusted R2 of the parsimonious model was 95% of the full model. The performance of the parsimonious model was evaluated in the derivation and validation cohorts by C statistics and validation plots. We also evaluated model discrimination and calibration in three clinically meaningful subgroups (as with the full model): first-time ICD recipients, prior ICD excluding those undergoing generator replacements, and CRT-D devices. We based the risk score system on the beta coefficients for the risk factors in the parsimonious model. Categorical variables were assigned numeric values proportional to their associated beta coefficients.
Continuous variables of the risk score model were classified into several categories and these categories were assigned numeric values proportional to the product of the beta coefficient and the distance from the base category to these categories (9). For each patient, the ICD complication risk score was calculated as the simple arithmetic sum of point values assigned to each risk factor. We calculated the adverse event rates observed within the population based on risk score values by intervals of 10 in both the derivation and validation cohorts to evaluate the performance of risk scores.
Data from derivation and validation cohorts were then combined in order to calculate risk-standardized complication rates. We used hierarchical logistic regression models to account for clustering of patient admissions within hospitals (10,11). The assumption with this approach is that after adjusting for patient risk factors, the remaining variation is attributable to hospital-level factors. The predicted number of complications at each hospital was estimated given its patient mix and using its own hospital-specific intercept, and the expected number of complications in each hospital was estimated using its patient mix and average hospital-specific intercept based on all hospitals in the sample. The risk-standardized complication rate for each hospital was computed by the ratio of number of predicted complications to the number of expected complications, multiplied by the unadjusted overall complication rate. We computed a 95% interval estimate of the risk-standardized complication rate to characterize the level of uncertainty around the point estimate using bootstrapping simulation. The risk-standardized complication rates and associated interval estimates can be used to characterize and compare hospitals’ performance with the Registry average. As an illustrative example, we used the risk-standardized complication rate and interval estimates to characterize hospital performance as better than Registry average, no different than the Registry average, and worse than Registry average.
A P value of <0.05 was considered statistically significant for all tests. Analyses were performed using SAS version 9.2 (SAS institute, Cary, NC).
RESULTS
Patient Characteristics
During the study period, 263,284 procedures were performed. Procedures involving lead only placement (N=9855, 3.7%) epicardial lead placement (N=5677, 2.2%), and lead extraction (N=7120, 2.9%) were excluded, leaving an analytic sample of 240,632 procedures. Of these, the majority (59.5%) represented first-time ICD implants. Over three quarters of implants (76.6%) were for primary prevention indications. Mean age of the study population was 67.3 years, and 27.3% were female. The most common medical conditions were hypertension (78.3%), prior myocardial infarction (50.7%), heart failure (42.4%), diabetes (37.9%), and chronic lung disease (21.6%). The most common device type was CRT-D (42.5%), followed by dual-chamber ICD (38.3%), then single-chamber ICD (19.0%).
Complications
Overall, 4388 patients (1.8%) experienced an adverse event. The characteristics of patients with and without adverse events are shown in Table 1. On average, patients who experienced an adverse event were older (68.4 vs. 67.3 years, P<0.0001), more often female (33.2% vs. 27.2%, P<0.0001), and less likely to have “ICD implantation” listed as the primary reason for admission (52.7% vs. 73.6%, P<0.0001). Patients with adverse events were also more likely to have experienced a hospitalization for heart failure within the last six months (25.4% vs. 15.6%, P<0.0001).
Table 1.
Characteristics of patients with and without complications
| Complication or in-hospital death* | P-value | ||
|---|---|---|---|
| No (N=236,244) | Yes (N=4,388) | ||
| Admission characteristics | |||
| Age, mean ± SD | 67.3±13.2 | 68.4±13.2 | <0.0001 |
| Female, N (%) | 64,205 (27.2%) | 1459(33.9%) | <0.0001 |
| Reason for admission, N (%) | <0.0001 | ||
| Admitted for procedure | 173,561 (73.6%) | 2304 (52.7%) | |
| Heart failure | 19,325 (8.2%) | 731 (16.7%) | |
| Other | 42,819 (18.2%) | 1336 (30.6%) | |
| History of heart failure, N (%) | <0.0001 | ||
| No | 136,357 (57.9%) | 2218 (50.6%) | |
| Yes – HF hospitalization within 6 months | 36,639 (15.6%) | 1112 (25.4%) | |
| Yes – no HF hospitalization within 6 months |
62,456 (26.5%) | 1050 (24.0%) | |
| NYHA class, N (%) | <0.0001 | ||
| I/II | 121,884(51.8%) | 1489 (34.6%) | |
| III | 106,680 (45.4%) | 2652 (58.6%) | |
| IV | 6665 (2.8%) | 319 (7.3%) | |
| Non-ischemic dilated cardiomyopathy, N (%) |
79,587 (33.8%) | 1682 (38.4%) | <0.0001 |
| Syncope, N (%) | 38,212 (16.2%) | 799 (18.2%) | 0.0003 |
| Atrial fibrillation/flutter, N (%) | 84,992 (36.0%) | 1748 (39.8%) | <0.0001 |
| Ventricular tachycardia, N (%) | 90,193 (38.2%) | 1611 (36.8%) | 0.0483 |
| Cardiac arrest, N (%) | 22,004 (9.3%) | 515 (11.7%) | <0.0001 |
| Previous ICD, N (%) | 96,587 (40.9%) | 943 (21.5%) | <0.0001 |
| Previous pacemaker, N (%) | 25,322 (10.7%) | 562 (12.8%) | <0.0001 |
| Prior myocardial infarction, N (%) | 119,904 (50.8%) | 2103 (48.0%) | 0.0002 |
| Prior PCI, N (%) | 76,604 (32.5%) | 1405 (32.0%) | 0.5542 |
| Prior CABG, N (%) | 77,955 (33.0%) | 1266 (28.9%) | <0.0001 |
| Primary valvular heart disease, N (%) | 28,610 (12.1%) | 674 (15.4%) | <0.0001 |
| Cerebrovascular disease, N (%) | 37,324 (15.8%) | 806 (18.4%) | <0.0001 |
| Diabetes, N (%) | 89,436 (37.8%) | 1734 (39.6%) | 0.0233 |
| Currently on dialysis, N (%) | 6373 (2.7%) | 243 (5.5%) | <0.0001 |
| Chronic lung disease, N (%) | 50683 (21.5%) | 1180 (26.9%) | <0.0001 |
| Hypertension, N (%) | 184876 (78.3%) | 3503 (79.9%) | 0.0139 |
| Sleep apnea, N (%) | 28526(12.1%) | 550 (12.6%) | <0.0001 |
| Diagnostics | |||
| Cardiac rhythm, N (%) | <0.0001 | ||
| Sinus | 153546 (65.2%) | 2866 (65.5%) | |
| Atrial fibrillation/flutter | 34027 (14.5%) | 789 (18.0%) | |
| Paced | 44698 (19.0%) | 586 (13.4%) | |
| Atrioventricular block (2nd and 3rd
degree) |
1606 (0.7%) | 77 (1.8%) | |
| 1487 (0.6%) | 57(1.3%) | ||
| Other | |||
| Abnormal conduction, N (%) | <0.0001 | ||
| No | 110141 (46.8%) | 1556 (35.6%) | |
| Yes – left bundle branch block | 52957 (22.5%) | 1417 (32.4%) | |
| Yes – other | 72114 (30.7%) | 1402 (32.0%) | |
| Procedure type, N (%) | <0.0001 | ||
| Initial implant | 139630 (59.2%) | 3445 (78.5%) | |
| Generator replacement-end of battery life | 80790 (34.2%) | 463 (10.6%) | |
| Generator replacement-infection | 1943 (0.8%) | 83 (1.9%) | |
| Generator replacement-device relocation | 379 (0.2%) | 11 (0.3%) | |
| Generator replacement-upgrade | 10151 (4.3%) | 312 (7.1%) | |
| Generator replacement-malfunction | 912 (0.4%) | 20 (0.5%) | |
| Generator change-other | 2134 (0.9%) | 52 (1.2%) | |
| ICD type, N (%) | <0.0001 | ||
| Single chamber | 45312 (19.2%) | 514 (11.7%) | |
| Dual chamber | 90585 (38.4%) | 1459 (33.3%) | |
| CRT-D | 99875 (42.4%) | 2404 (54.9%) | |
| Body mass index, mean ± SD | 29.8 ± 13.5 | 29.3 ± 14.5 | 0.0182 |
| Left ventricular ejection fraction†, mean ± SD |
30.4 ± 12.7 | 27.7 ± 11.5 | <0.0001 |
| Systolic blood pressure mmHg, mean ± SD |
131.6 ± 22.7 | 129.2 ± 23.3 | <0.0001 |
| Hemoglobin g/dL, mean ± SD | 13.1 ± 2.0 | 12.5 ± 2.1 | <0.0001 |
| Glomerular filtration rate, mean ± SD | 65.1 ± 25.7 | 60.3 ± 27.5 | <0.0001 |
| Blood urea nitrogen mg/dL, mean ± SD | 23.7 ± 13.0 | 27.5 ± 16.9 | <0.0001 |
| Sodium mEq/L, N (%) | <0.0001 | ||
| <135 | 22316 (9.6%) | 683 (15.7%) | |
| 135-145 | 207895 (89.4%) | 3608 (83.0%) | |
| >145 | 2305 (1.0%) | 58 (1.3%) | |
| Potassium mEq/L, N (%) | 0.0009 | ||
| <3.5 | 7829 (3.4%) | 189 (4.3%) | |
| 3.5-4.5 | 167388 (71.9%) | 3128 (71.9%) | |
| >4.5 | 57627 (24.7%) | 1031 (23.7%) | |
Complications: include any of the following: cardiac arrest, cardiac perforation, coronary venous dissection, hemothorax, device-related infection, lead dislodgement, myocardial infarction, pericardial tamponade, pneumothorax, stroke/transient ischemic attack, urgent cardiac surgery, hematoma, or set screw problem.
Ejection fraction data missing for 8.8% of patients
Abbreviations: CABG = coronary artery bypass grafting, CRT-D = cardiac resynchronization therapy with defibrillation, HF = heart failure, ICD = implantable cardioverter-defibrillator, NYHA = New York Heart Association, PCI = percutaneous coronary intervention, SD = standard deviation
The individual in-hospital adverse events (complications or mortality) are listed in Table 2. The most common events were lead dislodgement (1580 patients, 0.66%), hematoma (723 patients, 0.30%), and pneumothorax (573 patients, 0.24%). In-hospital death occurred in 637 patients (0.26%).
Table 2.
Prevalence of complications and mortality among study sample (N=240,632)
| N (%) | |
|---|---|
| Complications | |
| Lead dislodgement | 1580 (0.66%) |
| Hematoma | 723 (0.30%) |
| Pneumothorax | 573 (0.24%) |
| Cardiac arrest | 482 (0.20%) |
| Coronary venous dissection | 230 (0.10%) |
| Device-related infection | 194 (0.08%) |
| Pericardial tamponade | 147 (0.06%) |
| Cardiac perforation | 107 (0.04%) |
| Stroke or transient ischemic attack | 106 (0.04%) |
| Hemothorax | 60 (0.02%) |
| Set screw problem | 67 (0.03%) |
| Urgent cardiac surgery | 38 (0.02%) |
| Myocardial infarction | 41 (0.02%) |
| Mortality | 637 (0.26%) |
| Complications or mortality | 4388 (1.82%) |
Risk Model
The full model for adverse events after ICD implantation included 21 variables (Table 3). The greatest strength of association was seen with procedure type. Patients undergoing an initial ICD implant were over three times as likely to experience complications as those undergoing a generator replacement for end of battery life (odds ratio [OR] 3.57, 95% confidence interval [CI] 3.13-4.08), and placement of either a CRT-D or dual chamber device were more likely to experience complications compared with single chamber devices (CRT-D vs. single chamber: OR 1.73, 95% CI 1.51-1.98; dual chamber vs. single chamber: OR 1.45, 95% CI 1.28-1.64). Increasing severity of heart failure was also strongly associated with complications (NYHA class IV vs. class I/II: OR 2.01, 95% CI 1.71-2.36). The model demonstrated good discrimination in both the derivation and validation cohorts (C statistics 0.724 and 0.722 for derivation and validation cohorts, respectively), and for primary and secondary prevention subgroups in the derivation cohort (C statistics 0.713 and 0.763, respectively).
Table 3.
Risk model for complications after ICD implantation
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age (10 year increase) | 1.086 (1.049 - 1.126) | <0.0001 |
| Sex | ||
| Male | Reference | --- |
| Female | 1.289 (1.187 - 1.400) | <0.0001 |
| Reason for admission | ||
| Admitted for procedure | Reference | --- |
| Heart failure | 1.450 (1.299 - 1.620) | <0.0001 |
| Other | 1.571 (1.430 - 1.725) | <0.0001 |
| NYHA class | ||
| I/II | Reference | --- |
| III | 1.346 (1.231 - 1.473) | <0.0001 |
| IV | 2.007 (1.709 - 2.357) | <0.0001 |
| Non-ischemic dilated cardiomyopathy | 1.117 (1.017 - 1.227) | 0.0206 |
| Cardiac arrest | ||
| No | Reference | --- |
| Yes – bradycardic | 1.512 (1.132 - 2.019) | 0.0052 |
| Yes - tachycardic | 1.209 (1.062 - 1.376) | 0.0040 |
| Prior PCI | 1.098 (1.008 - 1.197) | 0.0324 |
| No prior CABG | 1.225 (1.117 - 1.344) | <0.0001 |
| Cerebrovascular disease | 1.131 (1.027 - 1.244) | 0.0120 |
| Diabetes | 0.913 (0.845 - 0.987) | 0.0218 |
| Current dialysis | 1.557 (1.298 - 1.868) | <0.0001 |
| Chronic lung disease | 1.211 (1.115 - 1.315) | <0.0001 |
| Cardiac rhythm | 0.0167 | |
| Sinus | Reference | --- |
| Atrial fibrillation/flutter | 1.118 (1.010 - 1.237) | 0.0317 |
| Paced | 1.029 (0.907 - 1.167) | 0.6615 |
| AV block (2nd or 3rd degree) | 1.352 (0.992 - 1.842) | 0.0564 |
| Other | 1.399 (1.051 - 1.863) | 0.0215 |
| Abnormal conduction | ||
| No | Reference | --- |
| Yes – LBBB | 1.256 (1.129 - 1.396) | <0.0001 |
| Yes – other | 1.228 (1.117 - 1.350) | <0.0001 |
| Procedure type | ||
| Initial implant | 3.573 (3.132 - 4.075) | <0.0001 |
| Generator replacement-end of battery life | reference | --- |
| Generator replacement-infection | 5.631 (4.188 - 7.572) | <0.0001 |
| Generator replacement-device relocation | 5.980 (3.135 - 11.408) | <0.0001 |
| Generator replacement-upgrade | 3.162 (2.626 - 3.809) | <0.0001 |
| Generator replacement-malfunction | 3.735 (2.168 - 6.434) | <0.0001 |
| Generator replacement-other | 3.984 (2.830 - 5.610) | <0.0001 |
| ICD type | ||
| Single chamber | reference | --- |
| Dual chamber | 1.450 (1.282 - 1.641) | <0.0001 |
| CRT-D | 1.727 (1.508 - 1.977) | <0.0001 |
| Sodium | ||
| <135 | 1.292 (1.164 - 1.433) | <0.0001 |
| 135 - 145 | reference | |
| >145 | 1.213 (0.874 - 1.685) | 0.2482 |
| Systolic blood pressure (1 point increase) | 0.980 (0.964 - 0.997) | 0.0184 |
| Hemoglobin (5 g/dL increase) | 0.755 (0.680 - 0.838) | <0.0001 |
| Glomerular filtration rate (10 unit increase) | 1.029 (1.007 - 1.051) | 0.0079 |
| BUN (10 mg/dL increase) | 1.123 (1.089 - 1.158) | <0.0001 |
Full model (odds ratios from derivation cohort)
C statistic = 0.724
Abbreviations: AV = atrioventricular, BUN = blood urea nitrogen, CABG = coronary artery bypass grafting, CRT-D = cardiac resynchronization therapy with defibrillation, ICD = implantable cardioverter-defibrillator, LBBB = left bundle branch block, NYHA = New York Heart Association, PCI = percutaneous coronary intervention
Among first-time ICD implants (patients without prior ICD or pacemaker, N=128,749) and patients with a prior ICD excluding those undergoing generator replacements (N=30,630), model discrimination was lower (C statistics 0.666 and 0.639, respectively). Among the subgroup of patients receiving CRT-D devices (N=102,279), the C statistic was 0.703.
Parsimonious Model
The parsimonious risk model retained 12 characteristics from the full model (Table 4). The parsimonious model had similar discrimination (C statistics 0.721 and 0.718 for derivation and validation cohorts, respectively). A point system was developed based on regression coefficients. The highest number of points was attributable to procedure type; for example, a patient received 18 points for undergoing device relocation (compared with zero points for an end of battery life generator replacement). The distribution of risk scores among the study population is shown in Figure 1. Based on this distribution, patients with a risk score of 10 or below were at very low risk of complications (0.3%). In contrast, patients with a risk score of 30 or above had a considerably increased risk of complications (4.2%) (Figure 2).
Table 4.
Parsimonious risk scoring system for complications after ICD implantation
| Beta coefficient | Reference value | Points | |
|---|---|---|---|
| Sex | |||
| Male | 0 | 0 | |
| Female | 0.2307 | 2 | |
| Reason for admission | |||
| Admitted for procedure | 0 | 0 | |
| Heart failure | 0.3846 | 4 | |
| Other | 0.5006 | 5 | |
| NYHA Class | |||
| I/II | 0 | 0 | |
| III | 0.2990 | 3 | |
| IV | 0.7102 | 7 | |
| No prior CABG | 0.2293 | 2 | |
| Current dialysis | 0.3149 | 3 | |
| Chronic lung disease | 0.2018 | 2 | |
| Abnormal conduction | |||
| No | 0 | 0 | |
| Yes – LBBB | 0.2376 | 2 | |
| Yes – other | 0.2296 | 2 | |
| Procedure type | |||
| Initial implant | 1.2462 | 13 | |
| Generator replacement-end of battery | 0 | 0 | |
| life | 1.6851 | 17 | |
| Generator replacement-infection | 1.7789 | 18 | |
| Generator replacement-device | 1.1349 | 12 | |
| relocation | 1.2964 | 13 | |
| Generator replacement-upgrade | 1.3568 | 14 | |
| Generator replacement-malfunction | |||
| Generator replacement-other | |||
| ICD type | |||
| Single chamber | 0 | 0 | |
| Dual chamber | 0.3737 | 4 | |
| CRT-D | 0.5745 | 6 | |
| Sodium* | |||
| <135 | 0.2600 | 3 | |
| 135-145 | 135-145 | 0 | |
| >145 | 0.2274 | 2 | |
| Hemoglobin* | −0.3029 | ||
| <12 | 3 | ||
| 12-14 | 2 | ||
| >14 | >14 | 0 | |
| BUN* | 0.0971 | ||
| <20 | <20 | 0 | |
| 20-40 | 2 | ||
| >40 | 4 |
Risk score based on parsimonious model
C statistic for risk score = 0.721
Abbreviations: BUN = blood urea nitrogen, CABG = coronary artery bypass grafting, CRT-D = cardiac resynchronization therapy with defibrillation, ICD = implantable cardioverter-defibrillator, LBBB = left bundle branch block, NYHA = New York Heart Association
Sodium analyzed as 3-level categorical variable (nonlinear relationship); Hemoglobin and BUN analyzed as continuous variables (linear relationship)
Figure 1. Frequency of risk scores among the study sample.
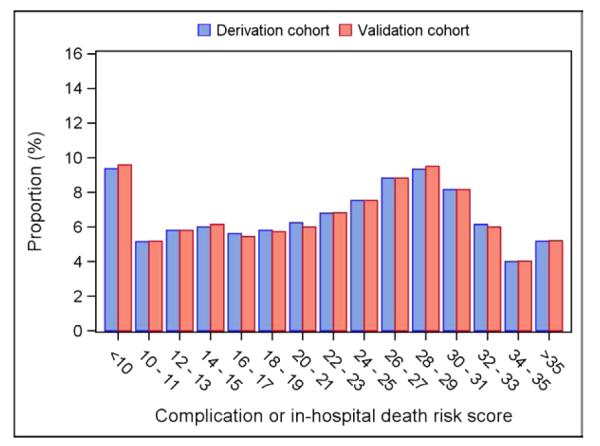
Each patient in the study sample was assigned a numeric score based on a parsimonious risk model that included 12 patient and procedural characteristics that independently predicted procedure-related adverse events. There was a relatively even distribution of scores across a wide range of values.
Figure 2. Risk of complications for in-hospital adverse events based on risk scoring system.

The risk of procedure-related adverse events (complications or in-hospital death) increased in a roughly linear matter as the numeric risk score increased.
Hospital Distribution
The distribution of risk-standardized complication rates among participating hospitals (N=1428) is shown in Figure 3. The median risk-standardized complication rate was 1.77 (interquartile range, 1.54 to 2.14, 5th and 95th percentiles 1.16 and 3.15 respectively). Compared with the Registry average, 54 hospitals (3.8%) were worse than expected, and 15 hospitals (1.1%) were better than expected.
Figure 3. Distribution of risk-standardized complication rates among hospitals.
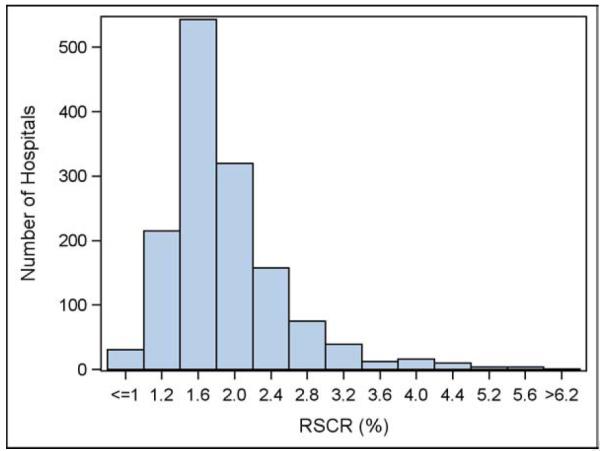
In general there was clustering around the median risk-standardized complication rate, which was 1.77% (interquartile range, 1.54% to 2.14%). Compared with the Registry average, 54 hospitals (3.8%) were worse than expected, and 15 hospitals (1.1%) were better than expected.
DISCUSSION
We developed a risk model to predict in-hospital adverse events after ICD implantation using clinical characteristics that are readily available at the time of procedure. Our efforts had two main purposes: first, the risk model can be used to benchmark hospital complication rates and therefore be a useful tool in quality improvement efforts. Second, the risk score derived from the model can be used to facilitate shared decision-making with patients by incorporating an individual’s expected risks of ICD implantation.
Our model provides adverse event rates that are risk standardized (risk-standardized complication rates) which we believe will have higher clinical significance for participating sites. To date, hospitals submitting data to the ICD Registry have received quarterly feedback reports listing their crude ICD complication rates compared with average rates for participating hospitals, and this approach has failed to account for differences in case mix. Sites may therefore dismiss higher crude complication rates as due to patient complexity rather than physician or system-wide factors that may be targeted for quality improvement. The use of risk-standardized complication rates in future reports should have higher face validity and allow a more compelling argument for internal evaluation of the quality of care among sites that are low performing.
The strongest risk factors for adverse events in our model were procedure type (any procedure other than simple generator replacement), ICD type (other than single lead), heart failure severity, renal dysfunction, and non-elective admission. Using our risk model, a patient with all five risk factors (for example: undergoing initial device implantation, dual-chamber device, NYHA Class IV, BUN = 45, admitted for primary reason other than device placement) would have an expected complication risk of at least 4%. Conversely, other patients (for example: elective admission for a single-lead ICD with mildly symptomatic heart failure and normal renal function) would have an expected complication risk of less than 1%. Our model may therefore be used for informed discussions between clinicians and patients about the individualized risk of ICD implantation. In addition, the model may potentially be used to identify particularly low-risk patients who may require less intensive post-procedure monitoring (for example, same-day discharge).
Several other findings are worthy of note. Our observed adverse event rate (1.8%) was lower than in most prior studies, including those using ICD Registry data (12,13,14). For example, a study of 268,701 ICD Registry patients undergoing procedures from 2006-2008 found a complication rate of 3.2% (12). Older studies generally reported even higher in-hospital complication rates – up to 10% in several reports (13,14). The low rate of adverse events in our study sample may represent differences between our datasets and others (i.e. some prior studies using claims data rather than Registry data), or a secular trend towards improved in-hospital outcomes among ICD recipients over time. We do not think our low adverse event rate would negatively affect the performance of our risk model; however, since data are self-reported from sites, with the potential for under-reporting of complications at certain hospitals, we believe it is premature for our risk-standardized complication rates to be used for public reporting. However, the information can be used by hospitals to promote internal quality improvement efforts to improve the outcomes associated with their ICD implantations.
An additional finding of interest is that patient age did not enter into our parsimonious risk model as its strength of association with adverse events was weak. Based on our investigation and others (12,13,15), age does not appear to be a strong predictor of in-hospital adverse events. Therefore older patients with few comorbidities may be expected to have reasonably good post-procedural outcomes.
There are several limitations to our study that deserve consideration. Our results were limited to in-hospital adverse events, and therefore our model is unable to predict risk of post-discharge complications (such as device infection) that may require rehospitalization. Also, as some hospitals do not submit data to the registry, or submit poor quality data, the in-hospital adverse event rate in practice may be higher than reported in our study sample, and there may be risk factors for adverse events that we were unable to identify. In addition, our model only provides information about the expected risk of device implantation, not potential benefits. Nevertheless we believe this information will prove useful in promoting shared decision-making. Another potential barrier to widespread use of the risk score is the number of covariates in our parsimonious model (twelve) which is more than several other risk scores. Nevertheless, these characteristics are generally readily available on all patients undergoing ICD implantation. Furthermore, the growth of electronic medical records may facilitate the calculation of a risk score by identifying risk factors in an automated fashion. Finally, given that data are self-reported, enhanced efforts to verify complication rates with external chart audits of participating hospitals may improve the validity of our observations.
CONCLUSIONS
We developed a simple model that predicts risk for in-hospital adverse events among patients undergoing ICD placement. This can be used for both shared decision making and to benchmark hospital performance for quality improvement efforts.
Acknowledgments
Funding Sources:
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
Dr. Dodson was supported by a Training Grant in Epidemiologic Research on Aging (T32 AG000158-24) from the NIH/NIA and a Clinical Research Loan Repayment Award from the NIH/NHLBI during the writing of this manuscript and is currently supported by the NIA grant R03AG045067 and a T. Franklin Williams Scholarship Award (funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American College of Cardiology).
Partners & Sponsors:
ICD Registry™ is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
ABBREVIATIONS
- ACC
American College of Cardiology
- BUN
blood urea nitrogen
- CRT
cardiac resynchronization therapy
- GFR
glomerular filtration rate
- ICD
implantable cardioverter defibrillator
- LVEF
left ventricular ejection fraction
- NCDR®
National Cardiovascular Data Registry
- NYHA
New York Heart Association
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Jeptha P. Curtis receives salary support from the American College of Cardiology National Cardiovascular Data Registry.
REFERENCES
- 1.Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: Expanding indications and technologies. JAMA. 2006;295:809–18. doi: 10.1001/jama.295.7.809. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among Medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493–7. doi: 10.1016/j.jacc.2006.02.049. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DS, Krahn AD, Healey JS, et al. Evaluation of early complications related to de novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774–82. doi: 10.1016/j.jacc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Hammill SC, Stevenson LW, Kadish AH, et al. Review of the Registry's first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–3. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 5.NCDR ICD Registry . http://www.ncdr.com/webncdr/ICD/Elements.aspx. [Google Scholar]
- 6.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–65. doi: 10.1016/j.hrthm.2013.01.035. 5. [DOI] [PubMed] [Google Scholar]
- 7.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular DataRregistry (NCDR) data quality brief: The NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SK, Frutkin AD, Lindsey JB, et al. Bleeding in patients undergoing percutaneous coronary intervention: The development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–9. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan LM, Massaro JM, D'Agostino RBS. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 10.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–92. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 11.Curtis JP, Geary LL, Wang Y, et al. Development of 2 registry-based risk models suitable for characterizing hospital performance on 30-day all-cause mortality rates among patients undergoing percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2012;5:628–37. doi: 10.1161/CIRCOUTCOMES.111.964569. [DOI] [PubMed] [Google Scholar]
- 12.Haines DE, Wang Y, Curtis J. Implantable Cardioverter-Defibrillator Registry risk score models for acute procedural complications or death after implantable cardioverter-defibrillator implantation. Circulation. 2011;123:2069–76. doi: 10.1161/CIRCULATIONAHA.110.959676. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002-2005. Circ Arrhythm Electrophysiol. 2008;1:240–9. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–32. doi: 10.1111/j.1540-8159.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 15.Mandawat A, Curtis JP, Mandawat A, Njike VY, Lampert R. Safety of pacemaker implantation in nonagenarians: An analysis of the healthcare cost and utilization project-nationwide inpatient sample. Circulation. 2013;127:1453–65. doi: 10.1161/CIRCULATIONAHA.113.001434. [DOI] [PubMed] [Google Scholar]


