Abstract
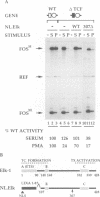
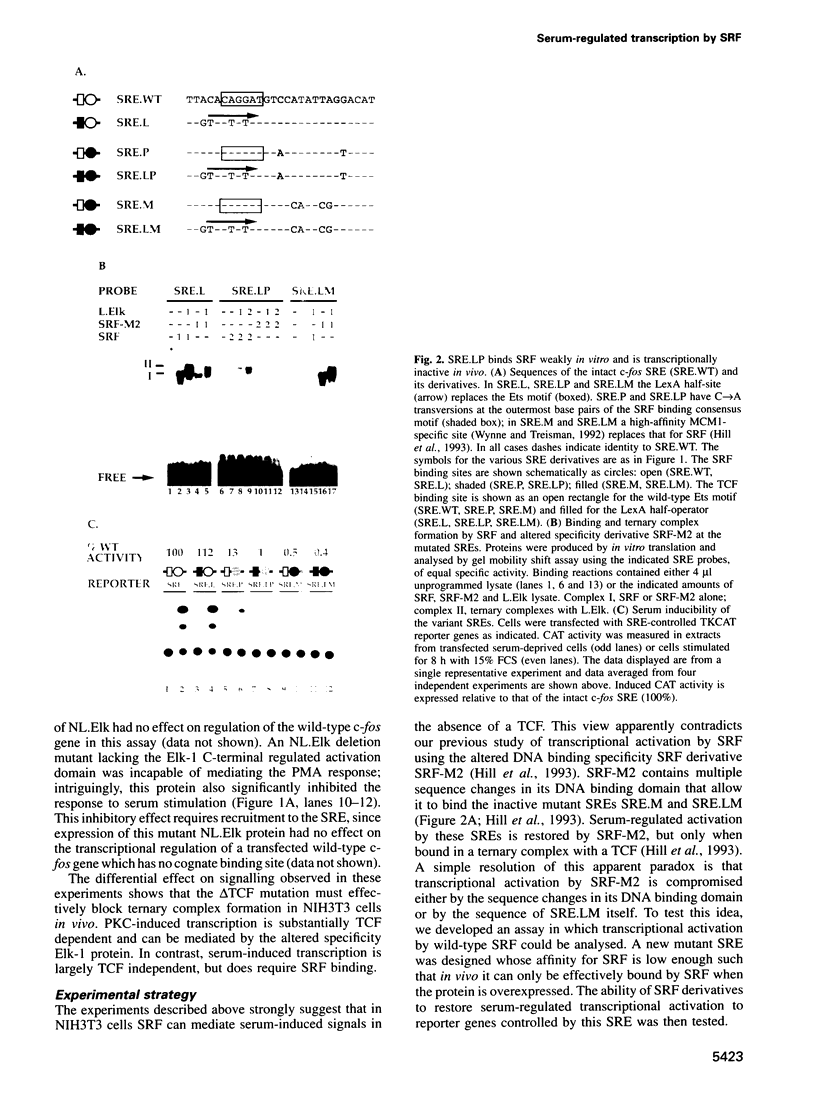
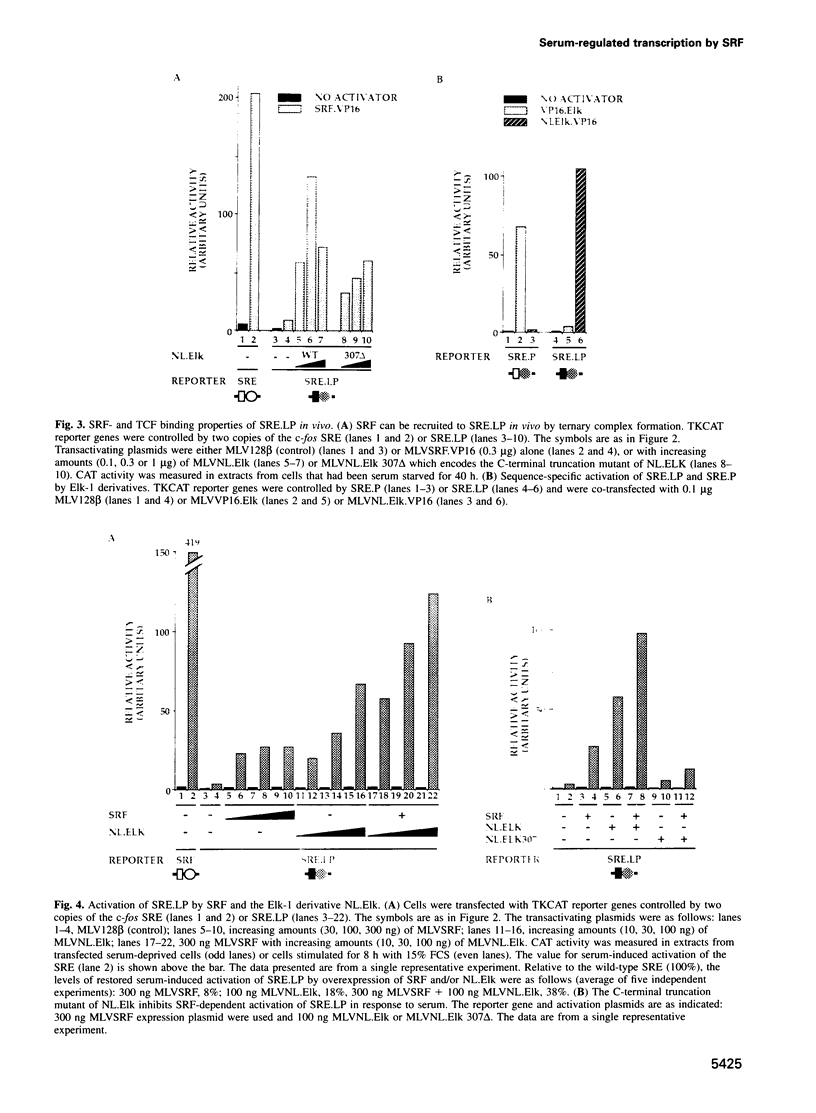
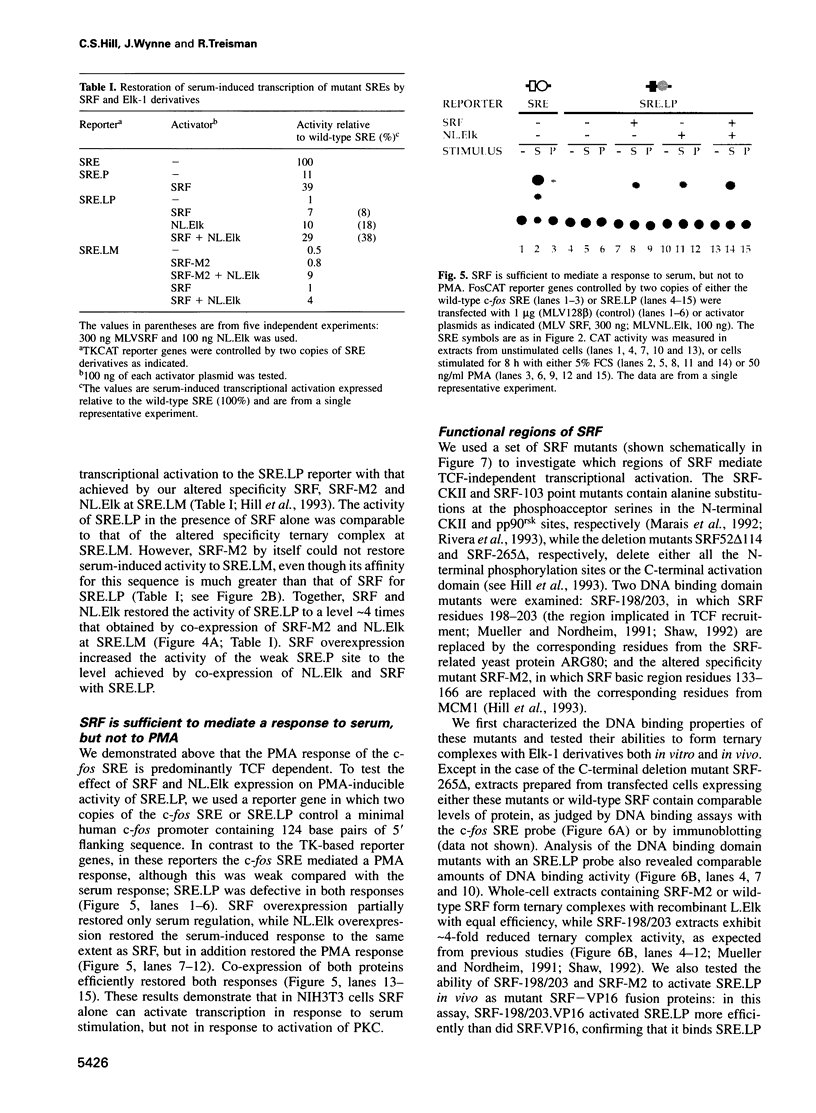
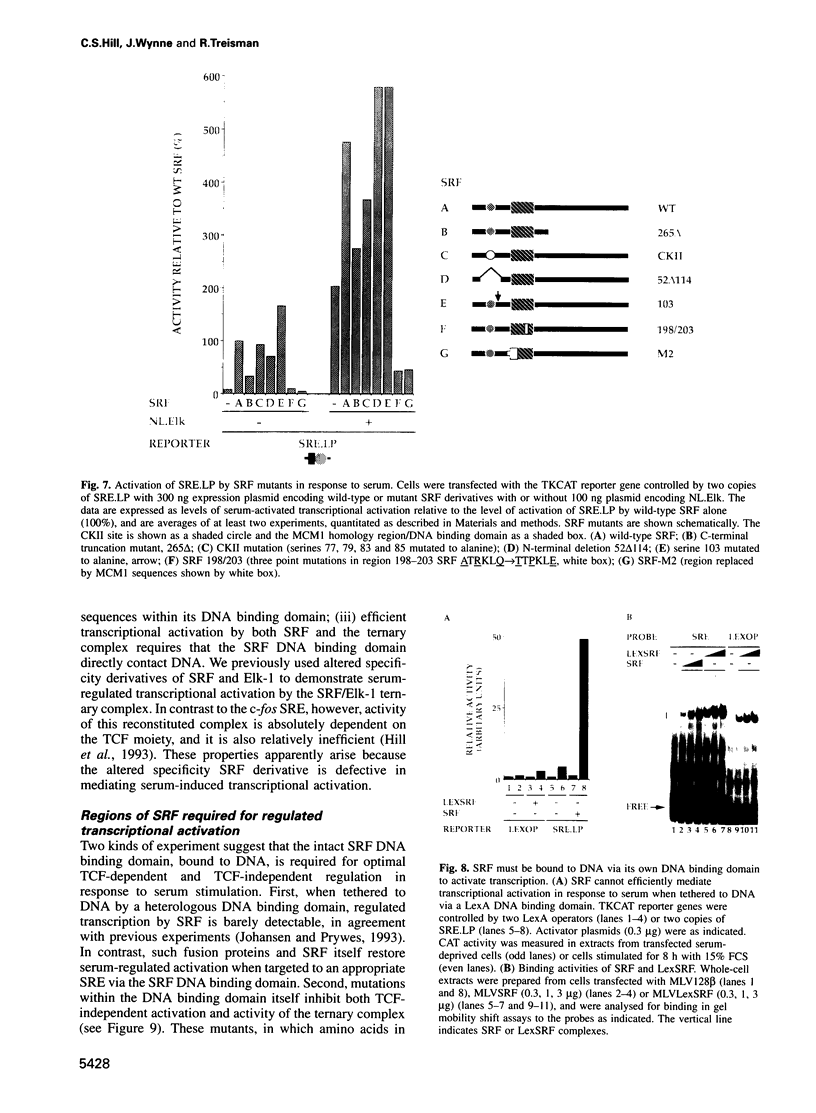
The transcription factors Serum Response Factor (SRF) and Ternary Complex Factor (TCF) form a ternary complex at the c-fos Serum Response Element (SRE). We show that in NIH3T3 cells TCF binding is required for regulated transcription in response to stimulation by phorbol myristate acetate (PMA), but not by whole serum. We constructed a novel transcriptionally inactive SRE variant whose serum-regulated activity can be partially restored by overexpression of SRF in the absence of bound TCF. Activation by SRF does not require the SRF N-terminal phosphorylation sites, but is potentiated 2- to 3-fold by the SRF C-terminal activation domain. Mutations in the SRF DNA binding domain, which do not affect the ability of SRF to bind DNA, abolish its ability to mediate TCF-independent serum-regulated activation and reduce activation by the SRF/TCF(Elk-1) ternary complex. Efficient activation requires that SRF be targeted to DNA via its own DNA binding domain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias J., Alberts A. S., Brindle P., Claret F. X., Smeal T., Karin M., Feramisco J., Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994 Jul 21;370(6486):226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- Bruhn L., Hwang-Shum J. J., Sprague G. F., Jr The N-terminal 96 residues of MCM1, a regulator of cell type-specific genes in Saccharomyces cerevisiae, are sufficient for DNA binding, transcription activation, and interaction with alpha 1. Mol Cell Biol. 1992 Aug;12(8):3563–3572. doi: 10.1128/mcb.12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn L., Sprague G. F., Jr MCM1 point mutants deficient in expression of alpha-specific genes: residues important for interaction with alpha 1. Mol Cell Biol. 1994 Apr;14(4):2534–2544. doi: 10.1128/mcb.14.4.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ C., Tye B. K. Functional domains of the yeast transcription/replication factor MCM1. Genes Dev. 1991 May;5(5):751–763. doi: 10.1101/gad.5.5.751. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Du W., Thanos D., Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993 Sep 10;74(5):887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- Eckner R., Birnstiel M. L. Cloning of cDNAs coding for human HMG I and HMG Y proteins: both are capable of binding to the octamer sequence motif. Nucleic Acids Res. 1989 Aug 11;17(15):5947–5959. doi: 10.1093/nar/17.15.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Tsuchiya H., Chuhjo T., Akizawa T., Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992 Nov;6(11):2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nolan G. P., Ghosh S., Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-kappa B. Genes Dev. 1992 May;6(5):775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Giovane A., Pintzas A., Maira S. M., Sobieszczuk P., Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994 Jul 1;8(13):1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Taylor A., Kedes L. DNA bending is induced by a transcription factor that interacts with the human c-FOS and alpha-actin promoters. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2162–2166. doi: 10.1073/pnas.86.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Hipskind R. A., Nordheim A. Functional dissection in vitro of the human c-fos promoter. J Biol Chem. 1991 Oct 15;266(29):19583–19592. [PubMed] [Google Scholar]
- Hipskind R. A., Rao V. N., Mueller C. G., Reddy E. S., Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Hipskind R. A., Houthaeve T., Nordheim A., Stunnenberg H. G. Identification of multiple SRF N-terminal phosphorylation sites affecting DNA binding properties. EMBO J. 1992 Mar;11(3):1045–1054. doi: 10.1002/j.1460-2075.1992.tb05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. doi: 10.1093/nar/20.13.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Zinck R., Ernst W. H., Nordheim A. Functional dissection of the transcription factor Elk-1. Oncogene. 1994 Apr;9(4):1273–1278. [PubMed] [Google Scholar]
- Johansen F. E., Prywes R. Identification of transcriptional activation and inhibitory domains in serum response factor (SRF) by using GAL4-SRF constructs. Mol Cell Biol. 1993 Aug;13(8):4640–4647. doi: 10.1128/mcb.13.8.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Vaillancourt R. R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol. 1994 Apr;6(2):230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Kortenjann M., Thomae O., Shaw P. E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H. Cell-type specific multiprotein complex formation over the c-fos serum response element in vivo: ternary complex formation is not required for the induction of c-fos. Nucleic Acids Res. 1991 Jul 11;19(13):3607–3611. doi: 10.1093/nar/19.13.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic B. V., O'Brien T. P., Lau L. F. Promoter function and structure of the growth factor-inducible immediate early gene cyr61. Nucleic Acids Res. 1991 Jun 25;19(12):3261–3267. doi: 10.1093/nar/19.12.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. H., Ma J. T., Yueh A. Y., Lees-Miller S. P., Anderson C. W., Ng S. Y. The carboxyl-terminal transactivation domain of human serum response factor contains DNA-activated protein kinase phosphorylation sites. J Biol Chem. 1993 Oct 5;268(28):21147–21154. [PubMed] [Google Scholar]
- Lopez M., Oettgen P., Akbarali Y., Dendorfer U., Libermann T. A. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization, and differential expression during B-lymphocyte development. Mol Cell Biol. 1994 May;14(5):3292–3309. doi: 10.1128/mcb.14.5.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Hsuan J. J., McGuigan C., Wynne J., Treisman R. Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 1992 Jan;11(1):97–105. doi: 10.1002/j.1460-2075.1992.tb05032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994 Feb;4(1):82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- McCormick F. Activators and effectors of ras p21 proteins. Curr Opin Genet Dev. 1994 Feb;4(1):71–76. doi: 10.1016/0959-437x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Miltenberger R. J., Cortner J., Farnham P. J. An inhibitory Raf-1 mutant suppresses expression of a subset of v-raf-activated genes. J Biol Chem. 1993 Jul 25;268(21):15674–15680. [PubMed] [Google Scholar]
- Mohun T., Garrett N., Treisman R. Xenopus cytoskeletal actin and human c-fos gene promoters share a conserved protein-binding site. EMBO J. 1987 Mar;6(3):667–673. doi: 10.1002/j.1460-2075.1987.tb04806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. G., Nordheim A. A protein domain conserved between yeast MCM1 and human SRF directs ternary complex formation. EMBO J. 1991 Dec;10(13):4219–4229. doi: 10.1002/j.1460-2075.1991.tb05000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Prywes R., Fisch T. M., Roeder R. G. Transcriptional regulation of c-fos. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):739–748. doi: 10.1101/sqb.1988.053.01.084. [DOI] [PubMed] [Google Scholar]
- Prywes R., Zhu H. In vitro squelching of activated transcription by serum response factor: evidence for a common coactivator used by multiple transcriptional activators. Nucleic Acids Res. 1992 Feb 11;20(3):513–520. doi: 10.1093/nar/20.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. elk-1 domains responsible for autonomous DNA binding, SRE:SRF interaction and negative regulation of DNA binding. Oncogene. 1992 Nov;7(11):2335–2340. [PubMed] [Google Scholar]
- Rivera V. M., Miranti C. K., Misra R. P., Ginty D. D., Chen R. H., Blenis J., Greenberg M. E. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993 Oct;13(10):6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993 Apr;12(4):1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P., Cochran B. H. MAT alpha 1 can mediate gene activation by a-mating factor. Genes Dev. 1991 Oct;5(10):1924–1934. doi: 10.1101/gad.5.10.1924. [DOI] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Shaw P. E. Ternary complex formation over the c-fos serum response element: p62TCF exhibits dual component specificity with contacts to DNA and an extended structure in the DNA-binding domain of p67SRF. EMBO J. 1992 Aug;11(8):3011–3019. doi: 10.1002/j.1460-2075.1992.tb05371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Treisman R., Marais R., Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994 Feb;4(1):96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Dwarki V. J., Verma I., Davis R., Hollenberg S., Snider L., Lassar A., Tapscott S. J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991 Aug;5(8):1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- Wynne J., Treisman R. SRF and MCM1 have related but distinct DNA binding specificities. Nucleic Acids Res. 1992 Jul 11;20(13):3297–3303. doi: 10.1093/nar/20.13.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Joliot V., Prywes R. Role of transcription factor TFIIF in serum response factor-activated transcription. J Biol Chem. 1994 Feb 4;269(5):3489–3497. [PubMed] [Google Scholar]
- Zhu H., Roy A. L., Roeder R. G., Prywes R. Serum response factor affects preinitiation complex formation by TFIID in vitro. New Biol. 1991 May;3(5):455–464. [PubMed] [Google Scholar]
- Zinck R., Hipskind R. A., Pingoud V., Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993 Jun;12(6):2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]