Abstract
Influenza infection of humans remains an important public health problem. Vaccine strategies result in a significant but only partial control (65-85%) of infection. Thus, chemotherapeutic approaches are needed to provide a solution both for vaccine failures and to limit infection in the unvaccinated population. Previously (Walsh et al. 2011, Teijaro et al. 2011) documented that sphingosine-1-phosphate 1 receptor (S1P1R) agonists significantly protected mice against pathogenic H1N1 influenza virus by limiting immunopathologic damage while allowing host control of the infection. Here we extend that observation by documenting S1P1R agonist can control pathogenic H1N1 influenza infection in ferrets. S1P1R agonist was more effective in reducing pulmonary injury than the antiviral drug oseltamivir but, importantly, combined therapy was significantly more effective than either therapy alone.
Keywords: H1N1 influenza, Ferrets, S1P1 Rec agonist, RP-002, Oseltamivir, Pathogenesis
Introduction
Newly emerging and re-emerging influenza virus infections provide considerable medical, public health, and economic hardships. These viral infections loom as important zoonotic human diseases with the threat of human-to-human transmission and excessive high infectivity and mortality rates. In the current century, the first influenza virus pandemic occurred in 2009, H1N1 influenza infection rapidly infected millions worldwide with estimates exceeding 290,000 deaths (Itoh et al., 2009; Shieh et al., 2010). In addition, non-pandemic seasonal influenza viruses cause approximately 36,000 deaths yearly in the United States. Vaccination is useful in providing protection of both uninfected (naïve) or previously vaccinated individuals although the vaccine is only 65 to 85% effective (www.CDC.gov). Thus, a complementary approach is required to protect those who do not respond to vaccination and others who are unvaccinated against a currently circulating influenza virus.
The end result of any viral infection reflects the balance between the virulence of the infecting agent and the resistance of the host. Over time, influenza virus survival relies on escape from host resistance through antigenic alteration arising via genetic drift by point mutations in the viral HA or through reassortment by genetic shift of its current HA ligand with that of viruses retained in non-human animals. The defining event in host resistance is the efficiency and effectiveness of its antiviral immune response. However, in certain instances the immune response becomes exaggerated and an over-abundant extreme innate and adaptive immune response termed “cytokine storm” leads to immune response-mediated tissue and cellular injury.
Pulmonary injury associated with influenza virus infection results from the combined effect of two factors. First, viruses own intrinsic virulence in lysing cells it infects and, second, the intensity of the immune response elicited by the virus that itself also damages tissues and cells. Anti-influenza drugs like neuraminidase inhibitors are effective therapies to diminish ongoing infections. However, such treatment has a negligible effect on cytokine storm and exerts selective pressure on viral progeny promoting their mutation and selection creating a new generation of influenza viruses that is more fit and resistant to the antiviral drug (2009; Poon et al., 2010; Vijaykrishna et al., 2010).
Earlier we provided the first definitive experimental evidence that cytokine storm rather than being an event accompanying influenza infection, by itself, was a major contributor to the resultant pulmonary injury and hence an important player in the morbidity and mortality of influenza virus pathogenesis (Marsolais et al., 2009; Teijaro et al., 2011; Walsh et al., 2011). Utilizing mice infected with a non-adapted human pathogenic H1N1 influenza 2009 viruses we showed that the resultant cytokine storm was chemically treatable utilizing immunomodulatory small molecules, either sphingosine-1-phosphate (S1P) receptor (R) agonist that signals through four of the five S1PR, R1, 3, 4, 5 (Marsolais et al., 2009; Walsh et al., 2011) or S1P1R specific agonists (Teijaro et al., 2011). These immunomodulating molecules dramatically blunted the production of cytokines/chemokines and the innate cellular immune response. Such therapies did not alter the host’s ability to control the viral infection but successfully limited immunopathologic injury and proved more effective than optimal therapy with the anti-influenza neuraminidase drug oseltamivir, 82% vs. 50% protection from mortality (Walsh et al., 2011). More important, combination therapy with both drugs provided greater than 96% survival rate (Walsh et al., 2011).
Mice and ferrets are the most useful and productive small animal models to study influenza virus pathogenesis. While the mouse cost is low and provides availability of reagents, well-defined genetics, and transgenic models, it fails to mimic clinical signs in humans. Further, its respiratory tract/pulmonary anatomy/physiology differs from humans and is a poor choice to study virus transmission. In contrast, ferrets display clinical signs found in humans following influenza infection as elevated body temperatures, nasal discharge and sneezing, can transmit infection to both healthy ferrets and humans, and share similar lung physiology and patterns of binding to the receptor for the virus that is throughout the respiratory tract (Belser et al., 2011; Maher and DeStefano, 2004; Smith and Sweet, 1988). Thus, the pathogenesis of influenza virus in ferrets more closely resembles that of humans than mice. Further, the initial transmission and isolation of influenza from humans occurred via ferrets with both animal species cross-transmitting to each other (Smith, 1933). For these reasons we studied in ferrets the therapeutic efficiency of S1P1R agonist alone and in combination with high dose of oseltamivir in the pathogenesis of human pathologic H1N1 influenza infection. Our results clearly show that S1P1R agonist was beneficial in altering the clinical course and was significant in blunting the pulmonary injury caused by the H1N1 influenza virus. As anticipated S1P1R signaling did not blunt viral loads in the lung on the third day of infection. Further, as in the mouse, the combination therapy of the S1P1R agonist which primarily treats the immune-mediated injury and antiviral neuraminidase oseltamivir which controls viral replication, gave the greatest therapeutic benefit.
Results and Discussion
Thirty-five male ferrets, average weights of 417 ± 23 gms, were randomly assigned to one of four groups of eight, or one group of three animals. On study day 0, all animals in groups comprising eight ferrets were challenged intranasally with 1 × 106 TCID50 of pathogenic human A/California/04/09 H1N1 influenza virus. The group of three ferrets which received no virus were euthanized at day 0 as controls for tissue analysis (negative control). Of the ferrets receiving virus, one group received only vehicle (positive control), a second group received oseltamivir 12.5 mg/kg administered orally two hours following virus inoculation, and then twice a daily on study days 1 through 5. A third group was treated orally with the S1P1 receptor agonist RP-002 at 6 mg/kg at 1, 25, and 49 hours post-infection. The fourth group received a combination of RP-002 and oseltamivir at the same time and dosages listed above. Of the eight ferrets in each group, four were randomly selected for sacrifice at day 3 post-infection for histopathologic examination while the remaining four ferrets in each group were followed for up to two weeks.
Clinical findings
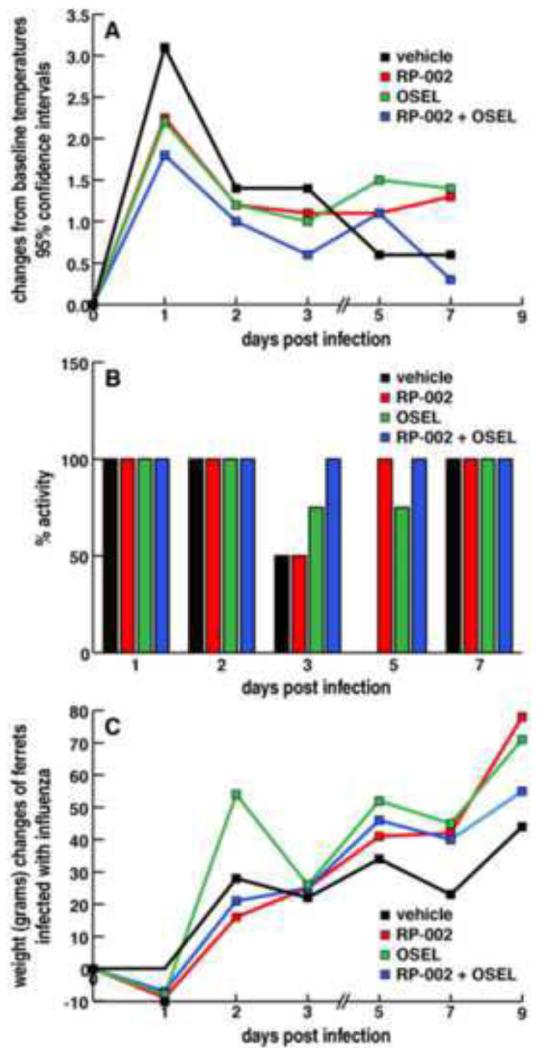
All ferrets were challenged with human 2009 pandemic influenza virus administered at Battelle due to biosafety concerns. Observations were measured in eight ferrets per group for the first three days. At day 3, four ferrets from each group were randomly selected for euthanasia to obtain tissues for study. Thereafter clinical findings were recorded in the four remaining ferrets. Temperatures (Fig. 1A) were recorded daily at two sites (shoulder blade and hip) and displayed highest peak at 24 hours post-infection. Ferrets receiving only vehicle recorded temperatures higher than the other three groups with ferrets receiving only S1P1 receptor agonist RP-002 or only oseltamivir showing similar temperature increases that were consistently less than ferrets that received neither therapy. The smallest increase in temperature occurred among ferrets that received combined therapy of RP-002 and oseltamivir. This trend continued throughout the first three days of infection, after which low-grade temperatures persisted until the termination of the study.
Figure 1.
Combined RP-002 S1P1R agonist and Oseltamivir therapy, RP-002 or Oseltamivir alone blunt the clinical manifestation of disease in ferrets following infection with 1 × 106 TCID50 dose of A/California/09/04 H1N1 given intranasally. Four ferrets in each group tested. Panel A: Temperatures recorded daily at two sites (shoulder blade and hip). Data plotted as degree of temperature change from baseline level of ferrets prior to initiation of infection or therapies. Panel B: Daily activity recorded twice daily as alert and playful, alert and playful only when stimulated, or neither alert or playful at any time. Bar graphs record percent of ferrets in each group that display activity (100%) to those showing no activity (0%). Panel C plots weight changes from the baseline measurement of each group prior to infection and thereafter during the course of infection with the various therapeutic treatments. Ferrets in each group followed for 12 days.
The second quantifiable examination was clinical activity and plotted in Figure 1B as % activity compared to baseline control. Each experimental group was studied twice daily from the afternoon after initiating infection and then each morning. Activity was recorded as alert and playful, alert but only playful when stimulated, or neither alert nor playful at any time. As shown in Figure 1B, for the first two and one-half days post-infection, none of the eight ferrets in any of the four groups displayed a loss of activity. By day 3 post-infection, two of four ferrets treated with vehicle or with RP-002 lost activity compared to one of four ferrets treated with oseltamivir. None of the four ferrets given a combination therapy with RP-002 and oseltamivir exhibited any loss of activity. By day 5 post-infection and thereafter ferrets treated with RP-002 only or RP-002 combined with oseltamivir displayed no loss of activity. However at day 5, one out of four infected ferrets treated only with oseltamivir showed a loss of activity but thereafter activity was present in all ferrets in this group up to day 12 (data not shown). By contrast, all ferrets receiving only vehicle appeared ill with only one of four showing activity on day 4 (data not shown) and none of the four ferrets in this group displayed activity at days 5 and 6 post-infection. Recovery occurred in the vehicle only treated group at day 7 post-infection. Body weights were measured day −1, day 0, and every day thereafter and shown in Figure 1C. There was a slight loss of weight in ferrets receiving RP-002, oseltamivir, or the combined RP-002 + oseltamivir therapy 24 hours after influenza challenge, but by day 2 and thereafter, weights in all four experimental groups increased throughout the observation period.
In summary, ferrets provided combination therapy of a S1P1 receptor agonist along with antiviral oseltamivir, with the exception of an elevated temperature on day 1 post-infection, showed negligible clinical signs resulting from the H1N1 human pathogenic influenza virus infection. In contrast, providing infected ferrets with either S1P1 agonist or the antiviral agent oseltamivir, while successfully blunting part of the manifestations of the influenza infection as recorded by lowered temperatures and less loss of activity when compared to vehicle only treated ferrets were not as successful as those receiving the combined therapy. Reasons for differences in kinetics concerning somnolence with loss of spontaneous or stimulated activity seen with either S1P1 receptor agonist or oseltamivir singular therapy is not clear but may reflect differences in blunting of innate immune response in lungs and/or different kinetics in viral replication or different pharmacologic side-effects of the drugs. The next series of studies utilized histopathologic study of lungs of ferrets sacrificed at day 3 post-infection.
Histopathologic findings
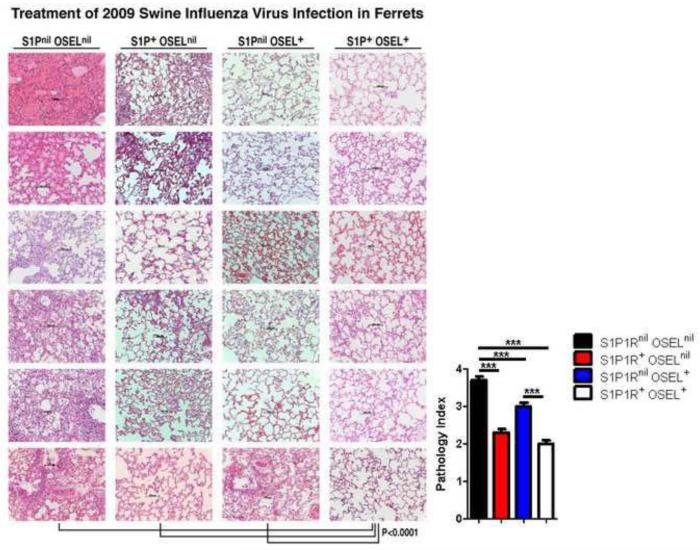
To evaluate the impact of RP-002 or oseltamivir therapy alone or in combination on ferrets infected with human pandemic influenza virus, four ferrets from each of these three groups and four infected ferrets receiving only vehicle were sacrificed three days post-infection. Pulmonary lobes from each ferret were studied in terms of upper, lower, and medial areas with at least six to seven different sites from each of these areas analyzed to determine the degree of pulmonary injury, extent and composition of immune cellular infiltrate, anatomy of the alveolar air sac and the presence or absence of exudate into the alveolar sac. As seen in Figures 2 and 3, ferrets treated with vehicle (S1P1Rnil oselnil) showed massive pulmonary injury in terms of pulmonary tissue consolidation, exudates in the alveolar air sacs, thickening of alveolar walls, and massive interstitial and intra-alveolar inflammatory infiltrates when compared to similarly infected ferrets receiving either RP-002 alone (S1P1R+ oselnil) or oseltamivir alone (S1P1Rnil osel+). Minimal injury was recorded in ferrets receiving both therapies combined (S1P1R+ osel+). Utilizing a score of 0-1 = <10% lung involvement; 1-2 = 10-30% lung involvement; 2-3 = 30-60% lung involvement; 3-4 = >60% lung involvement, we evaluated six to seven separate random fields per tissue section from the portions of right and left upper, middle, and lower lobes.
Figure 2.
Histopathologic analysis of pulmonary tissues on day 3 post-influenza infection revealed significantly less injury in ferrets receiving both S1P1R and Oseltamivir therapy than in ferrets receiving either treatment separately. Tissue injury was significantly less in ferrets receiving only the S1P1R agonist when compared to ferrets treated with Oseltamivir. Ferrets receiving combined S1P1R agonist and Oseltamivir therapy showed diminished polymorphonuclear cells, mononuclear cells, histiocyte and plasma cell infiltrates in interstitial tissues and intra-alveolar spaces, negligible exudate in alveolar air spaces and minimal alveolar wall thickening than displayed in ferrets in the other three groups. Vehicle only (S1P1Rnil oselnil) treated ferrets, by contrast, portrayed massive lung consolidation, exudate in alveolar air space, thickening of alveolar walls, vascular hemorrhage and excessive interstitial and intra-alveolar inflammatory infiltrates. Four ferrets comprised each group with samples obtained from right and left upper, middle, and lower lobes. Tissue inflammatory score numbers (path index) are derived from the mean lung involvement of at least six random fields per tissue section for each lung lobe sample ± SEM. Numbers correspond: 0-1 = ≤ 10% or less lung involvement; 1-2 = 10-30% lung involvement; 2-3 = 30-60% lung involvement; 3-4 = ≥ 60% lung involvement. Magnification 200× with scale bars of 100 μm shown. P values comparing each of the three treatment groups to the non-treatment groups was P < 0.0001. Significance (P < 0.0001) occurred between ferrets receiving combined S1P1R+ osel+ therapy vs. either vehicle, S1P1R+ oselnil or S1P1Rnil osel+, and S1P1R+ oselnil or S1P1Rnil osel+ compared to vehicle. Pulmonary injury score was significantly better in ferrets receiving S1P1R+ only compared to those only receiving Oseltamivir (P = 0.001).
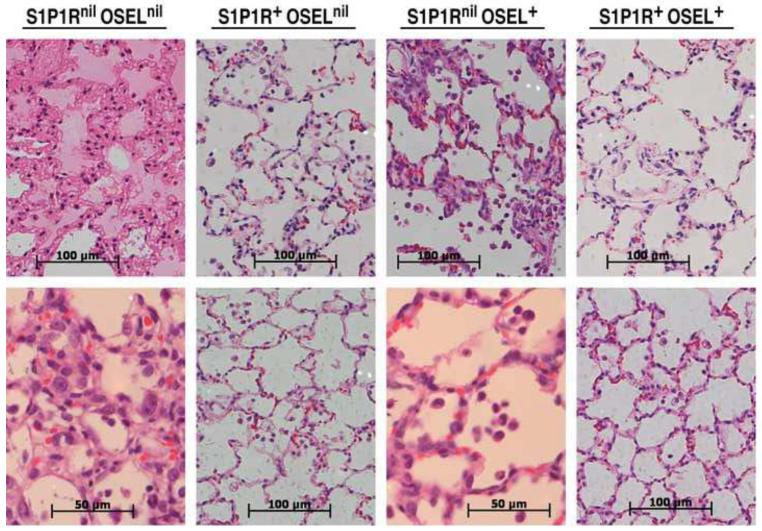
Figure 3.
Higher resolution analysis of representative samples displayed in Figure 2. Magnification 400× with scale bars of 100 μm, or 600× with scale bars of 50 μm.
The differences found were highly significant (P <0.0001) when comparing ferrets using vehicle only to those treated with either S1P1 receptor agonist or oseltamivir only or to ferrets receiving a combination of both therapies. Ferrets treated with RP-002 displayed significantly less pulmonary injury than ferrets treated with oseltamivir alone (P < 0.001). However, by far, ferrets that received a combination of both drugs showed the least degree of pulmonary injury (P < 0.0001) when compared to the other groups of treated ferrets.
Figure 3 displays higher resolution microscopy of representative pulmonary tissue from each of the four ferret groups. The upper panel for ferrets only receiving vehicle (S1P1Rnil oselnil) showed exudates that obstruct alveolar sacs and massive thickening of the alveolar walls, while the lower panel showed considerable inflammatory cells in interstitial pulmonary tissue and in the air sac spaces. Ferrets treated with only S1P1R agonist or only with oseltamivir displayed more open alveolar air spaces and fewer inflammatory infiltrates than vehicle treated ferrets, although inflammation in oseltamivir treated ferrets was greater than in the S1P1 agonist treated ferrets. Inflammatory infiltrates consisted primarily of polymorphonuclear cells, but also abundant monocytes/lymphocytes with occasional histiocytes and fewer plasma cells. By contrast, ferrets receiving combined S1P1R agonist RP-002 and oseltamivir therapy portrayed the most abundant open and anatomically normal alveolar air spaces along with the fewest number of inflammatory cells.
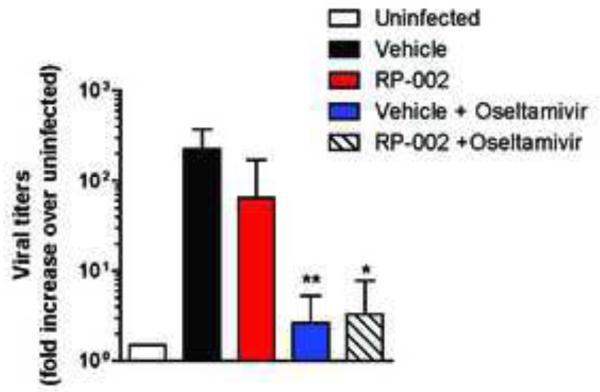
The last series of studies measured levels of influenza virus RNA from ferret lung samples from all treatment groups on day 3 post-infection. Using uninfected ferret lung RNA as a baseline, we observed significant levels of influenza virus RNA in the lungs of vehicle treated ferrets (Figure 4). Similar to our previous results, treatment with S1P1R agonist did not significantly affect the levels of viral RNA detected on day 3 post-infection (Figure 4). Further, in agreement with the antiviral activity of Oseltamivir, we observed significant (> 2 log) reductions in viral RNA in the lungs of ferrets treated with either Oseltamivir alone or combined RP-002 and Oseltamivir (Figure 4). Taken together, our results demonstrate that the S1P1R agonist, RP-002, can alleviate clinical symptoms and lung pathology independent of virus replication. However, maximal protection occurs with combined therapy blunting both immune pathology and suppressing viral replication.
Figure 4.
Viral loads of A/California/09/04 H1N1 on day 3 post-infection in ferrets treated with either vehicle, RP-002, Oseltamivir or combined RP-002 and Oseltamivir. Data are presented as fold increase over uninfected ferret lung tissue and are representative of 4 individual ferrets. ** p <0.01, * p<0.05 as determined by two-tailed student t-test.
In summary, this paper documents in a second animal model, the ferret, the applicability of S1P1 receptor agonist in blunting disease caused by a human pathologic H1N1 infection. This blunting is reflected in the S1P1 receptor agonist ability to down-regulate and control the massive innate inflammatory response despite minimally altering viral replication. Attempts to biochemically measure cytokine/chemokine message or molecules associated with cytokine storm was unsuccessful because of poor test reproducibility due to the unreliability of reagents and assays currently in use for the ferret. A weakness of the ferret model at present is the absence of ferret reagents required to give robust reproducible results required to quantitate these inflammatory molecules.
In conclusion, the major advantage of the ferret model is its close relationship to the clinical course and the respiratory architecture and physiology of humans for study of human influenza virus infection. Using the ferret model we showed here the utility of S1P1 receptor agonist as a potential therapy combined with administration of an antiviral drug to successfully treat pathogenic human influenza and established that such findings in the ferret closely mimicked our previous studies in the mouse (Teijaro et al., 2011; Walsh et al., 2011). Injury and disease resulting from influenza virus infection depends on two separate but intertwined components, the virus infection itself which can be blunted by antiviral therapy and excessive inflammatory immune response that can be controlled by the immunomodulating S1P1R agonist molecule. Thus, both the mouse and ferret model demonstrated the importance of utilizing combined antiviral Oseltamivir and S1P1 receptor agonist therapy for optimal results in treatment of pathogenic human H1N1 swine influenza infection. Cytokine storm plays a prominent and commanding role in other acute severe respiratory disorders like hantavirus (Macneil et al., 2011), SARS (Thiel and Weber, 2008), respiratory syncytial viral infection (Kawashima et al., 2012), and other respiratory viral infections suggesting the potential utility of S1P agonist immunomodulatory therapy to inhibit the excessive and disorganized immune responses in these illnesses.
Materials and Methods
Virus and Infection
Influenza virus A/California/04/2009 was amplified and plaqued on Madin-Darby canine kidney (MDCK) cells. Influenza infection was performed in a BSL-3 biocontainment facility at Battelle Labs. Influenza virus was inoculated via the intranasal route following Telazal anesthesia (16-22mg/kg via intramuscular injection). 1×106 plaque forming units (PFU) in 600ul of phosphate buffered saline (PBS) was introduced slowly into the nasal cavity of ferrets by 4 150ul aliquots inoculated alternatively in each nostril.
Viral RNA Quantification
Influenza A/California/04/2009 viral RNA was measured using the standard real-time RT-PCR assay for detection of the influenza A virus matrix gene from the World Health Organization’s manual for molecular diagnosis of influenza virus in humans, annex 2 protocol 1. For the real time PCR reaction, cDNA was made from RNA isolated from ferret lung tissue. We used 100ng of cDNA from lung tissue for each reaction. As a standard we purified viral RNA from cellular supernatants of stock virus with a known titer (6 × 106 PFU/mL) using the Qiagen RNeasy mini kit and made cDNA and 10-fold serial dilutions using no cDNA as a negative control. Viral RNA quantities for the vehicle control were in the range of 102-103 PFU/100ng cDNA based on our standard.
Drug treatment
Ferrets were treated with the S1P1 receptor-selective agonist RP-002 dissolved in water by oral gavage at a dose of 6mg/kg/day on days 0, 1 & 2 post-infection. The selectivity, characterization, and synthesis of RP-002 were described by Teijaro et al. 2011. Control ferrets received the same volume of water by oral gavage. Oseltamivir was dissolved in water to a final concentration of 12mg/mL. Oseltamivir (25mg/kg/day) was administered to ferrets 1 hour post-infection and on days 1-5 post-infection twice per day (12.5mg/kg/dose).
Ferret monitoring
Thirty-five neutered and de-scented male ferrets were purchased from Triple F Farms for this study. Prior to infection ferrets were determined to be in good health and were seronegative for A/California/4/09 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008/. Ferrets were weighed on days −1 & 0. Body weight from day 0 served as the baseline. Ferrets were weighed daily throughout the study until sacrifice. For body temperature measurements, anesthetized ferrets were recorded from implants in the shoulder and rump area. Temperatures were taken on days −2, −1 & 0 pre-infection and temperatures averaged from −2, −1 & 0 to serve as the baseline reading. Temperature readings were taken daily from both transponders, average taken from both sites and compared to the baseline reading until the end of the experiment.
Observations: Observations were taken twice daily upon arrival of ferrets prior to infection. Following challenge, observations were made twice daily until the end of the experiment and were done blind by an independent observer. The following scoring system was used: 0 - alert and playful, 1 - alert but playful only when stimulated, 2 - alert but not playful when stimulated, and 3 - neither alert or playful when stimulated.
Tissue sample collection and processing
All animals had a gross necropsy performed on the day of sacrifice. Ferrets were euthanized by first sedating with Telazol followed by a euthanasia solution approved by the American Veterinary Medical Association (AVMA). Lungs were collected and processed into 1 cm3 portion from three right lung lobes. Three complete sets of three were collected and one set stored in 10% neutral buffered formalin. A second set was snap frozen in a dry ice/alcohol bath. The final set of tubes was placed in pre-weighed tubes containing RNAlater. All samples were frozen at −70°C. Lungs were blocked in paraffin, and 10-m tissue sections were cut from each section of the ferret lung, placed on glass slides, and stained with hematoxylin and eosin.
Highlights.
S1P1R agonist reduces pulmonary injury following ferret influenza infection
S1P1R agonist does not suppress virus replication
S1P1R agonist improves clinical symptoms following ferret influenza infection
Acknowledgements
Supported by NIH grants AI057160 and AI009484 (MBAO), AI005509 and MH084512 (HR), AI074564, AI069274 (YK), and NIAID Contract # HHSN272201000003I (MBAO, HR, JPL, KPT, GVS), and American Heart Association Fellowship 11POST7430106 (JRT). We thank Edward Roberts (Department of Chemistry, TSRI), Marcus Boehm, Liming Huang, and Bryan Clemons (Receptos, Inc.) for helping provide RP-002 as a chemical tool. HR is a founder of Receptos, Inc. The authors acknowledge the technical assistance of Stephanie Rice (TSRI), Gerald Bordin (Division of Pathology, Scripps Clinic and Scripps Green Hospital), and Mariah Baughn (Department of Pathology, Scripps Memorial Hospital).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nature immunology. 2007 Nov;8(11):1188–93. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubenberger JK, Reid AH, Krafft AE, Bijwaard KE, Fanning TG. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997 Mar 21;275(5307):1793–6. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- 3.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bulletin of the history of medicine. 2002 Spring;76(1):105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 4.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009 Aug 20;460(7258):1021–5. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. The American journal of pathology. 2010 Jul;177(1):166–75. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, et al. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science. 2010 Jun 18;328(5985):1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon LL, Mak PW, Li OT, Chan KH, Cheung CL, Ma ES, et al. Rapid detection of reassortment of pandemic H1N1/2009 influenza virus. Clinical chemistry. 2010 Aug;56(8):1340–4. doi: 10.1373/clinchem.2010.149179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2009 Feb 5;85(6):37–40. Update on Oseltamivir-resistant pandemic A (H1N1) 2009 influenza virus: January 2010. [Google Scholar]
- 9.Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jul 19;108(29):12018–23. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011 Sep 16;146(6):980–91. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2009 Feb 3;106(5):1560–5. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher JA, DeStefano J. The ferret: an animal model to study influenza virus. Lab animal. 2004 Oct;33(9):50–3. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 13.Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Disease models & mechanisms. 2011 Sep;4(5):575–9. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith H, Sweet C. Lessons for human influenza from pathogenicity studies with ferrets. Reviews of infectious diseases. 1988 Jan-Feb;10(1):56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Macneil A, Nichol ST, Spiropoulou CF. Hantavirus pulmonary syndrome. Virus research. 2011 Dec;162(1-2):138–47. doi: 10.1016/j.virusres.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Thiel V, Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine & growth factor reviews. 2008 Apr;19(2):121–32. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima H, Kashiwagi Y, Ioi H, Morichi S, Oana S, Yamanaka G, et al. Production of chemokines in respiratory syncytial virus infection with central nervous system manifestations. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2012 Dec;18(6):827–31. doi: 10.1007/s10156-012-0418-3. [DOI] [PubMed] [Google Scholar]