Abstract
The intrahepatic biliary epithelium is a three-dimensional tubular system lined by cholangiocytes, epithelial cells that in addition to modify ductal bile are also the targets of vanishing bile duct syndromes (i.e., cholangiopathies) such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) that are characterized by the damage/proliferation of cholangiocytes. Cholangiocyte proliferation is critical for the maintenance of the biliary mass and secretory function during the pathogenesis of cholangiopathies. Proliferating cholangiocytes serve as a neuroendocrine compartment during the progression of cholangiopathies, and as such secrete and respond to hormones, neurotransmitters and neuropeptides contributing to the autocrine and paracrine pathways that regulate biliary homeostasis. The focus of this review is to summarize the recent findings related to the role of melatonin in the modulation of biliary functions and liver damage in response to a number of insults. We first provide a general background on the general function of cholangiocytes including their anatomic characteristics, their innervation and vascularization as well the role of these cells on secretory and proliferation events. After a background on the synthesis and regulation of melatonin and its role on the maintenance of circadian rhythm, we will describe the specific effects of melatonin on biliary functions and liver damage. After a summary of the topics discussed, we provide a paragraph on the future perspectives related to melatonin and liver functions.
Keywords: Angiogenesis, biliary epithelium and growth, gastrointestinal hormones, liver damage, transduction pathways
General background on liver functions
The liver is composed by several types of cells such as hepatocytes (parenchyma cells) and a non-parenchymal cell fraction that includes several cell types including intrahepatic bile duct cells (i.e., cholangiocytes), Kupffer cells, sinusoidal and vascular endothelial cells, hepatic stellate cells, dendritic cells, etc. Hepatocytes and cholangiocytes are the two hepatic epithelial cells that play key roles in biliary secretion and the maintenance of liver function/homeostasis (1,2). In rodents, hepatocytes comprise 70% of the total liver mass, whereas intrahepatic cholangiocytes form 3-5% of the total liver cell population (1). The liver maintains the balance of the metabolism of the entire body synthesizing different types of proteins and enzymes. The liver also regulates the energetic metabolism, and it participates in the detoxification and elimination of a wide variety of xenobiotics (1).
Anatomical characteristics of the biliary epithelium
The intrahepatic bile ductal apparatus (that extends from the canals of Hering to the extrahepatic hepatic ducts) is composed of interconnecting tubular structures that are lined by cholangiocytes of different diameters and functions (1,3-6). According to the Ludwig’s classification (4), the human intrahepatic biliary epithelium has been defined according to bile duct diameter: small bile ductules (<15 μm in diameter), interlobular ducts (15 to 100 μm in diameter), septal ducts (100 to 300 μm in diameter), area ducts (300 to 400 μm in diameter), segmental ducts (400 to 800 μm in diameter), and hepatic ducts (>800 μm in diameter). Recently, we have begun defining the anatomical and morphological characteristics of the rodent biliary epithelium that we have classified as small (<15 μm diameter) and large (>15 μm diameter) bile ducts, lined by small and large cholangiocytes, respectively (3,7-9).
Vascularization of biliary ductal system
In addition to an autocrine loop regulated by cholangiocytes (10-12), the bile duct system is regulated by a number of growth factors [e.g., vascular endothelial growth factor (VEGF)] that are secreted by a complex network of minute vessels (i.e., peribiliary vascular plexus, PBP) originating from the branches of the hepatic artery (10,13). PBP flow mainly into the hepatic sinusoids, directly (lobular branch) or through the portal vein branches (prelobular branches) (10,14,15). A well-defined PBP is present around large bile ducts, but PBP is less visible around small bile ducts since it gets smaller proportionally to the size of bile ducts (10). In cholestatic rodents, concomitant with enhanced biliary hyperplasia there is proliferation of PBP, to support the nutritional and functional demand of the proliferating biliary epithelium (10). Consistent with the concept that cholangiocytes secrete angiogenic factors such as VEGF regulating biliary homeostasis by autocrine mechanisms, a study has shown that the proliferation of the PBP only occurs after that one of the bile ductal system (10).
General background on cholangiocytes
After secretion at the canalicular domain, bile reaches small ductules via Hering canals (2). Before reaching the small intestine, canalicular bile is modified by large bile ducts by a series of reabsorptive and secretory events (9,16,17) that are regulated by parasympathetic, sympathetic and dopaminergic innervation, gastrointestinal hormones such as secretin, gastrin, somatostatin and peptides (e.g., endothelin-1, ET-1) (9,16-24). The reabsorptive/secretory activity of the biliary epithelium is heterogeneous and depends on the size of the bile ducts and the anatomical site within the length of the biliary tree (3,7,9,25,26). For example, we have shown that large cholangiocytes (lining large bile ducts) (7,9) are the only cell types in the liver to express secretin receptor (SR), the somatostatin receptor subtype 2 (SSTR2), cystic fibrosis transmembrane conductance regulator (CFTR) and Cl-/HCO3- anion exchanger AE2 (7,9,20,27,28) and to respond to secretin with changes in the secretion of water and electrolytes (7,9,20,26,27). Conversely, small cholangiocytes in small ducts do not express SR, SSTR2, CFTR and Cl-/HCO3- anion exchanger AE2 and do not respond to secretin and somatostatin (7,9,20,26-28).
In addition to heterogeneity with regard to secretory activity, cholangiocytes proliferate or are damaged differentially in a number of pathological models of cholestasis such as extrahepatic bile duct ligation (BDL), acute administration of carbon tetrachloride (CCl4) and chronic administration of gamma-aminobutyric acid (GABA) (29-31). In these models, large more-differentiated cholangiocytes are more susceptible to these pathological maneuvers with loss of proliferative capacity, increase in biliary apoptosis and loss of responsiveness to hormones such as secretin (29-31). Following damage of large bile ducts, small cholangiocytes replenish the biliary epithelium by amplification of Ca2+-dependent signaling and acquisition of large cholangiocyte phenotypes by activation of Ca2+/CaMK I-dependent adenylyl cyclase 8 (29-31).
Background on melatonin
Melatonin [i.e., chemically as N-acetyl-5-methoxytryptamine, whose synthesis is mainly regulated by the rate-limiting enzyme, arylalkylamine N-acetyltransferase (AANAT)] (32), is a hormone found in animals, plants, and microbes (33,34). Melatonin is synthesized in the brain by the pineal gland from the amino acid tryptophan (35). The synthesis and release of melatonin are stimulated by darkness and reduced by light (detected by the photosensitive ganglion cells of the retina) (36), which suggest that melatonin plays a role in the modulation of the circadian rhythm and diverse body functions (32,35,36). In addition, melatonin secretion is also dependent on food consumption (37). A number of studies have shown that melatonin is produced in various extrapineal sites including bone marrow cells (38), lymphocytes (39), mast cells (40) and gastrointestinal tract including the biliary epithelium (41,42). The melatonin concentration in these cells is much higher than that found in the blood and contributes to the changes in melatonin concentration found in the peripheral blood (41,43). Melatonin synthesis decreases progressively with aging as the elderly population has lower levels of melatonin (44,45). Other factors related to aging (e.g., depression, physical impairment) may contribute to the alteration of the circadian rhythm contributing to the reduction in melatonin levels in old people (44). Melatonin is mainly metabolized in the liver, where it is hydroxylated in the sixth carbon position by cytochrome P450 mono-oxygenases. Then, it is conjugated to sulfate and released as 6-sulfatoxymelatonin (46).
Regulation of melatonin synthesis and downstream signaling pathways following melatonin release
As stated above, melatonin synthesis in the pineal gland is modulated by light/dark information that is detected by the photosensitive ganglion cells of the retina (36). Specifically, the signal passes through the suprachiasmatic nucleus to the pineal gland where specific “dark” and “light”-induced neural and endocrine signals co-coordinately regulate melatonin secretion. Synthesis of melatonin is inhibited by light and permitted by darkness peaking in the middle of the night in both diurnal and nocturnal animals. The function of melatonin released from the pineal gland may be also modulated by the local release of gonadotropin-releasing hormone (GnRH) from the hypothalamus (47) (Figure 1). We will discuss below some preliminary data that we have generated in our laboratory showing that chronic exposure of cholestatic rats to complete dark for one week reduces biliary proliferation and ameliorates liver function (48). GnRH also acts on the anterior pituitary to bring about the release of follicle-stimulating hormone (FSH) at low frequency GnRH pulses and luteum hormone (LH) at high frequency GnRH pulses. Therefore, melatonin effects may also due to inhibition of the GnRH-mediated release of LH (49) and FSH (50), the latter also affecting biliary functions (51). Central melatonin synthesis has been also shown to be dysregulated in a number of cholestatic liver diseases. For example, abnormal melatonin circadian rhythms have been demonstrated in patients with hepatic cirrhosis and correlated with the severity of liver injury (52). Melatonin arrhythmia has been shown to be corrected following liver transplantation (53).
Figure 1.
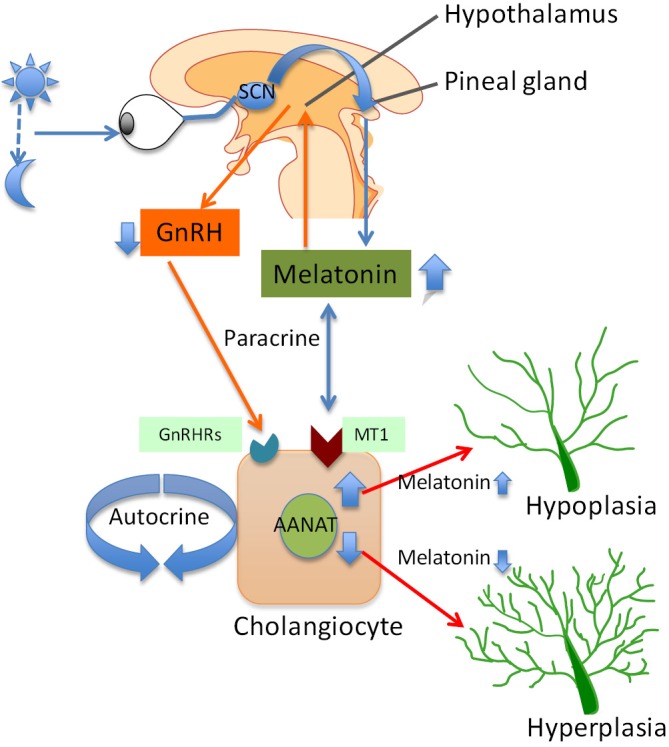
Melatonin production in the pineal gland is regulated by the light/dark signals that are detected by the retina. These signals pass through the retino-hypothalamic tract and the superachiasmatic nucleus to the pineal gland, where the melatonin is synthesized and secreted by pinealocytes. The release of melatonin from pineal gland interacts with melatonin receptors that are located in the hypothalamus to inhibit the production of GnRH. Inhibition of GnRH release may partly inhibits the biliary hyperplasia in cholestatic livers by interacting with the GnRH receptors. The release of melatonin from pineal gland inhibits cholangiocyte proliferation via interaction with the MT1 in a paracrine fashion. On the other hand, AANAT, the rate-limiting enzyme for melatonin synthesis is expressed by cholangiocytes. Changes in melatonin secretion from cholangiocytes modulate biliary growth/loss by an autocrine loop. Abbreviations: AANAT, arylalkylamine N-acetyltransferase; GnRH, gonadotropin-releasing hormone.
Melatonin exerts its effects by interacting with melatonin receptor subtypes, MT1, MT2 and MT3 [part of a G-protein coupled receptor (GPCR) family sharing a common seven-transmembrane structure] that are expressed in the gastrointestinal tract by the ileum, colon and in the liver by hepatocytes, extrahepatic biliary cells in gallbladder and cholangiocytes (54-59).
Role of melatonin on biliary growth/damage
We have recently demonstrated that melatonin inhibits cholangiocyte hyperplasia in BDL rats by interaction with MT1 receptors (56) (Figure 1). Specifically, administration of melatonin to cholestatic BDL rats decreased ductal mass and improved serum chemistry and reduced the expression of the clock genes (CLOCK, BMAL1, CRY1, and PER1 all upregulated after BDL), cAMP levels, and PKA phosphorylation in cholangiocytes (56). We propose that melatonin may be important in the management of cholestatic liver diseases.
In addition to the above described paracrine mechanism, changes in melatonin synthesis (regulated by the enzyme, AANAT, that is expressed by cholangiocytes) regulates biliary function by an autocrine mechanism (42) (Figure 1). Specifically, we have shown that AANAT expression and melatonin secretion increased in BDL and decreased in normal and BDL rats treated with AANAT Vivo-Morpholino, a treatment that decreases the biliary expression of AANAT. The decrease in AANAT expression, and subsequent lower melatonin secretion by cholangiocytes, was associated with increased biliary proliferation and increased ductal secretory activity (42) (Figure 1). Overexpression of AANAT in cholangiocytes decreased proliferative capacity of these cells (42). Local modulation of melatonin synthesis may be important for management of the balance between biliary proliferation/loss in cholangiopathies. Another study has shown that oral administration of melatonin protects from hepatotoxicity induced by α-naphthylisothiocyanate (ANIT) (60,61), a toxin that also induces biliary damage (62-64).
There is growing information regarding the role of melatonin in the regulation of the growth of cholangiocarcinoma, a devastating tumor of the biliary epithelium (54,65). In fact, melatonin has been shown to reduce cholangiocarcinoma growth and liver injury in Opisthorchis viverrini-infected and N-nitrosodimethylamine-treated hamsters (54,65). We have recently shown that dysregulation of the enzymatic machinery AANAT/ASMT (N-Acetylserotonin O-methyltransferase that regulates melatonin synthesis) in cholangiocarcinoma that leads to inhibition of melatonin secretion and subsequently enhanced cholangiocarcinoma growth (54). Modulation of the AANAT/ASMT/melatonin—melatonin receptor axis may be important in the management of cholangiocarcinoma growth. Some of the effects of melatonin on biliary functions are summarized in Table 1.
Table 1. Melatonin regulation of biliary functions.
| Parameter | Mechanisms | References |
|---|---|---|
| Inhibition of biliary hyperplasia in BDL rats | Reduced expression CLOCK genes by downregulation of cAMP levels, and PKA phosphorylation by interaction with MT1 | (56) |
| Modulation of biliary hyperplasia in normal and BDL rats | In vivo and in vitro overexpression of AANAT in cholangiocytes decreased biliary proliferation | (42) |
| Modulation of biliary hyperplasia in ANIT-fed rats | Anti-oxidant activity | (60,61) |
| Inhibition of cholangiocarcinoma growth | Decreased AANAT expression and melatonin secretion leads to enhanced cholangiocarcinoma growth | (54) |
Abbreviations: AANAT, arylalkylamine N-acetyltransferase; ANIT, α-naphthylisothiocyanate; BDL, bile duct ligation.
Role of melatonin on liver damage
In support of the concept that melatonin protects the liver from selected pathological perturbations, a recent study has shown that this hormone protects against apoptosis during acetaminophen-induced acute liver failure (66). Another study has shown that melatonin decreases BDL-induced liver fibrosis that was evidenced by reduced levels of malondialdehyde, glutathione, luminal and lucigenin in tissue homogenates compared to BDL animals. The findings suggest that melatonin may be an effective antioxidant agent able to reduce liver fibrosis (67). Furthermore, melatonin is able to reduce dimethylnitrosamine-induced liver fibrosis in rats suggesting that this hormone may be used as a therapeutic strategy for managing liver fibrosis (68). Another study has demonstrated that melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects the liver against thioacetamide-induced damage (69).
Furthermore, oral administration of melatonin (10 or 100 mg/kg body weight) has been shown to prevent the disruption of hepatic antioxidant status in cholestatic rats by its direct and indirect antioxidant action (70). Similarly, a protective role of melatonin against cholestatic oxidative stress has been demonstrated in BDL rats (71). Moreover, melatonin reduced the negative parameters of cholestasis, the degree of oxidative stress and provided a hepatoprotective effect against BDL-induced liver damage (72). Similarly, a protective effect of melatonin against oxidative stress induced by BDL was also described in a different study (73). Consistent with beneficial effect of melatonin on liver function, melatonin has been shown to attenuate oxidative stress, lessen liver damage, and improve liver histology in rats with high fat diet-induced non-alcoholic fatty liver disease (NAFLD), when given simultaneously with the diet (74). Recent studies have demonstrated that chronic melatonin administration protects against liver damage by attenuating oxidative stress, inflammatory responses, and apoptosis in animal models of hepatic cirrhosis and fibrosis (75-78). Melatonin has also been shown to improve oxidative damage and rat liver mitochondrial dysfunction during hyperglycemia-induced liver injury (79,80). The data suggests that melatonin may be an important nutritional supplement for diabetic patients.
Melatonin has also been shown to display a hepatoprotective effect against liver injury secondary to methanol intoxication (81). Another study has shown that liver mitochondrial damage (following acute or chronic CCl4-induced intoxication) was improved by melatonin and cranberry flavonoids (82). Another interesting study has shown that administration of melatonin before and after irradiation reduces liver damage caused by gamma irradiation (83). The protective effect of melatonin on liver function has also been demonstrated during ethanol administration. For example, melatonin reduces alcoholic liver injury by reducing oxidative stress, inflammatory response, and apoptosis (75). In support of these concepts, a study has demonstrated a protective role for melatonin during ischemia reperfusion (I/R) hepatic damage by maintaining preventing activation of apoptotic cell death (84,85). Another study has shown that melatonin protection against I/R occurred by inhibition of toll-like receptor signaling pathway (86). Another study has shown that melatonin protects from I/R induced injury by inhibition of IκB kinase (IKK) and c-Jun NH2-terminal kinase (JNK) pathways and modification of cell proliferation (87). Melatonin can also improve liver function and hepatic perfusion after hemorrhagic shock (88). The protective role of melatonin has been also observed in rats treated with CCl4. In fact, the study showed that except for mild hydropic degeneration of the hepatocytes, a normal lobular appearance was seen in the animals treated with melatonin (89). Moreover, melatonin has been shown to play a role as potential anti-aging agent (90,91). For example, has been shown to restore hepatic mitochondrial physiology in old mice (90). The finding suggests that melatonin may be useful to reduce the deteriorative oxidative changes in mitochondria that normally occur in advanced age. Also, the hepatic mitochondrial respiratory chain activity observed in senescence-accelerated mice was improved by melatonin treatment (91). Some of the effects of melatonin on liver damage are summarized in Table 2.
Table 2. Melatonin regulation of liver damage.
| Parameter | Mechanisms | References |
|---|---|---|
| Protection against acetaminophen-induced acute liver failure | Reduction of apoptosis | (66) |
| Decreased BDL-induced liver fibrosis | Reduced levels of malondialdehyde, glutathione, luminal and lucigenin in tissue homogenates | (67) |
| Protects the liver against thioacetamide-induced damage | Inhibition of nuclear factor kappa B activation and oxidative stress | (69) |
| Prevention of the disruption of hepatic antioxidant status in cholestatic rats | Direct and indirect antioxidant action | (70) |
| Hepatoprotective effect against BDL-induced liver damage, and animal models of fibrosis and cirrhosis | Reduction of the degree of oxidative stress | (72,75-78) |
| Improvement of liver histology in rats with high fat diet-induced NAFLD | Attenuation of oxidative stress | (74) |
| Improvement of oxidative damage and rat liver mitochondrial dysfunction during hyperglycemia-induced liver injury | Attenuation of oxidative damage and rat liver mitochondrial dysfunction | (79,80) |
| Protective role during I/R hepatic damage | Prevention of activation of apoptosis Inhibition of IKK and JNK pathways |
(86,87) |
Abbreviations: BDL, bile duct ligation; NAFLD, non-alcoholic fatty liver disease; I/R, ischemia reperfusion; IKK, IκB kinase; JNK, c-Jun NH2-terminal kinase.
Summary and future perspectives
In summary, we have, following a general background on anatomical features of the biliary epithelium, discussed the vascularization of bile ducts followed by a general discussion on the secretory and proliferative response of the biliary tree with regarding to their heterogeneous profile. After a general background on melatonin synthesis, distribution and metabolism, we discuss the role of melatonin on biliary hyperplastic and neoplastic growth followed by a summary of the findings related to the protective role of melatonin against liver damage induced by a number of pathological insults. Further studies are necessary to evaluate the role of melatonin in the regulation of CLOCK genes and the role of CLOCK genes in the modulation of biliary functions.
Acknowledgements
Portions of this review were supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Hospital, and a NIH grant DK062975 to Dr. Alpini and Glaser.
Disclosure: The authors declare no conflict of interest.
References
- 1.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al. eds. The Liver; Biology & Pathobiology, 4E. [Google Scholar]
- 2.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology 1991;14:551-66 [PubMed] [Google Scholar]
- 3.Kanno N, LeSage G, Glaser S, et al. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 2000;31:555-61 [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J.New concepts in biliary cirrhosis. Semin Liver Dis 1987;7:293-301 [DOI] [PubMed] [Google Scholar]
- 5.Sasaki H, Schaffner F, Popper H.Bile ductules in cholestasis: morphologic evidence for secretion and absorption in man. Lab Invest 1967;16:84-95 [PubMed] [Google Scholar]
- 6.Schaffner F, Popper H.Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol 1961;38:393-410 [PMC free article] [PubMed] [Google Scholar]
- 7.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110:1636-43 [DOI] [PubMed] [Google Scholar]
- 8.Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74 [DOI] [PubMed] [Google Scholar]
- 10.Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology 1996;111:1118-24 [DOI] [PubMed] [Google Scholar]
- 11.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415-31 [DOI] [PubMed] [Google Scholar]
- 12.Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 2004;127:1198-209 [DOI] [PubMed] [Google Scholar]
- 13.Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 2006;291:G307-17 [DOI] [PubMed] [Google Scholar]
- 14.Ohtani O, Kikuta A, Ohtsuka A, et al. Microvasculature as studied by the microvascular corrosion casting/scanning electron microscope method. I. Endocrine and digestive system. Arch Histol Jpn 1983;46:1-42 [DOI] [PubMed] [Google Scholar]
- 15.Terada T, Ishida F, Nakanuma Y.Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol 1989;8:139-49 [DOI] [PubMed] [Google Scholar]
- 16.Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 1988;81:569-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 2001;281:G612-25 [DOI] [PubMed] [Google Scholar]
- 18.Glaser SS, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol 1997;273:G1061-70 [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri A, Glaser S, Rodgers RE, et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol 1998;275:G835-46 [DOI] [PubMed] [Google Scholar]
- 20.Tietz PS, Alpini G, Pham LD, et al. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol 1995;269:G110-8 [DOI] [PubMed] [Google Scholar]
- 21.Alvaro D, Alpini G, Jezequel AM, et al. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest 1997;100:1349-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser S, Alvaro D, Francis H, et al. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol 2006;290:G813-26 [DOI] [PubMed] [Google Scholar]
- 23.Glaser S, Alvaro D, Roskams T, et al. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol 2003;284:G683-94 [DOI] [PubMed] [Google Scholar]
- 24.LeSage GD, Alvaro D, Glaser S, et al. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology 2004;40:1116-27 [DOI] [PubMed] [Google Scholar]
- 25.Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74 [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Glaser S, Meng F, et al. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp Biol Med (Maywood) 2013;238:549-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol 1997;272:G289-97 [DOI] [PubMed] [Google Scholar]
- 28.Banales JM, Arenas F, Rodríguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 2006;43:266-75 [DOI] [PubMed] [Google Scholar]
- 29.LeSage GD, Glaser SS, Marucci L, et al. Acute Carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol 1999;276:G1289-301 [DOI] [PubMed] [Google Scholar]
- 30.Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol 2010;176:1790-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancinelli R, Franchitto A, Glaser S, et al. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+) /CaMK I-dependent adenylyl cyclase 8. Hepatology 2013;58:251-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep 2009;61:383-410 [DOI] [PubMed] [Google Scholar]
- 33.Caniato R, Filippini R, Piovan A, et al. Melatonin in plants. Adv Exp Med Biol 2003;527:593-7 [DOI] [PubMed] [Google Scholar]
- 34.Paredes SD, Korkmaz A, Manchester LC, et al. Phytomelatonin: a review. J Exp Bot 2009;60:57-69 [DOI] [PubMed] [Google Scholar]
- 35.Altun A, Ugur-Altun B.Melatonin: therapeutic and clinical utilization. Int J Clin Pract 2007;61:835-45 [DOI] [PubMed] [Google Scholar]
- 36.Klein DC, Ganguly S, Coon S, et al. 14-3-3 Proteins and photoneuroendocrine transduction: role in controlling the daily rhythm in melatonin. Biochem Soc Trans 2002;30:365-73 [DOI] [PubMed] [Google Scholar]
- 37.Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 2011;62:591-922314561 [Google Scholar]
- 38.Conti A, Conconi S, Hertens E, et al. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res 2000;28:193-202 [DOI] [PubMed] [Google Scholar]
- 39.Carrillo-Vico A, Calvo JR, Abreu P, et al. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 2004;18:537-9 [DOI] [PubMed] [Google Scholar]
- 40.Maldonado MD, Mora-Santos M, Naji L, et al. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res 2010;62:282-7 [DOI] [PubMed] [Google Scholar]
- 41.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002;47:2336-48 [DOI] [PubMed] [Google Scholar]
- 42.Renzi A, DeMorrow S, Onori P, et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology 2013;57:1130-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stebelová K, Herichová I, Zeman M.Diabetes induces changes in melatonin concentrations in peripheral tissues of rat. Neuro Endocrinol Lett 2007;28:159-65 [PubMed] [Google Scholar]
- 44.Mishima K, Okawa M, Shimizu T, et al. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J Clin Endocrinol Metab 2001;86:129-34 [DOI] [PubMed] [Google Scholar]
- 45.Pandi-Perumal SR, Bahammam AS, Brown GM, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res 2013;23:267-300 [DOI] [PubMed] [Google Scholar]
- 46.Claustrat B, Brun J, Chazot G.The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005;9:11-24 [DOI] [PubMed] [Google Scholar]
- 47.Gillespie JM, Roy D, Cui H, et al. Repression of gonadotropin-releasing hormone (GnRH) gene expression by melatonin May involve transcription factors COUP-TFI and C/EBP beta binding at the GnRH enhancer. Neuroendocrinology 2004;79:63-72 [DOI] [PubMed] [Google Scholar]
- 48.Dusio G, Renzi A, Meng F, et al. Increased synthesis of melatonin from pineal gland and cholangiocytes (by prolonged exposure of cholestatic rats to complete dark) leads to inhibition of biliary hyperplasia by autocrine/paracrine mechanisms. Gastroenterology 2012;142:S-930 [Google Scholar]
- 49.Vanecek J, Klein DC. Melatonin inhibition of GnRH-induced LH release from neonatal rat gonadotroph: involvement of Ca2+ not cAMP. Am J Physiol 1995;269:E85-90 [DOI] [PubMed] [Google Scholar]
- 50.Damian E, Ianăs O, Bădescu I, et al. Inhibitory action of pineal extract on LH and FSH. Endocrinologie 1978;16:257-62 [PubMed] [Google Scholar]
- 51.Mancinelli R, Onori P, Gaudio E, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 2009;297:G11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velissaris D, Karanikolas M, Kalogeropoulos A, et al. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J Gastroenterol 2008;14:4190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordoba J, Steindl P, Blei AT. Melatonin arrhythmia is corrected after liver transplantation. Am J Gastroenterol 2009;104:1862-3 [DOI] [PubMed] [Google Scholar]
- 54.Han Y, DeMorrow S, Invernizzi P, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 2011;301:G623-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CQ, Fichna J, Bashashati M, et al. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 2011;17:3888-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renzi A, Glaser S, DeMorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 2011;301:G634-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.León J, Casado J, Carazo A, et al. Gender-related invasion differences associated with mRNA expression levels of melatonin membrane receptors in colorectal Cancer. Mol Carcinog 2012;51:608-18 [DOI] [PubMed] [Google Scholar]
- 58.Poon AM, Choy EH, Pang SF. Modulation of blood glucose by melatonin: a direct action on melatonin receptors in mouse hepatocytes. Biol Signals Recept 2001;10:367-79 [DOI] [PubMed] [Google Scholar]
- 59.Aust S, Thalhammer T, Humpeler S, et al. The melatonin receptor subtype MT1 is expressed in human gallbladder epithelia. J Pineal Res 2004;36:43-8 [DOI] [PubMed] [Google Scholar]
- 60.Ohta Y, Kongo-Nishimura M, Imai Y, et al. alpha-Tocopherol protects against alpha-naphthylisothiocyanate-induced hepatotoxicity in rats less effectively than melatonin. Chem Biol Interact 2006;161:115-24 [DOI] [PubMed] [Google Scholar]
- 61.Ohta Y, Kongo M, Kishikawa T.Preventive effect of melatonin on the progression of alpha-naphthylisothiocyanate-induced acute liver injury in rats. J Pineal Res 2003;34:185-93 [DOI] [PubMed] [Google Scholar]
- 62.Lesage G, Glaser S, Ueno Y, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 2001;281:G182-90 [DOI] [PubMed] [Google Scholar]
- 63.Alpini G, Lenzi R, Zhai WR, et al. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol 1989;257:G124-33 [DOI] [PubMed] [Google Scholar]
- 64.Mazzanti L, Del Tacca M, Lopez M.Effect of caerulein on the ANIT-induced proliferation of the rat intrahepatic bile duct cells. A histological study. Arzneimittelforschung 1974;24:785-90 [PubMed] [Google Scholar]
- 65.Laothong U, Pinlaor P, Boonsiri P, et al. Melatonin inhibits cholangiocarcinoma and reduces liver injury in Opisthorchis viverrini-infected and N-nitrosodimethylamine-treated hamsters. J Pineal Res 2013;55:257-66 [DOI] [PubMed] [Google Scholar]
- 66.Liang YL, Zhang ZH, Liu XJ, et al. Melatonin protects against apoptosis-inducing factor (AIF)-dependent cell death during acetaminophen-induced acute liver failure. PLoS One 2012;7:e51911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tahan G, Akin H, Aydogan F, et al. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg 2010;53:313-8 [PMC free article] [PubMed] [Google Scholar]
- 68.Tahan V, Ozaras R, Canbakan B, et al. Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res 2004;37:78-84 [DOI] [PubMed] [Google Scholar]
- 69.Bruck R, Aeed H, Avni Y, et al. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J Hepatol 2004;40:86-93 [DOI] [PubMed] [Google Scholar]
- 70.Ohta Y, Imai Y, Matsura T, et al. Successively postadministered melatonin prevents disruption of hepatic antioxidant status in rats with bile duct ligation. J Pineal Res 2005;39:367-74 [DOI] [PubMed] [Google Scholar]
- 71.Esrefoglu M, Gül M, Emre MH, et al. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol 2005;11:1951-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montilla P, Cruz A, Padillo FJ, et al. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res 2001;31:138-44 [DOI] [PubMed] [Google Scholar]
- 73.López PM, Fiñana IT, De Agueda MC, et al. Protective effect of melatonin against oxidative stress induced by ligature of extra-hepatic biliary duct in rats: comparison with the effect of S-adenosyl-L-methionine. J Pineal Res 2000;28:143-9 [DOI] [PubMed] [Google Scholar]
- 74.Hatzis G, Ziakas P, Kavantzas N, et al. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J Hepatol 2013;5:160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu S, Yin S, Jiang X, et al. Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Eur J Pharmacol 2009;616:287-92 [DOI] [PubMed] [Google Scholar]
- 76.Rosa DP, Bona S, Simonetto D, et al. Melatonin protects the liver and erythrocytes against oxidative stress in cirrhotic rats. Arq Gastroenterol 2010;47:72-8 [DOI] [PubMed] [Google Scholar]
- 77.Hong RT, Xu JM, Mei Q. Melatonin ameliorates experimental hepatic fibrosis induced by Carbon tetrachloride in rats. World J Gastroenterol 2009;15:1452-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cruz A, Padillo FJ, Torres E, et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J Pineal Res 2005;39:143-50 [DOI] [PubMed] [Google Scholar]
- 79.Zavodnik IB, Lapshina EA, Cheshchevik VT, et al. Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J Physiol Pharmacol 2011;62:421-7 [PubMed] [Google Scholar]
- 80.Korkmaz GG, Uzun H, Cakatay U, et al. Melatonin ameliorates oxidative damage in hyperglycemia-induced liver injury. Clin Invest Med 2012;35:E370-7 [DOI] [PubMed] [Google Scholar]
- 81.Koksal M, Kurcer Z, Erdogan D, et al. Effect of melatonin and n-acetylcysteine on hepatic injury in rat induced by methanol intoxication: a comparative study. Eur Rev Med Pharmacol Sci 2012;16:437-44 [PubMed] [Google Scholar]
- 82.Cheshchevik VT, Lapshina EA, Dremza IK, et al. Rat liver mitochondrial damage under acute or chronic Carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol 2012;261:271-9 [DOI] [PubMed] [Google Scholar]
- 83.Shirazi A, Mihandoost E, Ghobadi G, et al. Evaluation of radio-protective effect of melatonin on whole body irradiation induced liver tissue damage. Cell J 2013;14:292-7 [PMC free article] [PubMed] [Google Scholar]
- 84.Koh PO. Melatonin prevents hepatic injury-induced decrease in Akt downstream targets phosphorylations. J Pineal Res 2011;51:214-9 [DOI] [PubMed] [Google Scholar]
- 85.Kim SH, Lee SM. Cytoprotective effects of melatonin against necrosis and apoptosis induced by ischemia/reperfusion injury in rat liver. J Pineal Res 2008;44:165-71 [DOI] [PubMed] [Google Scholar]
- 86.Kang JW, Koh EJ, Lee SM. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J Pineal Res 2011;50:403-11 [DOI] [PubMed] [Google Scholar]
- 87.Liang R, Nickkholgh A, Hoffmann K, et al. Melatonin protects from hepatic reperfusion injury through inhibition of IKK and JNK pathways and modification of cell proliferation. J Pineal Res 2009;46:8-14 [DOI] [PubMed] [Google Scholar]
- 88.Mathes AM, Kubulus D, Pradarutti S, et al. Melatonin pretreatment improves liver function and hepatic perfusion after hemorrhagic shock. Shock 2008;29:112-8 [DOI] [PubMed] [Google Scholar]
- 89.Kus I, Ogeturk M, Oner H, et al. Protective effects of melatonin against Carbon tetrachloride-induced hepatotoxicity in rats: a light microscopic and biochemical study. Cell Biochem Funct 2005;23:169-74 [DOI] [PubMed] [Google Scholar]
- 90.Okatani Y, Wakatsuki A, Reiter RJ, et al. Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int J Biochem Cell Biol 2003;35:367-75 [DOI] [PubMed] [Google Scholar]
- 91.Okatani Y, Wakatsuki A, Reiter RJ. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J Pineal Res 2002;32:143-8 [DOI] [PubMed] [Google Scholar]


