Abstract
We analyzed a comprehensive telephone log of pest infestation reports to assess the spatial and temporal trends in Cimex lectularius L. (bed bug) reporting throughout Philadelphia, PA. Citywide spatial analyses of reports from September 2011 to June 2012 revealed several statistically significant bed bug hotspots. However, these were small and diffuse. Temporal analyses of reports from December 2008 to May 2011 detected prominent seasonality in bed bug reporting, peaking in August and reaching a nadir in February each year. Controlling for seasonal cycling, the number of bed bug reports in Philadelphia increased steadily at a rate of ≈4.5% per month (or 69.45% per year) from December 2008 to May 2011. While it may be difficult to spatially target citywide bed bug control measures because of the insects’ widespread migration, interventions informed by seasonal trends may enhance efforts to curb the recent increases in urban bed bug populations.
Keywords: bed bug, Cimex lectularius, Philadelphia, seasonality, spatial
Cimex lectularius L., commonly known as bed bug, is a hematophagous ectoparasite that has spread throughout the United States in recent years (Anderson and Leffler 2008). Research suggests that increased international travel, changes in pest control practices, and increased resistance to pyrethroid insecticides have fostered a global resurgence of bed bugs, stimulating renewed interest in bed bug biology and migration patterns (Potter 2005, Boase 2007, Romero et al. 2007). Although bed bugs have not been identified as pathogen vectors, they often cause substantial emotional distress and physical discomfort, and also require costly extermination and control services (Goddard 2009, Fallen and Gooderham 2011).
Philadelphia, PA, has consistently ranked as one of the highest bed bug-infested cities in the United States, and was deemed the most bed bug-infested city in the nation by pest management professionals in 2012 (Wassmer and Crawford 2012). To better understand bed bug reporting trends in urban environments, we analyzed a telephone log of pest reports made to the Philadelphia Department of Public Health’s Vector Control Services (VCS) between December 2008 and June 2012, with the exception of a gap in the report data from June 2011 to August 2011. We describe temporal patterns of bed bug reporting across the city, and generate maps of infestation reports to identify spatial hotspots of infestation.
Materials and Methods
Temporal Analysis
To evaluate temporal trends in bed bug reporting in Philadelphia, we first reviewed a call log of bed bug infestation reports registered to the Philadelphia Department of Public Health’s VCS between December 2008 and May 2011. We modeled the number of reports over time using a cosinor analysis (Halberg 1969, Nelson et al. 1979, Naser and James 2006). Briefly, the cosinor approach captures seasonal cycles with a sinusoid term as a covariate in a generalized linear regression model. We included an additional covariate term to capture any temporal trends in the data beyond the seasonal cycles. We compare the complete model, comprising both seasonal and trend covariates, with a reduced version without seasonal covariates using the Akaike information criteria (Akaike 1973). The reduced version is equivalent to a traditional Poisson regression.
Spatial Analysis
To assess spatial clustering of recent bed bug reports, we mapped households that reported any pest infestations to VCS between September 2011 and June 2012, as this was our most recent, continuous set of report data. Reports included complaints about bed bugs, raccoons, roaches, rats, feral cats, and dogs, and a wide range of other vertebrate and invertebrate pests (Table 1). Mosquito reports are not present because VCS has a separate phone line dedicated to mosquito complaints. Levels of pest reporting are unlikely to be spatially homogenous throughout Philadelphia, as some geographic sections of the city are more densely populated than others; if the pest report data were assessed directly for spatial trends, we would likely see artificial clustering in locations where there are higher concentrations of people. To account for this heterogeneity, we compared the citywide spatial distribution of bed bug reports with that of other nuisance pest reports, thereby controlling for spatial inconsistencies in reporting.
Table 1.
Reports to VCS between September 2011 and June 2012
| Reason for report | No. reports |
|---|---|
| Bed bugs | 236 |
| Raccoons | 53 |
| Rats/mice | 38 |
| Roaches | 24 |
| Fleas | 22 |
| Birds | 24 |
| Bees/wasps | 14 |
| Cats | 13 |
| Dogs | 10 |
| Squirrels | 9 |
| Opossums | 9 |
| Horses | 7 |
| Flies | 4 |
| Spiders | 3 |
| Gnats | 2 |
| Termites | 2 |
| Foxes | 2 |
| Mites | 1 |
| Slugs | 1 |
| Groundhogs | 1 |
| Other/unknown | 24 |
This comparative approach, using bed bug reports as cases and other pest reports as controls, allows us to determine whether bed bug reports are significantly clustered relative to all other reports (Lawson and Williams 1993, Kelsall and Diggle 1995, Bithell 2006). We spatially smoothed the density of cases using a smoothing function based on a Gaussian kernel with a bandwidth of 500 m. We then smoothed the density of controls using the same function, and calculated the ratio of the two sets of smoothed densities. To test whether the density of bed bug reports was significantly greater than that of other pests, we used a random label permutation test with 999 simulations (Kelsall and Diggle 1995, Waller and Gotway 2004).
Results
Temporal Analysis
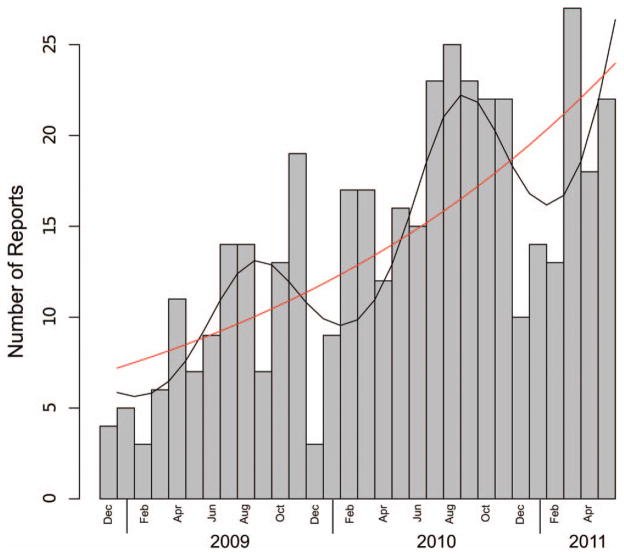
Between December 2008 and May 2011, VCS received 382 reports regarding bed bug infestations. We generated a time-series plot depicting the number of reports made each month (Fig. 1). Our cosinor model, which included seasonal and aggregate trend covariates, detected significant seasonality (P < 0.025) and fit the data better than the reduced (Poisson) model, which excluded the seasonality term (AIC 176.8 vs. 188.2). A comparison of the two models is shown in Fig. 1. Controlling for seasonal cycling, the model demonstrates that the number of reports increased steadily at a rate of 4.5% per month (or 69.45% per year) over the course of the study. The seasonal cycle in the cosinor model peaked in August and reached a nadir in February.
Fig. 1.
Time series of the number of bed bug reports made to the Philadelphia Department of Public Health VCS from December 2008 to June 2011 in Philadelphia, PA, with cosinor (black) and reduced Poisson (red) models.
Spatial Analysis
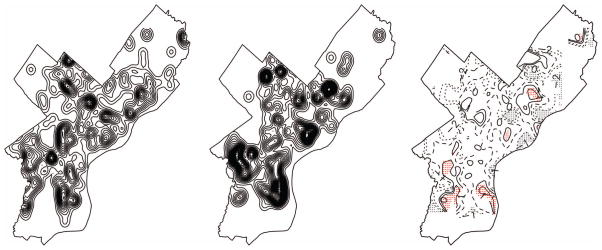
From September 2011 to June 2012, there were 497 reports to VCS regarding various pest infestations. Of these reports, 236 (47%) involved bed bugs; these reports were widely distributed throughout the residential areas of the city. Kernel smoothing analysis identified several significant hotspots of bed bug reports in Philadelphia (compared with other reports), particularly in southern Philadelphia (Fig. 2). Several smaller hotspots were also distributed throughout the city.
Fig. 2.
Spatial distribution of pest reports to the Philadelphia Department of Public Health VCS from September 2011 to June 2012 in Philadelphia, PA. Left, smoothed density of all reports not pertaining to bed bugs; center, smoothed density of bed bug reports; right, ratio of the two smoothed densities with significant bed bug reporting hotspots highlighted in red.
We observed that a large proportion of the pest reports to VCS regarded raccoons. Therefore, we were concerned that the spatial pattern of raccoon occurrence could affect our analysis of bed bugs. As a sensitivity analysis, we repeated our kernel smoothing analysis, excluding raccoons from the control group, and found no significant change in the number or structure of bed bug hotspots throughout Philadelphia. All statistical tests were performed using R (cran.r-project.org).
Discussion
We find a marked upward trend in the number of bed bug reports made to the Philadelphia health authorities throughout the past several years, corroborating reports of recent bed bug resurgences in worldwide urban environments (Hwang et al. 2005). We also find a steep and significant seasonal cycle in bed bug reporting, with peaks in the summer and troughs occurring throughout winter.
Surprisingly, little is known about seasonal trends in bed bug activity levels or population dynamics. Only three previous studies—all from Europe— have attempted to describe such seasonal patterns for indoor bed bug populations, yet none of these studies tested seasonal trends for statistical significance. In a Bulgarian study of poultry farms, bed bug populations exhibited seasonal fluctuations, reaching a maximum peak between July and September (Monov and Topalski 1980). A similar study conducted in Great Britain by the multinational pest control company Rentokil illustrated that residential and industrial bed bug infestations fluctuated throughout the year, reaching a maximum in September and declining throughout the winter (Cornwell 1974). A Danish study found similar results: the average number of monthly bed bug reports made to local health authorities over a period of 11 yr displayed seasonal variation, with the incidence of reports typically rising throughout the year, reaching a maximum in October, and declining throughout the winter (Hallas et al. 1977).
We propose the following two scenarios to explain seasonality in bed bug reporting: bed bugs may have different levels of mobility depending on the season or their population sizes may fluctuate throughout the year. In our first scenario, we propose that bed bugs move more frequently within households during warmer months to feed on human hosts, as higher temperatures increase insect metabolism (Lehane 2005, Pinto et al. 2007, Reinhardt and Siva-Jothy 2007). Higher insect mobility would stimulate more frequent contact between bed bugs and humans, instigating more reports to VCS. While household climate control systems would presumably prevent any major shifts in temperature, air conditioning systems are not always present in city residences, and are unlikely to homogenously cool entire buildings, including the cracks and crevasses in which bed bugs reside (Usinger 1966). Thus, it is reasonable to assume that residential bed bug populations are sometimes subject to external temperatures, which may drive an increase in insect mobility during warmer months. Conversely, anecdotal evidence suggests that bed bugs may be less mobile during colder months, when we typically observe fewer bed bug reports (Potter 2011).
Our second scenario revolves around cycles in bed bug population levels; higher temperatures during the summer may instigate rapid bed bug development and population growth (Johnson 1940, Monov and Topalski 1980). Bed bugs develop faster when the temperature is warm and slower when it is cold, which has implications for the detection of new infestations (Powell and Logan 2005). There may be increased travel during winter and summer because of vacation periods, increasing the likelihood of travelers encountering bed bugs and causing new infestations (Goddard 2009). While it may take time for these infestations to grow to detectable levels, increased developmental and reproduction rates during the summer may speed up detection. Variable detection rates across seasons, driven by changing population growth rates, could potentially explain the relative increase in reports we observe in the summer.
Our analysis of seasonality has disparate implications for the design of bed bug control strategies. By carrying out pest control during summer, it may be easier to eliminate bed bug infestations if they are more mobile. Because bed bugs hide in areas where chemical treatments are often unable to reach, the insects typically have to vacate harborages to come into contact with insecticides (Usinger 1966). Therefore, increased mobility in the summer would enhance the effectiveness of chemical treatment. However, higher bed bug populations during warmer months are likely to counteract this advantage. Control may instead be more effective in the winter if bed bugs are less active during these months because the insects may tend to disperse less, even if insecticides are applied (Potter 2011). In addition, if bed bug populations are lower during certain months, it may be easier for treatments or stochastic events to significantly reduce population sizes and increase the probability of a local extinction (Lande 1993).
Our spatial analysis points to a widespread distribution of bed bugs throughout the city, with a number of small hotspots. This is somewhat surprising because our data suggest that bed bugs have only recently resurged in Philadelphia; typically, when a species emerges in a new habitat, there is a clear spatial pattern to its spread, at least early in the course of dispersion (Skellam 1951, Kareiva 1983). However, in Philadelphia, bed bugs have traversed the entire city in a relatively short period of time, leaving widely distributed hotspots and no clear pattern of dispersion. This rapid spatial expansion, lacking any clear sequential pattern, suggests that bed bugs mainly migrate on people and personal effects capable of traveling long distances (Boase 2007, Pfiester et al. 2009). Active migration by bed bugs is unlikely to be driving the spread of bed bugs in Philadelphia, as such movement is typically confined to a household or between adjacent households (Reinhardt and Siva-Jothy 2007).
There are several limitations to our analyses. First, the datasets obtained from VCS only include households for which an address was provided during the filing of an infestation report. As a result, these data do not fully characterize the actual number or distribution of pest reports in Philadelphia. Second, seasonality in bed bug reporting may be artificially produced by seasonal occurrences unrelated to bed bug dispersal or biology. The yearly nadir in bed bug reporting in December may occur because of increased travel during the holidays, or because the VCS office is briefly closed during the holiday season. In addition, majority of the reports made to VCS were not verified, so residents may have mistakenly filed bed bug reports because of the discovery of similar-looking insects or unfamiliar dermatological irritations. Using telephone reports as a proxy for biological phenomena is problematic for several other reasons; not everyone has a phone, and not all households with bed bug infestations are likely to call the local health department. However, we have attempted to control for these issues by using a case– control framework for our spatial analyses. Finally, the cosinor model assumes that seasonal patterns are symmetrical. Perhaps for this reason, the model overestimated the number of reports in December and underestimated the number in the late summer.
We have found evidence of a bed bug resurgence in Philadelphia that is subject to significant seasonality. While seasonality is a relatively common occurrence across insect species, in the case of bed bugs, it may be a critical attribute that can aid control measures. Our spatial analysis has revealed several modest, yet widely distributed, hotspots of bed bug reporting, a finding that could greatly complicate attempts to design targeted control measures. It may be difficult to spatially target bed bug populations, but it may be possible to opportunistically target them during certain parts of the year in accordance with their seasonal dynamics. Bed bug populations continue to grow as a pervasive public health problem worldwide, and careful temporal design of bed bug control programs may prove invaluable in reducing the incidence of bed bug infestations.
Acknowledgments
We thank the Philadelphia Department of Public Health’s Vector Control Services for providing data for analysis, and for continuing to provide information regarding bed bug infestations to area residents. We also thank Kimberly Nemzoff for her technical assistance in developing the statistical analyses.
References Cited
- Akaike H. International symposium on information theory. 2. Armenian SSR; Tsahkadsor: 1973. Information theory and an extension of the maximum likelihood principle; pp. 267–281. [Google Scholar]
- Anderson AL, Leffler K. Bedbug infestations in the news: a picture of an emerging public health problem in the United States. J Environ Health. 2008;70:24–27. 52–53. [PubMed] [Google Scholar]
- Bithell J. An application of density estimation to geographical epidemiology. Stat Med. 2006;9:691–701. doi: 10.1002/sim.4780090616. [DOI] [PubMed] [Google Scholar]
- Boase C. 15. Bed bugs: research and resurgence. In: Takken W, Knols BGJ, editors. Emerging Pests and Vector-Borne Diseases in Europe. Vol. 1. Wageningen Academic Publishers; Wageningen, The Netherlands: 2007. p. 261. [Google Scholar]
- Cornwell PB. The incidence of fleas and bed bugs in Britain. Int Pest Control. 1974;16:17–20. [Google Scholar]
- Fallen RS, Gooderham M. Bedbugs: an update on recognition and management. Skin Ther Lett. 2011;16:5–7. [PubMed] [Google Scholar]
- Goddard J. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358–1366. doi: 10.1001/jama.2009.405. [DOI] [PubMed] [Google Scholar]
- Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–726. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- Hallas T, Mourier H, Winding O. Seasonal variation and trends for some indoor insects in Denmark. Entomol Medd. 1977;45:77–88. [Google Scholar]
- Hwang SW, Svoboda TJ, De Jong IJ, Kabasele KJ, Gogosis E. Bed bug infestations in an urban environment. Emerg Infect Dis. 2005;11:533. doi: 10.3201/eid1104.041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. Development, hatching and mortality of the eggs of Cimex lectularius L. (Hemiptera) in relation to climate, with observations on the effects of preconditioning to temperature. Parasitology. 1940;32:127–173. [Google Scholar]
- Kareiva P. Local movement in herbivorous insects: applying a passive diffusion model to mark-recapture field experiments. Oecologia. 1983;57:322–327. doi: 10.1007/BF00377175. [DOI] [PubMed] [Google Scholar]
- Kelsall JE, Diggle PJ. Kernel estimation of relative risk. Bernoulli (Andover) 1995;1:3–16. [Google Scholar]
- Lande R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat. 1993;142:911–927. doi: 10.1086/285580. [DOI] [PubMed] [Google Scholar]
- Lawson A, Williams F. Applications of extraction mapping in environmental epidemiology. Stat Med. 1993;12:1249–1258. doi: 10.1002/sim.4780121306. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The biology of blood-sucking in insects. 2. Cambridge University Press; Cambridge, United Kingdom: 2005. [Google Scholar]
- Monov M, Topalski E. Distribution and seasonal dynamics of bed-bugs in poultry farms. Veterinarna Sbirka. 1980;78:21–22. [Google Scholar]
- Naser E, James M. Modeling biological rhythms in failure time data. J Circadian Rhythms. 2006;4:14. doi: 10.1186/1740-3391-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305. [PubMed] [Google Scholar]
- Pfiester M, Koehler PG, Pereira RM. Effect of population structure and size on aggregation behavior of Cimex lectularius (Hemiptera: Cimicidae) J Med Entomol. 2009;46:1015–1020. doi: 10.1603/033.046.0506. [DOI] [PubMed] [Google Scholar]
- Pinto L, Cooper R, Kraft S. Bed bug handbook: the complete guide to bed bugs and their control. Pinto & Associates Inc; Mechanicsville, MD: 2007. [Google Scholar]
- Potter M. A bed bug state of mind: emerging issues in bed bug management. Pest Control Technol. 2005;33:82–85. [Google Scholar]
- Potter MF. The history of bed bug management—with lessons from the past. Am Entomol. 2011;57:14–25. [Google Scholar]
- Powell JA, Logan JA. Insect seasonality: circle map analysis of temperature-driven life cycles. Theor Popul Biol. 2005;67:161–179. doi: 10.1016/j.tpb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annu Rev Entomol. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest’s sudden resurgence? J Med Entomol. 2007;44:175–178. doi: 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Skellam J. Random dispersal in theoretical populations. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- Usinger RL. Monograph of Cimicidae (Hemiptera, Heteroptera) Vol. 7. Entomological Society of America; College Park, MD: 1966. [Google Scholar]
- Waller LA, Gotway CA. Applied spatial statistics for public health data. Vol. 368. Wiley-Interscience; Hoboken, NJ: 2004. [Google Scholar]
- Wassmer K, Crawford K. Philadelphia takes top spot in annual bedbug-infested cities list. Terminix; Philadelphia, PA: 2012. [Google Scholar]