Abstract
Background/Rationale
Breast cancer is not a homogeneous disease, but several different and unique subtypes defined by gene expression analysis. Incidence and mortality rates vary by almost three fold between Alaska (highest) and the Southwestern tribes (lowest). We hypothesized that these differences may be due in part to varying levels of biologic tumor aggressiveness.
Methods
A bio-repository of the North Central Cancer Treatment Group with 95 cases of American Indian and Alaska Native women with adenocarcinoma of the breast surgically treated from 1990 to 2000 were tested for several biomarkers. Comparison distributions of biomarker values across state of residence using t-tests for continuous (p53, MIB-1, Cyclin D) and ordinally scaled markers (EGFR, BCL-2, Her2) and chi-square tests of significance for binary markers (ER, PR) were done.
Results
Significant regional differences in some biomarker expression levels were seen. No increase was observed in “triple negative” breast cancer or Her 2 overexpression in these cases.
Conclusions
Despite a three-fold difference in breast cancer mortality in Alaska Native vs. Southwestern American Indians, standard biomarkers such as ER, PR and Her 2 neu expression did not explain the disparity.
Impact
There is a need for research to understand the biologic basis of breast cancer disparities in AIAN women. Potential for a prospective trial will be explored with tribes.
Keywords: Breast cancer, biomarkers, American Indian/Alaska Native
Introduction
There has never been a prior pathologic series analyzing American Indian and Alaska Native breast cancer patterns. Breast cancer is a major cause of cancer mortality in American Indian and Alaska Native (AIAN) women. Previous studies had suggested that breast cancer rates are lower among AIAN women than among women of other racial and ethnic groups (1–6). However, breast cancer survival among AIAN women reportedly was lower than among non-Hispanic White women in SEER registry areas. The Spirit of Eagles Community Networks Program (CNP) (CNP U01 153604 and U54 153605) is the only national CNP working for more than a decade with American Indians and Alaska Natives on cancer prevention and control. The Principal Investigator for that CNP was a co-author on data published in 2008 that show striking regional differences in breast cancer incidence with lowest rates in Arizona and highest in Alaska with almost a threefold difference in incidence and mortality between the two states (1). The female breast cancer incidence rate in Alaska was 134.8/100,000 vs. 50.8/100,000 in the Southwest from 1999–2004, the most recent complete data published.
We hypothesized that these differences may be due in part to varying levels of biologic tumor aggressiveness. A bio-repository of paraffin embedded breast cancer tumors was created as collaboration between the North Central Cancer Treatment Group and the Spirit of E.A.G.L.E.S. CNP as a special project.
Breast cancer is not a homogeneous disease but a compilation of several different and unique subtypes defined by gene expression analysis. AIAN are rarely included in reviews of breast cancer. For example, differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older was found in 11 population-based cancer registries connected with the SEER Program. Significantly that review did not include American Indians and Alaska Natives because of small sample size for this population in the registries (7).
Epidemiologic features found in the Carolina Breast Cancer Study (8), a population-based, case-control study analyzed clinical associations showed African American women had a high prevalence of basal-like tumors, particularly among premenopausal women. The observation that so-called “triple-negative breast cancers” (ER-, PR- and Her2-) had worse prognosis has led to more attention to the molecular classification and forecasting for breast cancer (9). Other markers have been reputed to provide prognostic and predictive value in breast cancer patients and were analyzed in a panel of biomarkers (9–37) described in Table 1.
Table 1.
SELECT BIOMARKERS OF BREAST CANCER PROGNOSIS
We hypothesized that observed regional differences in breast cancer incidence and mortality in AIAN populations might also reflect underlying molecular biologic differences. This retrospective study is the first such evaluation of a significant number of American Indian and Alaska Native breast cancer patients from a bio-repository established to analyze these two regions with widely different incidence and survival rates. These cases were not enrolled in clinical trials and were accrued as a special project of the NCCTG and Spirit of E.A.G.L.E.S. CNP in conjunction with tribes across the country.
MATERIALS AND METHODS
The Study Population
All American Indian and Alaska Native women with primary adenocarcinoma of the breast surgically treated and with available paraffin-embedded tissue from 1990 to 2000 from the Phoenix Area of the Indian Health Service and the Alaska Native Medical Center were eligible for inclusion in this retrospective study. Those years were chosen by the tribal IRBs since the blocks were allowed to be stored permanently. All data reported here is aggregate and de-identified. Blocks could be returned to the pathologist if clinically required for treatment decisions for patients with late relapses.
A separate protocol at the Phoenix Indian Medical Center allowed for demographic data not present in the pathology reports to be abstracted from the clinical records by a nurse practitioner. That data is also presented in a table in aggregate format. (Table 2)
Table 2.
Demographic and clinical variables by state of residency
| Attribute | Alaska | Arizona |
|---|---|---|
| Age: Mean (N, SD) | 55.2 (42, 9.2) | 58.3 (52, 14.4) |
| Tumor Stage: N (%) | ||
| 0 | 1 (2) | 1 (2) |
| 1 | 16 (39) | 12 (30) |
| 2 | 18 (44) | 17 (42) |
| 3 | 5 (12) | 2 (5) |
| 4 | 1 (2) | 8 (20) |
SD, standard deviation; N, number of subjects with non-missing values; %, percent of subjects with tumor stage of interest in a given state of residence.
A small number of cases (total of 16 cases) from North Dakota and South Dakota were initially also accrued but because the pathology labs were outside of the Indian Health system, complete pathology and clinical correlates could not be released due to hospitals in those states concerned about HIPAA compliance.
Registration Procedures
IRB approval letters from the Phoenix Area Indian Health Service and Alaska Area were provided before pathologic review. Registration of the materials was done via the Materials Library maintained by the NCCTG Research pathology coordinator. The bio-repository tissues are stored per protocol in a secure area within the NCCTG Central Operations Office. Information regarding age and stage of these patients were abstracted from pathology reports.
Tissue Processing
Paraffin blocks were recut at the Mayo Clinic Pathology Department in 5 micron slices and reviewed for standard pathologic features. New sections were cut for the panel of molecular markers and interpreted by a board certified pathologist.
The panel of molecular markers that could be performed on paraffin imbedded specimens was selected based on literature review of their prognostic significance. Retrospective analysis of tissue blocks measured expression levels for the following panel of biomarkers: ER and PR (coded as positive vs. negative); her2, BCL-2, and EGFR (ordinally scaled as 0, 1+, 2+, and 3+) and P53, MIB-1 and Cyclin D (continuous percent of cells stained).
Statistical Methods
The overall objective of these analyses was to determine whether cancer-related biomarkers differ between Native American breast cancer cases residing in Alaska and those residing in Arizona. Data were descriptively summarized using means and standard deviations for continuous and ordinal variables, and frequencies and percents for binary variables. We compared distributions of biomarker values across state of residence using t-tests for continuous markers (p53, MIB-1, Cyclin D1) and chi-square tests of significance for binary markers (ER, PR). The ordinally scaled markers (EGFR, BCL-2, HER2) were also examined using t-tests to take into account the inherent ordering of the values, under the biological assumption that observed associations, if any, would exhibit a dose-response pattern with state of residence. Due to the small number of subjects in some groups, two sets of analyses were carried out: one based on the usual testing techniques that rely upon asymptotic assumptions, and one using non-parametric randomization tests that are robust to deviations from these assumptions(38). For these latter analyses, a standard t-test or chi-square test was run, and a test statistic calculated, on the observed data. Next, subject-specific state or village residency was randomly shuffled to simulate the null hypothesis, and a t-test or chi-square test was run on the resulting data set. This reshuffling step was repeated 10,000 times to generate an empirical distribution of test statistics under the null hypothesis (39). The test statistic based on the observed data was then compared to this empirical distribution, and a final randomization test p-value was calculated as the proportion of the null hypothesis test statistics that were more extreme than the observed one.
Many of the biomarkers of interest, such as ER and PR, are known to differ according to age. To rule out the possibility that the observed association between a given biomarker and residency was due to the confounding effects of age, we ran a series of age-adjusted analyses using analyses of covariance for continuous and ordinal variables and logistic regression models for binary variables. Two such sets of analyses were run: one for age and one for tumor stage. For each, we first subset subjects with non-missing values for the covariate of interest. We then fit models both before and after covariate adjustment and compared results. All statistical tests were two-sided, and all analyses were carried out using the SAS (SAS Institute, Inc. Cary, NC) software system.
RESULTS
A total of 95 breast cancer cases were included in the study: 53 from Arizona and 42 from Alaska. Mean age at diagnosis and the percentage of high stage tumors (i.e. stage 3 or 4) were similar across state of residence (Table 2).
Molecular characteristics
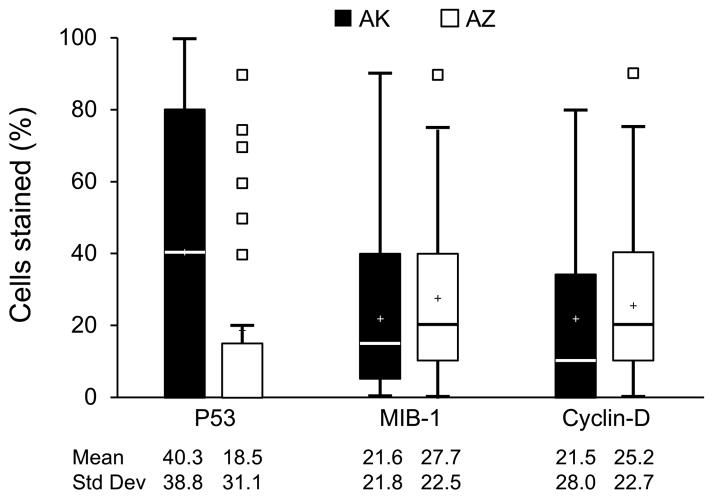
Cases from AK had higher levels of p53 staining (40.3 vs. 18.5, p=0.004) and lower levels of both EGFR (mean ordinal scaling 0.15 vs. 0.53, p=0.02) and Her2 (mean ordinal scaling 0.81 vs. 1.32, p=0.02) than those from AZ. No differences in distribution were observed for MIB-1, Cyclin D, BCL-2, ER or PR. When examined together, the triple negative combination of ER/PR/Her2 also did not differ across states (12% for AK vs. 13% for AZ, p=0.85). (Table 3) Non-parametric Monte Carlo based randomization tests and age- and stage-adjusted analyses yielded similar associations to those presented in Table 1 (data not shown).
Table 3.
Associations between biomarkers of interest and state of residency
| Biomarker | Alaska (N=42) | Arizona (N=53) | P-value4 |
|---|---|---|---|
| Continuous Markers1 | |||
| P53 | 40.3 (38.8) | 18.5 (31.1) | 0.004 |
| MIB-1 | 21.6 (21.8) | 27.7 (22.5) | 0.190 |
| Cyclin D1 | 21.5 (28.0) | 25.3 (22.7) | 0.476 |
| Ordinal Markers2 | |||
| EGFR | 0.15 (0.58) | 0.53 (0.82) | 0.015 |
| BCL-2 | 2.07 (1.30) | 2.17 (1.07) | 0.686 |
| HER2 | 0.81 (1.13) | 1.32 (1.01) | 0.023 |
| Binary Markers3 | |||
| ER | 0.722 | ||
| Negative | 9 (21) | 13 (25) | |
| Positive | 33 (79) | 40 (75) | |
| PR | 0.095 | ||
| Negative | 12 (29) | 24 (45) | |
| Positive | 30 (71) | 29 (55) | |
| HER2 (2 or 3+) | 0.087 | ||
| Negative | 33 (79) | 33 (62) | |
| Positive | 9 (21) | 20 (38) | |
| HER2 (3+) | 0.968 | ||
| Negative | 35 (83) | 44 (83) | |
| Positive | 7 (17) | 9 (17) | |
| ER/PR/HER2 (2 or 3+) | 0.930 | ||
| Triple Negative | 5 (12) | 6 (11) | |
| Positive | 37 (88) | 47 (89) | |
| ER/PR/HER2 (3+) | 0.849 | ||
| Triple Negative | 5 (12) | 7 (13) | |
| Positive | 37 (88) | 46 (87) | |
Values for continuous markers are based on percent staining and range from 0 to 100. Summary statistics provided are mean (standard deviation).
Values for ordinal markers are based on staining intensity and take on values of 0, 1, 2, or 3. Summary statistics provided are mean (standard deviation).
Binary markers are classified as negative or positive. Summary statistics provided are Number positive (percent).
Unadjusted p-value from two-sample t-test (for continuous or ordinal markers) or chi-square test (for binary markers). Age-adjusted analyses and randomization tests yielded similar results.
DISCUSSION
This is the first study to compare American Indian and Alaska Native women with breast cancer in regions with markedly distinctive patterns of incidence and mortality. We began this study to develop a tissue repository of AIAN breast cancer cases at a time when there was suspicion that breast cancer rates were rising. The data had not been analyzed until the Wingo (1) article appeared in a special report. Our study also began before the recognition of distinctive biomarker patterns such as “triple negative” breast cancer. This study did not have the luxury of the newer technology such as Oncotype Dx for defining those marker patterns (36). However, the relative contributions of standard clinical features such as immunohistochemical analysis of ER, PR, HER2 and Ki-67 in the absence of a controlled clinical trial are the most commonly used clinicopathologic assessment tools to predict recurrence (40).
We hypothesized that there might be differences in biomarker patterns of these breast cancers. We were particularly interested in finding out if there was a preponderance of either triple negative or Her2 positive cancers to explain these observations. However, neither of these patterns appears to explain the differences observed. Unfortunately there are very small numbers of American Indians and Alaska Natives in clinical trials, so only this retrospective review of molecular patterns was possible. In the absence of a clinical trial, data presented here is limited by the retrospective nature of the acquisition of paraffin embedded tissues. Alaska and Arizona were selected because most of the samples were able to be accessed through IRB agreements with tribal health boards in those states. Alaska is a SEER special registry as well. While we identified cases of breast cancer tissues from AI women in North Dakota and South Dakota, the pathology departments of many small hospitals were unwilling to send specimens due to their interpretation of HIPAA compliance. We found that regional differences in biomarker expression levels of P53, EGFR and HER2 may exist in AIAN women. While stage of disease would certainly affect mortality rates, the most comprehensive review of regional patterns of breast cancer in AIAN women found no difference in early vs. late stages of breast cancer in Alaska vs. the Southwest. However more Southwestern women had higher rates of being “unstaged”(1). Our staging data on the cases included in this review confirmed staging patterns previously reported. A new review of incidence and mortality patterns of breast cancer in AIAN women will be published in 2013 but unfortunately no data on the molecular markers of interest will be in that report (48). The current mortality data still confirms the dramatic regional differences in breast cancer seen in AK and AZ.
Currently, there are no data on genetic testing for BRCA1, BRCA2 in these populations. There are no genetic counselors within the Indian Health System and referrals for treatment to oncologists in private practice or academic health centers may not include or approve this service. Therefore studies of genetic association with breast cancer risk are quite limited for AIAN women. There are families where breast and ovarian cancer have been noted and there are other hereditary forms of cancer such as Lynch Syndrome documented in some tribes. While controversial, there is mixed evidence that breast cancer should be included in Lynch Syndrome. (49–52). No genetic information was available to evaluate for this study but it is unlikely that the three fold incidence and mortality rates between AK and AZ would be explained by heritable disease. We do note that the self-reported rate of first degree relatives with breast cancer is around 8 percent in both regions included in this study (53), which is quite similar to the general United States population. The higher percentage of AIAN women diagnosed before age 50 (30 percent [30.6 vs. 16.3 in NHW]) underscores the importance of providing culturally appropriate counseling about the value of genetic testing. Excess mortality from breast cancer in the 40–50 year old group of AIAN women requires more research and interventions that are scientifically and culturally appropriate. The genetic influence on breast cancer incidence and mortality in this study is unknown.
Certainly, there are many plausible explanations for the differences in AZ and AK such as screening rates and delay from diagnosis to treatment. A recent review of breast cancer in low income women showed a significant survival difference with delay in treatment for advanced stage patients greater than 60 days from diagnosis. (54). We do not know the time from diagnosis to treatment for this patient group. There other limitations to this study including small numbers and treatment details. Many issues relate to the fragmented care for AIAN cancer patients. A woman may have her mammogram in one location, biopsy in another, definitive surgery elsewhere and systemic therapy in yet another facility. Any future studies will need to overcome these obstacles in order to paint a clear biologic and clinical picture of breast cancer in this population. AI women in Arizona are more likely to present with a palpable mass and higher stages of breast cancer than other racial or ethnic groups. (Table 4) Chart review data was only available for Arizona due to IRB restrictions in Alaska.
Table 4.
Demographic and Clinical Characteristics for 50 Native American Breast Cancer Cases from Arizona.
| Attribute | N (%) |
|---|---|
| Menopausal status | |
| Pre-menopausal | 14 (28) |
| Post-menopausal | 32 (64) |
| Unknown | 4 (8) |
| Family history of breast cancer | |
| None | 38 (76) |
| First degree relative | 4 (8) |
| More distant relative | 3 (6) |
| Unknown | 5 (10) |
| Smoking status | |
| Never | 33 (66) |
| Ever | 12 (24) |
| Unknown | 5 (10) |
| Use of exogenous estrogens | |
| Never | 32 (64) |
| Ever | 13 (26) |
| Unknown | 5 (10) |
| Previous breast biopsy | |
| No | 33 (66) |
| Yes | 9 (18) |
| Unknown | 8 (16) |
| Clinical presentation | |
| Mass | 37 (74) |
| Mammogram | 10 (20) |
| Radical mastectomy | 1 (2) |
| Unknown | 2 (4) |
| Local therapy | |
| Biopsy only | 1 (2) |
| Lumpectomy only | 3 (6) |
| Lumpectomy plus nodal dissection | 16 (32) |
| Mastectomy only | 4 (8) |
| Unknown | 23 (46) |
| 3 (6) | |
We hope that future studies will find this pattern changing. The late stage diagnoses can only be reduced with new and innovative approaches tailored to increase mammographic or other appropriate screening among high risk women in the AIAN population. The U.S. Preventive Services Task Force recommends only biennial screening mammography in women aged 50 to 74 years old. The decision to start regular, biennial screening mammography “before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms”(55) There is no uniformity within the Indian Health System about referral for mammography. This group of women has had less access to screening mammography historically. The latest reports from the Indian Health Service in 2009 reported that the percentage of women ages 52–64 who had mammography screening in the prior two years was only 45 percent. It specifically noted that although there has been overall improvement in breast cancer mortality rates in the general population, “AIAN women have not shared these gains”(56).
In summary, we found differing patterns of p53, EGFR and Her2 tumor expression in AIAN breast cancer cases from Alaska compared to those from Arizona (Figure 1). These differences may explain some, but likely not all, of the previously observed differences in breast cancer mortality in AIAN populations. Understanding the excess burden of breast cancer in AIAN populations will require further research to confirm and expand our results and determine to what extent observed biomarker differences may explain known differences in mortality.
Figure 1.
Boxplots of continuous biomarker values by state. Values represent percent of cells that stained positive for the biomarker of interest. Upper and lower borders of the box represent the 75th and 25th percentiles, respectively. Median and mean values are represented by the blue line inside the box and plus sign, respectively. Whiskers represent the range of values contained within 1.5 times the width of the inter-quartile range above and below the 75th and 25th percentiles. Small boxes represent values lying outside borders of the whiskers.
Acknowledgments
Supported in part by NCI U01 114609 and U54 153605 Spirit of Eagles Community Network Program and the North Central Cancer Treatment Group (NCCTG 97-95-51)
Footnotes
Presented in poster session at the 3rd Annual AACR Cancer Health Disparities Conference in Miami, Florida, October 2010.
The authors have no conflict of interest.
References
- 1.Wingo PA, King J, Swan J, Coughlin SS, Kaur JS, Erb Alvarez JA, et al. Breast cancer incidence among American Indian and Alaska Native women: US, 1999–2004. Cancer. 2008;113(5 Suppl):1191–202. doi: 10.1002/cncr.23725. [DOI] [PubMed] [Google Scholar]
- 2.Espey D, Paisano R, Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska Natives, 1990–2001. Cancer. 2005;103(5):1045–53. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- 3.Cobb N, Paisano RE. Patterns of cancer mortality among Native Americans. Cancer. 1998;83(11):2377–83. doi: 10.1002/(sici)1097-0142(19981201)83:11<2377::aid-cncr18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Hebert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115(11):2539–52. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 6.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women. 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002 Jul;11(7):601–7. [PubMed] [Google Scholar]
- 8.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009 Jun;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005 Oct 10;23(29):7350–60. doi: 10.1200/JCO.2005.03.3845. Epub 2005 Sep 6. [DOI] [PubMed] [Google Scholar]
- 10.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995 Nov;7(6):541–6. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Joensuu H, Pylkkänen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994 Nov;145(5):1191–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston SR, MacLennan KA, Sacks NP, Salter J, Smith IE, Dowsett M. Modulation of Bcl-2 and Ki-67 expression in oestrogen receptor-positive human breast cancer by tamoxifen. Eur J Cancer. 1994;30A(11):1663–9. doi: 10.1016/0959-8049(94)00327-2. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31(20):2586–92. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eeles RA. Predictive testing for germline mutations in the p53 gene: are all the questions answered? Eur J Cancer. 1993;29A(10):1361–5. doi: 10.1016/0959-8049(93)90001-v. [DOI] [PubMed] [Google Scholar]
- 15.Eeles RA, Bartkova J, Lane DP, Bartek J. The role of TP53 in breast cancer development. Cancer Surv. 1993;18:57–75. [PubMed] [Google Scholar]
- 16.Eeles RA. Germline mutations in the TP53 gene. Cancer Surv. 1995;25:101–24. [PubMed] [Google Scholar]
- 17.Hollstein M, Hainaut P. Massively regulated genes: the example of TP53. J Pathol. 2010 Jan;220(2):164–73. doi: 10.1002/path.2637. Review. [DOI] [PubMed] [Google Scholar]
- 18.Hollstein M, Hergenhahn M, Yang Q, Bartsch H, Wang ZQ, Hainaut P. New approaches to understanding p53 gene tumor mutation spectra. Mutat Res. 1999 Dec 17;431(2):199–209. doi: 10.1016/s0027-5107(99)00162-1. Review. [DOI] [PubMed] [Google Scholar]
- 19.Hollstein M, Moeckel G, Hergenhahn M, Spiegelhalder B, Keil M, Werle-Schneider G, et al. On the origins of tumor mutations in cancer genes: insights from the p53 gene. Mutat Res. 1998 Sep 20;405(2):145–54. doi: 10.1016/s0027-5107(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein M, Bartsch H, Wesch H, Kure EH, Mustonen R, Mühlbauer KR, et al. p53 gene mutation analysis in tumors of patients exposed to alpha-particles. Carcinogenesis. 1997 Mar;18(3):511–6. doi: 10.1093/carcin/18.3.511. [DOI] [PubMed] [Google Scholar]
- 21.Hollstein M, Soussi T, Thomas G, von Brevern MC, Bartsch P53 gene alterations in human tumors: perspectives for cancer control. Recent Results Cancer Res. 1997;143:369–89. doi: 10.1007/978-3-642-60393-8_26. Review No abstract available. [DOI] [PubMed] [Google Scholar]
- 22.Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, et al. Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acids Res. 1996 Jan 1;24(1):141–6. doi: 10.1093/nar/24.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sørlie T, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994 Sep;22(17):3551–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. Review. [DOI] [PubMed] [Google Scholar]
- 25.Malkin D. Li-fraumeni syndrome. Genes Cancer. 2011 Apr;2(4):475–84. doi: 10.1177/1947601911413466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattoretti G, Becker MH, Key G, Duchrow M, Schlüter C, Galle J, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–63. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 27.Wintzer HO, Zipfel I, Schulte-Mönting J, Hellerich U, von Kleist S. Ki-67 immunostaining in human breast tumors and its relationship to prognosis. Cancer. 1991 Jan 15;67(2):421–8. doi: 10.1002/1097-0142(19910115)67:2<421::aid-cncr2820670217>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Sahin AA, Ro JY, Brown RW, Ordonez NG, Cleary KR, el-Naggar AK, et al. Assessment of Ki-67-derived tumor proliferative activity in colorectal adenocarcinomas. Mod Pathol. 1994 Jan;7(1):17–22. [PubMed] [Google Scholar]
- 29.Sahin AA, Ro JY, el-Naggar AK, Wilson PL, Teague K, Blick M, Ayala AG. Tumor proliferative fraction in solid malignant neoplasms. A comparative study of Ki-67 immunostaining and flow cytometric determinations. Am J Clin Pathol. 1991 Oct;96(4):512–9. doi: 10.1093/ajcp/96.4.512. [DOI] [PubMed] [Google Scholar]
- 30.Sahin AA, Ro J, Ro JY, Blick MB, el-Naggar AK, Ordonez NG, et al. Ki-67 immunostaining in node-negative stage I/II breast carcinoma. Significant correlation with prognosis. Cancer. 1991 Aug 1;68(3):549–57. doi: 10.1002/1097-0142(19910801)68:3<549::aid-cncr2820680318>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Veronese SM, Maisano C, Scibilia J. Comparative prognostic value of Ki-67 and MIB-1 proliferation indices in breast cancer. Anticancer Res. 1995 Nov-Dec;15(6B):2717–22. [PubMed] [Google Scholar]
- 32.Thor AD, Liu S, Moore DH, 2nd, Edgerton SM. Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol. 1999 Feb;17(2):470–7. doi: 10.1200/JCO.1999.17.2.470. [DOI] [PubMed] [Google Scholar]
- 33.Clahsen PC, van de Velde CJ, Duval C, Pallud C, Mandard AM, Delobelle-Deroide A, et al. The utility of mitotic index, oestrogen receptor and Ki-67 measurements in the creation of novel prognostic indices for node-negative breast cancer. Eur J Surg Oncol. 1999 Aug;25(4):356–63. doi: 10.1053/ejso.1999.0657. [DOI] [PubMed] [Google Scholar]
- 34.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005 Oct 1;23(28):7212–20. doi: 10.1200/JCO.2005.07.501. Review. [DOI] [PubMed] [Google Scholar]
- 35.de Azambuja E, Cardoso F, de Castro G, Jr, Colozza M, Mano MS, Durbecq V, et al. M.Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007 May 21;96(10):1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang G, Shak S, Paik S, Anderson SJ, Costantino JP, Geyer CE, Jr, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011 May;127(1):133–42. doi: 10.1007/s10549-010-1331-z. Epub 2011 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002 Mar-Apr;9(2):409–16. [PubMed] [Google Scholar]
- 38.Zhang X, Meng J, Wang ZY. A switch role of Src in the biphasic EGF signaling in ER-negative breast cancer cells. PLoS ONE. 2012;7(8):e61613. doi: 10.1371/journal.pone.0041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manly BJF. Randomization, bootstrap, and Monte Carlo methods in biology. 2. CRC Press LLC; Boca Raton, FL: 1997. [Google Scholar]
- 40.Lundin SH, Lundin M, Lehtimaki T, Ristimaki A, Holli K, Sailas L, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Can Res. 2011;13(5):R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tunca B, Egeli U, Cecener G, Tezcan G, Gokgoz S, Tasdelen I, et al. CK19, CK20, EGFR and HER2 status of circulating tumor cells in patients with breast cancer. Tumori. 2012;98(2):243–51. doi: 10.1177/030089161209800211. [DOI] [PubMed] [Google Scholar]
- 42.Chen SJ, Luan J, Zhang HS, Ruan CP, Ku KY, Li QQ, et al. EGFR-mediated G1/S transition contributes to the multidrug resistance in breast cancer cells. Molec Bio Re. 2012;39(5):5465–71. doi: 10.1007/s11033-011-1347-4. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa M, Bando Y, Nagao T, Takai C, Ohnishi T, Honda J, et al. Among triple-negative breast cancers, HER2(0) breast cancer shows a strong tendency to be basal-like compared with HER2 (1+) breast cancer: preliminary studies. Breast Cancer. 2012;19(1):54–9. doi: 10.1007/s12282-011-0265-6. [DOI] [PubMed] [Google Scholar]
- 44.Niwinska A, Olszewski W, Murawska M, Pogoda K. Triple-negative breast cancer with brain metastases: a comparison between basal-like and non-basal-like biological subtypes. J Neuro Oncol. 2011;105(3):547–53. doi: 10.1007/s11060-011-0616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002 Mar-Apr;9(2):409–16. [PubMed] [Google Scholar]
- 46.Musgrove EA, Lee CSL, Buckley MF, Sutherland RL. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA. 1994 Aug 16;91(17):8022–26. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. New Engl J Med. 2002 Nov 14;347(20):1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 48.White A, Richardson L, Kaur JS, Li C, Ekwueme DU. Breast Cancer Mortality among American Indian/Alaskan Natives, 1990–2009. Am J Public Health. 2013 doi: 10.2105/AJPH.2013.301720. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pande M, Wei C, Chen J, Amos CI, Lynch PM, Lu KH, et al. Cancer spectrum in DNA mismatch repair gene mutation carriers: results from a hospital based Lynch syndrome registry. Familial Cancer. 2012;11(3):441–7. doi: 10.1007/s10689-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grandval P, Barouk-Simonet E, Bronner M, Buisine MP, Moretta J, Tinat J, et al. Is the controversy on breast cancer as part of the Lynch-related tumor spectrum still open? Familial Cancer. 2012;11(4):681–3. doi: 10.1007/s10689-012-9562-2. [DOI] [PubMed] [Google Scholar]
- 51.Aung KW, Lindor NM, Jenkins MA. Risk of breast cancer in Lynch syndrome: a systematic review. Breast Cancer Research. 2013;15:R27. doi: 10.1186/bcr3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotsari JE, Gylling A, Abdel-Rahman WM, Nieminen TT, Aittomaki K, Friman M, et al. Breast carcinoma and Lynch syndrome: molecular analysis of tumors arising in mutation carriers, non-carriers, and sporadic cases. Breast Cancer Research. 2012;14(3):R90. doi: 10.1186/bcr3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur JS, Roubidoux MA, Sloan J, Novotny P. Can the Gail Model be useful in American Indian and Alaska Native populations? Cancer. 2004;100(5):906–12. doi: 10.1002/cncr.20047. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on Survival of Longer Intervals Between Confirmed Diagnosis and Treatment Initiation Among Low-Income Women With Breast Cancer. J Clin Oncol. 2012;30:4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.(http://www.uspreventiveservicestaskforce.org/uspstf09/breastcancer/brcanrs.htm
- 56.Indian Health Service. National Summary Government Performance & Results Act. 2009 at www.ihs.gov.