Abstract
Background
Chinese Americans are at increased risk for hepatitis B virus (HBV) infection. To reduce or eliminate disparities in HBV-related infection rates, participation in scientific investigations of HBV risk and treatment, including biospecimen sampling, is important. However, Asian Americans have low rates of participation in biospecimen research, and little is known about how educational interventions affect knowledge and participation in HBV-related biospecimen research.
Methods
Eight Chinese community-based organizations participated in a quasi-experimental, two-group design with education assessments at pre- and post-workshop and a three-month follow-up. Four sites were randomly assigned to receive the intervention (n = 175) and four sites to receive general health education (control; n = 240).
Results
Participant knowledge about biospecimen research increased from pre- to post-education in the intervention but not in the control condition. Of intervention participants, 83.4% (146/175) donated one tube of blood for future HBV biospecimen research, and 50.9% (89/175) donated another tube of blood for HBV testing. In contrast, only 1.1% of participants in the control condition reported donating a blood sample at follow-up assessment.
Conclusion
The intervention program significantly increased knowledge of and participation in HBV biospecimen research among Chinese Americans. Community-based participatory research (CBPR) methods featured active support by community leaders, a culturally specific curriculum, and convenient, immediate access to blood sampling, which resulted in high donation rates.
Impact
HBV-related morbidity and mortality is an urgent problem faced by Chinese Americans. CBPR provides a model for engaging communities in early detection, vaccination, and treatment that can reduce this health threat.
Keywords: biospecimen research, biobanking, Chinese Americans, Hepatitis B, cultural factors
Introduction
Biospecimen research is integral to studies that examine variations in disease risk or characteristics, including variations by race/ethnicity or ancestry informative markers (1-3). Nevertheless, studies of beliefs and practices related to biospecimen donation among racial/ethnic minority groups are rare.
Existing studies on donors’ beliefs about participation in biospecimen research, among various groups, have reported an unwillingness to consent to future unspecified research for fear that research results would stigmatize their community (4). For example, some ethnic group leaders have discouraged their community members from participating in genetics research because they fear the possible discrimination or stigmatization of the ethnic group as a consequence of research to identify their populations as being at higher risk for particular diseases (5). These beliefs have translated into generally lower rates of participation in such studies. This is also true among Asian Americans and Pacific Islanders, as studies have reported lower rates of participation in DNA sample donation in this population as compared with the rates of non-Hispanic Whites (40% vs. 61.1%, respectively) (6).
The low rates of racial/ethnic minority participation in biospecimen research may have a negative impact on advances in medical research and treatment pertinent to these groups. For example, the Chinese immigrant population is at high risk for hepatitis B virus (HBV) infection. HBV-related liver cancer rates among male Chinese Americans are six times higher than they are among white males (7). To reduce or eliminate health disparities in HBV between Chinese and mainstream populations, it is important to make scientific advances in HBV treatment that take into account cultural considerations. There is, however, a lack of information about Chinese Americans’ knowledge, perceptions, attitudes, and behaviors in regard to biospecimen collection and future use for hepatitis B and liver cancer research. Notably, little research has examined the circumstances under which people would be willing to donate biological samples for research.
In particular, there is a paucity of data on Asians’ knowledge and attitudes in regard to the donation of blood specimens for medical research. Many Asians are reluctant to donate blood because they believe that blood, once drawn, is not replenished (8). A Singapore-based study that examined individuals’ willingness to donate blood specifically for genetic research found that fear of discovery of disease or genetic weakness or of discrimination based on test results as well as no self-benefit were major concerns about blood donation (9). Although data on Asian American participation in biospecimen research is sparse, evidence from extant studies as well as from our preliminary interviews with community leaders and members indicate complex and culturally-based attitudes and behaviors that might prevent Asians, in general, and Chinese, in particular, from participating in this type of research. For example, among Chinese individuals, misperceptions about potential adverse health effects of blood donation and fear of discrimination have been found to influence willingness to participate in biospecimen donation (9). In addition, cultural beliefs shaped by Confucian philosophy or the balance between yin and yang (i.e., the interconnection and interdependence of contrary forces) were significant factors associated with Chinese individuals’ willingness to donate blood (9).
Although there is a growing body of literature on knowledge, perceptions, and attitudes in regard to biospecimen research, few studies have presented data on actual biospecimen donation behaviors in response to outreach and educational programs, particularly in Asian American populations. This dearth of data indicates an urgent need for well-designed studies, with culturally appropriate methods and measures, to establish key determinants of awareness, opportunity, and acceptance of participation in biospecimen research (10) and to develop and implement culturally appropriate promotion and education programs to increase knowledge, change attitudes and intent, and increase participation in biospecimen research among Chinese Americans.
To stimulate an effective, high-quality biospecimen collection, processing, and storing system within Chinese American communities, research that assesses psychosocial and cultural beliefs, barriers, and facilitators as well as an interdisciplinary scientific team with community partners to educate the public and to enhance their awareness and participation are urgently needed. To this end, the current pilot study was built upon an updated needs assessment of Chinese Americans in the greater Philadelphia area and used our long-standing community-based participatory research (CBPR) approach, which has been utilized in Asian communities region-wide.
Materials and Methods
This research program was approved by the Institutional Review Board (IRB) of Temple University and key partner organizations for the protection of human subjects.
Study Sites and Participant Recruitment
Using the CBPR approach, we have established long-term partnerships with more than 60 Chinese community organizations in the targeted geographic areas of this study. This study was conducted in eight community-based organizations (CBOs) whose leaders indicated a desire to participate in the pilot study. These CBOs have a long history of collaboration with the research team on numerous CBPR projects. A formal partnership agreement, to develop, implement, and deliver the education intervention and disseminate study findings, was signed. Detailed protocols for involving partners in this phase of the study included (1) planning meetings that involved Chinese community participating site leaders, a community advisory board, and project leaders and staff to review study aims, design, recruitment strategy, and protocols as well as individual and group partner responsibilities in this phase of the study; (2) review and discussion of on-site training for community health workers, (3) discussion of community leaders’ role in announcing and facilitating the study in the selected sites; and (4) review and discussion of participant eligibility screening and sign-in sheet (information and contact).
To balance the intervention and control groups, we stratified the CBOs by type of setting, such as community-service and action, community support groups, church groups, and educational groups. Then, from each stratum, we randomly selected one CBO for the intervention and one for the control. In this way, four sites were assigned to receive the intervention condition (n = 185) and four to the control condition (n = 264). Community leaders from each CBO were responsible for recruiting potential study participants. Recruitment procedures involved the use of a study flyer jointly developed by the researchers and community partners. The inclusion criteria for study participants were (1) self-identified Chinese ethnicity; (2) aged 18 or older; and (3) accessible by telephone or email.
Overall, a total of 449 Chinese Americans were recruited from the eight CBOs, with 185 participants in the intervention condition and 264 participants in the control condition. Of these, 432 participants attended the study workshops, and 415 completed the study assessments (175 participants in the intervention condition and 240 participants in the control condition). At follow-up, 359 participants completed the 3-month assessment (170 participants in the intervention condition and 189 participants in the control condition). Preliminary analyses indicated that those individuals lost to follow-up did not differ significantly on baseline characteristics from participants who completed the study.
Development of community-based participatory education intervention
The CBPR framework was used to guide the study process. Specifically, key CBPR principles of participation, relevance, empowerment, community competence, and issue selection were incorporated into the study (11-13).
To develop a culturally competent education intervention program to enhance Chinese American's knowledge of, attitudes toward, and intention to participate in hepatitis B (HepB) biospecimen research, first, we conducted a needs assessment study that involved two focus groups with Chinese community members (n = 20) and in-depth interviews with Chinese community leaders (n = 8). Community leaders and members were selected from two Chinese CBOs using a purposive sampling method. None of the CBOs involved in the needs assessment study were included in the pilot educational intervention study. The purpose of this preliminary study was to develop a better understanding of Chinese American cultural beliefs, attitudes, perceived barriers, and intentions in regard to participating in HepB biospecimen research as well as to attain recommendations for developing and implementing a culturally appropriate education intervention from the perspectives of both Chinese community leaders and lay members.
The major findings, based on the focus groups and in-depth interviews, were as follows: (1) 40% of the participants indicated that blood donation may be harmful to one's health and that it is better to follow traditional Chinese medicine's less invasive diagnostic approach, which uses observation and pulse taking; (2) 70% of the participants felt that, if the research had a close connection to, or would benefit directly a family member or themselves, they would be more willing to participate, i.e., it is more acceptable to donate blood for research if a family member has the disease of the research focus; (3) 35% of the participants expressed a willingness to donate blood for future HBV biospecimen research for the purpose of advancing science to improve health or for HepB prevention and treatment; and (4) all participants stated that the proposed culturally appropriate education was urgently needed for the Chinese community. They recommended that the education program should be consulted with community leaders to ensure its cultural appropriateness and success in participant recruitment. They also indicated the importance of training community health workers in the project protocols so that they could articulate the purpose of the proposed education intervention program to community members. All community leaders expressed their strong support for the proposed pilot program. The findings from the focus groups and in-depth interviews with community leaders and members provided us with evidence to support need for the current study.
Second, the investigators and Chinese community leaders jointly reviewed the needs assessment findings, with the aim of identifying psychosocial and cultural factors that prevent or facilitate participation in biospecimen research among Chinese Americans. These factors were incorporated into the educational curriculum and provided an update to a previously pilot-tested HepB intervention developed by Dr. Ma and colleagues (14-16). To identify additional relevant education materials, the National Cancer Institute (NCI) National Outreach Network and Education Network to Advance Clinical Trials (ENNACT) also was consulted.
Study Design and Procedures
This study utilized a two-group quasi-experimental design with baseline and post-intervention assessment and a 3-month follow-up on biospecimen research participation. Baseline and post-intervention assessments were administered on site just prior to and then immediately following the delivery of the group education sessions. The 3-month follow-up assessments were conducted through telephone interviews.
Intervention group
The education program included three major components: (1) small-group education on (a) blood sample collection, process and storage in biospecimen studies, and participant protection (adapted from NCI's National Biospecimen Network) (17, 18); (b) severity of HepB and liver cancer in the Chinese population, prevention, and treatment (adapted from our Chinese HepB intervention curriculum): and (c) the importance of biospecimen donation for biomedical research on HBV and liver cancer disease in the Chinese population, which might encompass genetic analysis and determination of serum enzymes; (2) discussion of opportunities for participating in biospecimen research; and (3) print materials on biospecimen research (i.e., what a biospecimen is, types of biospecimens used in research, importance of human biospecimens to disease therapy, detection and prevention, scientific discoveries through biospecimen blood samples, risks of donating a blood biospecimen, and how a participant's privacy and confidentiality are protected).
Key constructs of the Health Belief Model (HBM), affective factors, and cultural beliefs were incorporated into the education intervention, which emphasized participants’ cultural values and beliefs, knowledge, perceived barriers and benefits, attitudes, and intention in regard to biospecimen donation for future research. As delineated by the HBM (19), the likelihood that an individual will take action to prevent or detect disease is determined by several factors: perceived vulnerability to the health condition, perceived severity of the health threat, perceived benefits of performing the health behavior, and perceived costs of and barriers to performing this behavior. Guided by the HBM, the intervention program addressed perceived benefits of and perceived barriers to donating blood for biospecimen research and cultural beliefs in regard to blood draws as well as provided a cue to action (accessibility of biosample donation).
After the post-survey, participants who expressed willingness to donate blood for future biospecimen research were invited to sign an informed consent form and to donate either one tube of blood for future HBV-related biospecimen research or two tubes, including one for HBV testing at the study site.
Control group
Control group participants received small-group education on general health, nutrition, balanced diet, and the need for regular medical checkups and for cancer screening. Control group participants were given print material, provided by the Fox Chase Cancer Center (FCCC) Biosample Repository Core Facility (BRCF), that contained information on biospecimen research and instructions on how to participate as well as invited study participants to take part in biospecimen research by donating blood to the BRCF.
To balance the format and time across the intervention and control groups, the same small-group education delivery format was used. Both intervention and control education sessions lasted approximately 40 minutes. Seven of the eight education sessions were held on weekends to accommodate participants’ needs and the availability of participating community organizations. Small-group education sessions for both the intervention and control groups were held at collaborating community partner sites and presented in Chinese by trained Chinese community health educators.
Three-month follow up assessment for intervention and control groups
Three months after the education intervention, participants were contacted by phone by research assistants who inquired whether they had donated blood for future research since attending the workshops. If so, participants were asked where they donated their blood samples; if not, participants were asked to describe their reasons for not donating a sample.
Biosample Collection
The pathologists and biorepository scientists on our research team collectively managed the blood collection process to ensure high quality specimens. The National Cancer Institute Best Practices for Biospecimen Resources was used to guide the biospecimen collection protocols (20). With regard to biospecimen identification, each blood sample was collected, labeled, and handled in a manner that maintained each individual's confidentiality. Each sample was labeled with an identification number, date and time of collection, and specimen source (serum or plasma). Individual characteristics (race, age, and gender) also were documented. Staff members who performed blood collections were appropriately trained in blood collection techniques. One tube of blood (4.5 ml) was collected in red cap vacutainers with no additives. The serum from each vacutainer was stored for future analysis of HBV-related enzymes and biomarkers. A similar procedure was performed for the blood draw for HBV testing.
The HBV test samples were transported to a local clinical laboratory, and the results were returned to our clinicians within two weeks. The clinicians then sent the report to participants, which informed them of their results and, if needed, any further action. The biobanking samples were transported directly to the FCCC BRCF for processing and storage. Processing of samples was conducted within 8 hours of blood draw.
Processing and Banking Biosamples
Blood was processed and stored at the FCCC BRCF in compliance with the NCI's biospecimen best practice guidelines (20). Once the samples were delivered to the BRCF, sample data were entered into the BRCF database, including study consent date, date and time of blood draw, date and time of sample receipt, and processing and storage information. This database was used to track sample type, quantity, freezer location, and distribution. Tubes were centrifuged at 800 × g for 10 minutes at room temperature. Serum was removed and aliquotted into four 0.5 ml tubes and stored at −80° C.
Measures
Sociodemographic information was collected on participants’ age, marital status, educational level, and household income; acculturation measures concerned years living in the US, English proficiency, and healthcare.
Knowledge and awareness of biospecimen research and HepB
Questions included, “How much do you know about biospecimen research?” and “How much do you know about hepatitis B and liver cancer?” and had the following response choices: (1) Nothing, (2) Only heard the name, (3) Know some, (4) Know a lot. Participants were then asked to identify human biospecimens from a multiple-choice list: urine, blood, tissue, cells, DNA/RNA, protein. They also were asked to identify the use of biospecimens from a multiple-choice list: identify and validate ways to deliver drugs or agents to specific cells, identify how diseases progress and vary, group patients as more or less likely to respond to specific drugs, group patients to determine which treatment is appropriate, and develop screening tests to detect biomarkers that are associated with certain stages or subtypes of a disease.
Behavior and willingness to participate in blood donation for future HBV biospecimen research
Participants were asked about their willingness to donate blood to be stored for future biospecimen research. Questions included, “Have you ever donated blood for scientific research?” and “If you were invited to participate in a project that includes donating blood to be processed and stored for future HBV biospecimen studies, how would you feel?” Participants were asked to select a best answer from a 10-point Likert scale that ranged from “Not willing to participate” to “Willing to participate.” The behavior of blood donation was assessed in the following manner. Participants in the intervention group who provided a blood sample for biobanking, following the educational program, were categorized as having donated a sample. Those who did not provide a blood sample were contacted by phone three months later to determine whether they had donated in the interim. For participants in the control group, donation of blood was determined at the 3-month follow-up assessment, as described above.
Statistical Analyses
Descriptive statistics were reported for the demographic and acculturation variables and willingness to participate in biospecimen research at baseline. Discrete variables were reported as percentages and continuous variables as means and standard deviations. The score of willingness to donate blood for future HBV biospecimen research was calculated by subtracting the score on the 10-point Likert scale from 11 so that the higher the score, the more willingness to donate.
The comparison of differences in continuous variables, such as age and years lived in US, between intervention and control groups was conducted using a Student's t-test, while a chi-square test or Fisher's exact test, where appropriate, was used to evaluate potential differences in dichotomous or categorical variables between the two treatment groups.
The effectiveness of the education intervention was evaluated by comparing differences in participants’ knowledge between intervention and control groups after education. Paired t-tests were used to examine knowledge change within the intervention or control groups from pre- to post-education. Comparison of the knowledge change between intervention and control groups was performed using Student's t-tests. Multiple logistic regression was used to examine the difference in blood donation rates at 3-month post-intervention between the two groups, after adjusting for sociodemographic differences.
All analyses were performed using SAS, Version 9.2 (SAS Institute Inc, Cary, NC, USA); a two-sided significance level of α = 0.05 was used to determine statistical significance for all calculations.
Results
Sociodemographic characteristics of the intervention (n = 175) and control (n = 240) groups are presented in Table 1. No statistical differences were found between the intervention and control groups in terms of age, gender, whether born in the US, years lived in the US, marital status, annual household income, English proficiency, having health insurance, or having a regular physician (all p values > 0.05). However, compared to participants in the control group, those in the intervention group had higher education levels (63.9% vs. 47.4% for high school or above, p = 0.004). At baseline, participants’ levels of willingness to participate in biobanking did not statistically differ between the two groups (p = 0.084); the mean scores for willingness to participate in biospecimen research were 6.9 and 6.4, respectively.
Table 1.
Baseline Demographic Characteristics, Health Care Access, and Willingness to Participate in Biospecimen Research
| Intervention N (%) | Control N (%) | P-value | |
|---|---|---|---|
| Age (Mean, SD) | 63.7 (15.2) | 61.4 (17.6) | 0.1464 |
| Gender | 0.3270 | ||
| Male | 69 (39.4) | 83 (34.7) | |
| Female | 106 (60.6) | 156 (65.3) | |
| Born in the U.S. | 0.8727 | ||
| No | 168 (97.1) | 230 (98.3) | |
| Yes | 5 (2.9) | 4 (1.7) | |
| Years lived in U.S. (Mean, SD) | 12.8 (9.8) | 13.9 (11.4) | 0.2806 |
| Current Marital Status | 0.8249 | ||
| Married | 131 (76.2) | 173 (73.6) | |
| Never married | 10 (5.8) | 14 (6.0) | |
| Other | 31 (18.0) | 48 (20.4) | |
| Level of education | 0.0042 | ||
| Less than high school | 61 (36.1) | 121 (52.6) | |
| High School | 43 (25.4) | 40 (17.4) | |
| University/Graduate | 65 (38.5) | 69 (30.0) | |
| Employment | 0.6403 | ||
| Employed | 27 (16.2) | 45 (19.3) | |
| Unemployed | 14 (8.4) | 22 (9.5) | |
| Other | 126 (75.4) | 166 (71.2) | |
| Annual Household Income | 0.1505 | ||
| <$10,000 | 102 (71.3) | 136 (65.4) | |
| >$30,000-40,000 | 13 (9.1) | 13 (6.2) | |
| Health insurance | 0.1425 | ||
| No | 53 (31.4) | 87 (38.5) | |
| Yes | 116 (68.6) | 139 (61.5) | |
| Have regular physician | 0.7760 | ||
| No | 51 (30.9) | 71 (32.3) | |
| Yes | 114 (69.1) | 149 (67.3) | |
| Speak English well | 0.9079 | ||
| Not at all/not well | 154 (91.1) | 214 (91.4) | |
| Well/Very well | 15 (8.9) | 20 (8.9) | |
| Score of willing to participate in biospecimen research (Mean, SD) | 6.9 (2.7) | 6.4 (2.7) | 0.0839 |
Post-intervention Changes in Knowledge about Biospecimen Research
Responses to knowledge items were summed to obtain a composite score for knowledge of biospecimens and of biospecimen research. Within the intervention group, the results of paired t-tests suggested that knowledge of biospecimens and biospecimen research increased from pre- to post-education (both ps < 0.001), whereas knowledge change within the control group was not statistically significant (both ps > 0.05). The comparison between the intervention and control groups in terms of the knowledge change from pre- to post also was significant (both ps < 0.001).
Willingness to donate blood significantly increased from pre- to post-education in the intervention group (p < 0.05) but not in the control group (p > 0.05). However, the change between the two groups was not significant (p > 0.05) (Table 2).
Table 2.
Change in Knowledge About Biospecimen Research
| Variables | Intervention (N=175) | Control (N=240) | Comparison of change between intervention and control groups | ||||
|---|---|---|---|---|---|---|---|
| Pre- | Post- | Paired T-test P-value | Pre- | Post- | Paired T- test P-value | T-test test P-value | |
| Score of knowledge about biospecimen (mean(SD)) | 2.2(1.9) | 3.0(2.0) | <0.0001 | 2.0(1.8) | 2.2(1.8) | 0.0513 | <0.0001 |
| Score of knowledge about biospecimen research (mean(SD)) | 1.4(1.7) | 2.7(2.0) | <0.0001 | 1.4(1.5) | 1.6(1.6) | 0.0541 | <0.0001 |
| Willingness to donate blood (mean(SD)) | 6.9(2.7) | 7.3(2.7) | 0.0466 | 6.4(2.7) | 6.4(2.6) | 0.9130 | 0.0921 |
Blood Donation Rate
The results indicated that 83.4% (146/175) of the participants in the intervention group donated one tube of blood for future HBV biospecimen research at the study site. Among the 146 donors, 61% (89) also donated another tube of blood for HBV testing. Among the participants who did not donate blood at the study site, none reported blood donation after the educational program. In preliminary analyses, education level was noted to be significantly associated with blood donation among intervention group participants. Specifically, the donation rate was 57.3% among participants with less than a high school education, 83.3% among participants with a high school education, and 62.8% among participants with a college education or higher (p < 0.01).
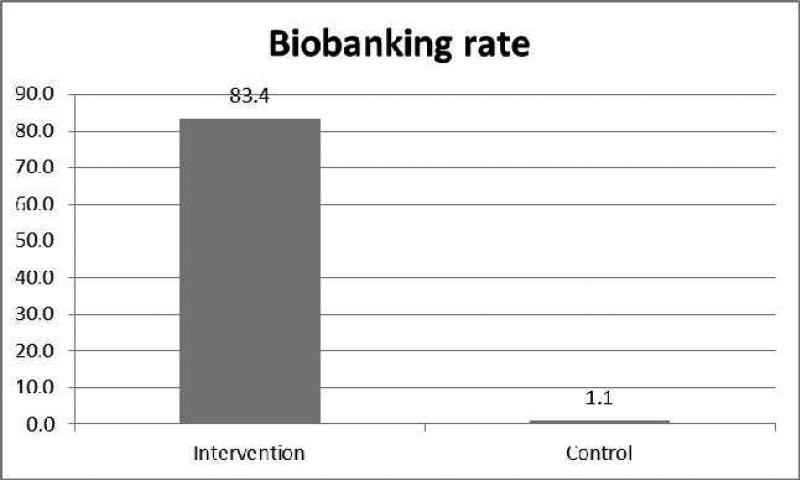
Biospecimen donation rates were significantly lower in the control group than in the intervention group (p < 0.001). Among the 189 control group participants who were able to be contacted by phone, only two self-reported blood donation, which represented 1.1% of control group participants. The difference in blood donation rates for future HBV biospecimen research between the intervention and treatment groups remained statistically significant after adjusting for education level (p < 0.001) (Figure 1).
Figure. 1.
Biobanking Rate of Blood Donation by Treatment Group
Discussion
Building on the findings of our early Chinese community-based HepB assessment study (14) and the existing culturally and linguistically appropriate intervention program that targets Chinese families, communities, and health providers to enhance HBV screening, vaccination, and treatment (21), this pilot study on the HBV biospecimen research education intervention was both a natural and innovative step for expanding our understanding of biospecimen donation in the target population. To the best of our knowledge, this is the first study that has used a CBPR approach to develop and implement a culturally appropriate education intervention on biospecimen research participation among Chinese Americans, a fast-growing population that is at high risk for HBV infection and liver cancer. The study design made it possible not only to compare the changes within each group but also between the groups. The results showed a knowledge increase in the intervention group after small-group education. Notably, the results exceeded our expectations: More than 83% of participants in the intervention group donated their blood for future biospecimen research. This rate was higher than the one that we obtained from the needs assessment interview; it also was higher than that seen in studies of African Americans and low-income urban Whites (22). Kiviniemi and colleagues (2013) reported a 74% participation rate for providing blood or saliva among study respondents (22). In their study, however, due to scheduling constraints, not all the group education interventions included on-site blood draw, although participants were invited to come back another time to donate blood or go to the biobanking laboratory to do so. This key difference may help explain the higher participation rates in the present study, as opportunities to participate in biobanking were provided immediately after education for the intervention groups.
The success of our pilot study may be attributed to a variety of factors. First, the intervention was culturally and linguistically adapted to this group, which thereby removed a substantial barrier to the provision of a health message. Second, the venue of the intervention as well as the support and encouragement of community leaders and family members (collectivism) inspired the participants. It is noteworthy that many community leaders not only facilitated the group education but also showed their support by standing at the front of the line to donate blood for the research. Third, our providing on-site blood draw eliminated the access barriers such as transportation, language, and time and was well received by the participants.
Lessons Learned
We have learned a few important lessons from this pilot study. First, a multi-disciplinary collaborative team with a common vision and shared responsibilities appeared to be key to the program's success. Our conducting this pilot study expanded our network's capacities to establish a multidisciplinary collaborative team of experienced scientists and community leaders with expertise in cancer control and biospecimen research. Their areas of expertise ranged from behavioral health, biobehavioral medicine, pathology, hepatology, and medical ethics to qualitative and qualitative research, epidemiology, biostatistics, and biosample acquisition. This team had a comprehensive understanding of the cultural, psychosocial, and logistical issues that may influence willingness to participate in biosample donation among Asians, which contributed to the success of the intervention program.
As one study found (22), it is essential to the CBPR partnership to be responsive and flexible to the community's requests for programs. With a common interest, researchers, church leaders, community health educators and workers, and health care providers volunteered their time to participate in the delivery of the intervention. In some cases, this meant that study-related programs were held on weekends and holidays to accommodate the community's needs. For example, due to the use of community setting, most of our programs were held on weekends. However, our clinical partners and biobanking laboratory are generally not available on such days. Through coordination and flexibility, however, we were able to mobilize all parties, including community health educators and workers, community leaders, phlebotomists, biobanking laboratory technologists, and a clinical laboratory delivery team, to adjust their regular schedule so that all activities, including group education sessions, blood draws, and blood processing could occur on weekends. By doing so, we avoided the challenges that some other studies encountered when they delivered workshops on weekends but scheduled blood draws for weekdays (22), which may have contributed to lower biospecimen donation rates due to logistical barriers for participants who needed to return to the site on a weekday or to go to a biobanking laboratory for the blood draw.
Second, community partners were instrumental in developing the intervention program, recruiting participants, organizing workshops, and conveying the culturally competent message to the community as well as in providing feedback from the community to the research team. A critical and essential element of our successful CBPR partnership was the establishment of trust and credibility within the targeted Chinese community, which was tied together with effective communication and a decision-making process characterized by equality during program development and implementation (16). Community leaders were actively involved in the intervention planning and development, and their views on cultural issues as well as their views on program settings were incorporated into the program curriculum. This approach ensured that the program was culturally appropriate and responsive to community needs as well as feasible for and acceptable and accessible to Chinese American community members. The established trust and credibility among research team and community partners inspired community leaders to take the initiative in setting up and promoting the intervention program and in bridging gaps in knowledge, attitudes, and participation in biospecimen research in the their communities.
Third, community members are more willing to participate in biospecimen research when the disease is relevant to them and relevant clinical testing is provided. Studies have indicated that people are more motivated to donate tissue if they have had the personal experience of a family member's being affected by the disease or hold a favorable view of the intrinsic merits of scientific or medical research (23). HepB infection and liver cancer are familiar to Asians, including the Chinese community. During our education sessions, participants actively took part in the discussion of the severity of HepB and liver cancer in the Chinese population, prevention, and treatment. Through the discussion, they learned the significance of donating a biospecimen for biomedical research in HBV and, subsequently, voluntarily participated in blood donation. Participants also were appreciative of the opportunity for HBV testing. More than half (61%) of those participants who donated blood for biospecimen research also requested HBV testing, as they had never had a prior opportunity to participate in HBV screening. Provision of such testing was integral to this partnership with community leaders and participants. It is worth noting, however, that our study by no means provided a purely clinical service, given that some of the participants had been tested previously. This also explains why the percentage of HBV testing was lower than that of blood donation for future research.
Limitations
This pilot study had some limitations. First, while we endeavored to recruit diverse populations from various CBOs such as community service and action groups, community support groups, church groups, and educational groups, our findings may not be generalizable to Chinese residents who are not closely engaged with their communities, and nonparticipants may have different views and patterns in regard to participating in biospecimen research. Second, because biobanking and biospecimen research is relatively new to the Chinese population, compared to research on cancer and chronic diseases, some participants felt overwhelmed by the educational program; others reported challenges with completing the detailed questionnaire.
Third, on-site blood draws were available only for the intervention group. As a result, it is not known whether rates of biospecimen donation would have been higher in the control group if on-site blood draws had been available. In the present study, the intervention program was designed to address both knowledge and access barriers that Chinese Americans reported facing with respect to biospecimen research; in contrast, the control condition was designed to resemble current practices with regard to general access to biospecimen research, and, therefore, it linked participants to the ongoing biospecimen donation procedures available at FCCC. Opportunities to participate in biospecimen donation and research at FCCC are widely advertised via various channels throughout the surrounding region and have been ongoing for many years. Thus, we emphasized this opportunity for the control group participants.
Future studies should evaluate whether cognitive and access barriers independently influence donation rates. This could be done, for example, by adding two more conditions: one in which participants are provided group education without on-site access to blood donation and one in which participants are provided blood draw opportunities on-site without the corresponding group education program. This would allow a more detailed exploration of the independent impact of access and cognitive factors as well as any synergistic effects. Finally, although the participating sites were randomly selected using a stratified method, participants’ demographic characteristics were not entirely balanced across the intervention and control groups. For example, participant education levels were higher in the intervention group compared to the control group, which could have contributed to the higher blood donation rates observed between the groups.
Despite these limitations, the present study offers a comprehensive model for recruiting a racial/ethnic minority population to participate in biospecimen research. The knowledge increase and high biospecimen research participation rate in the intervention group demonstrated the effectiveness of the education and specimen collection program. This study adds to the limited literature on developing and evaluating culturally appropriate biobanking educational programs among Chinese and other Asian American ethnic subgroups. Asian Americans have high incidence rates of HBV infection and liver cancer in the USA. Their increased awareness and participation in HBV-related biospecimen research will enhance medical research and contribute to the reduction or elimination of cancer health disparities in this fast-growing immigrant population.
Using the experience gained from the pilot program and feedback from community leaders and members, the multi-disciplinary team was able to revise the educational curriculum and questionnaire to remove jargon and to make these materials more concise. The program is currently being expanded and evaluated in Korean and other Asian communities. We anticipate that the results from these ongoing studies will further demonstrate the effectiveness of a culturally appropriate biospecimen intervention for Asian communities. In addition, this academic-community partnership guided by CBPR principles will be strengthened and expanded to make potentially sustainable contributions to reducing cancer health disparities.
Acknowledgments and Funding Source
This research is a pilot study supported by NIH-NCI's Community Network Program Center, ACCHDC- CNPC (U54CA153513, PI: Grace Ma; Pilot Study Leader is Wanzhen Gao). The authors wish to thank Asian Community Health Coalition for collaboration.
Footnotes
*Wanzhen Gao is a junior researcher trainee of training core under ACCHDC-CNPC (U54CA153513, PI: Grace Ma).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 2.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a singleinstitution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr., Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaught JB, Lockhart N, Thiel KS, Schneider JA. Ethical, legal, and policy issues: dominating the biospecimen discussion. Cancer Epidemiol Biomarkers Prev. 2007;16:2521–3. doi: 10.1158/1055-9965.EPI-07-2758. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg KH, Rutkin AB. Toward a framework of mutualism: the Jewish community in genetics research. Community Genet. 1998;1:148–53. doi: 10.1159/000016154. [DOI] [PubMed] [Google Scholar]
- 6.Crider KS, Reefhuis J, Woomert A, Honein MA. Racial and ethnic disparity in participation in DNA collection at the Atlanta site of the National Birth Defects Prevention Study. Am J Epidemiol. 2006;164:805–12. doi: 10.1093/aje/kwj264. [DOI] [PubMed] [Google Scholar]
- 7.Miller BA, Kolonel LN, Bernstein L, Young JL, Jr., Swanson GM, West D, et al. Racial/ethnic patterns of cancer in the United States 1988-1992. National Cancer Institute; Bethesda, MD: 1996. [Google Scholar]
- 8.Chin JL, Bigby J. Care of Asian Americans. In: Bigby J, editor. Cross-cultural medicine. American College of Physicians; Philadelphia: 2003. [Google Scholar]
- 9.Wong ML, Chia KS, Yam WM, Teodoro GR, Lau KW. Willingness to donate blood samples for genetic research: a survey from a community in Singapore. Clin Genet. 2004;65:45–51. doi: 10.1111/j..2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 11.Israel BA, Lichtenstein R, Lantz P, McGranaghan R, Allen A, Guzman JR, et al. The Detroit Community-Academic Urban Research Center: development, implementation, and evaluation. J Public Health Manag Pract. 2001;7:1–19. doi: 10.1097/00124784-200107050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: Assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 13.Ma GX, Toubbeh JI, Su X, Edwards RL. ATECAR: An Asian American community-based participatory research model on tobacco and cancer control. Health Promot Pract. 2004;5:382–94. doi: 10.1177/1524839903260146. [DOI] [PubMed] [Google Scholar]
- 14.Ma GX, Shive SE, Toubbeh JI, Tan Y, Wu D. Knowledge, attitudes, and behaviors of Chinese hepatitis B screening and vaccination. Am J Health Behav. 2008;32:178–87. doi: 10.5555/ajhb.2008.32.2.178. [DOI] [PubMed] [Google Scholar]
- 15.Ma GX, Lee S, Wang M, Tan Y, Gao W, Ma X, et al. Role of sociocultural factors in hepatitis B screening among Asian Americans. South Med J. 2011;104:466–72. doi: 10.1097/SMJ.0b013e31821f8ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma GX, Gao W, Tan Y, Chae WG, Rhee J. A community-based participatory approach to a hepatitis B intervention for Korean Americans. Prog Community Health Partnership. 2012;6:7–16. doi: 10.1353/cpr.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Research Advocacy Network [2009 September 3];“Why is it important for me to consider donating my tissue for research?”. Available from: http://www.researchadvocacy.org/publications/pdf/tissue_ConsiderDonating.pdf.
- 18.Research Advocacy Network [9/3/2009];Booklet publication “The Importance of Tissue Sample in Research”. at http://www.researchadvocacy.org/publications/posters.php.
- 19.Becker MH. The health belief model and personal health behavior. Slack; Thorofare, NJ: 1974. [Google Scholar]
- 20.National Cancer Institute National Cancer Institute best practices for biospecimen resources. 2007 [cited; Available from: http://biospecimens.cancer.gov/global/pdfs/NCI_Best_Practices_060507.pdf.
- 21.Hsu CE, Liu LC, Juon HS, Chiu YW, Bawa J, Tillman U, et al. Reducing liver cancer disparities: a community-based hepatitis-B prevention program for Asian-American communities. J Natl Med Assoc. 2007;99:900–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Kiviniemi MT, Saad-Harfouche FG, Ciupak GL, Davis W, Moysich K, Hargrave NC, et al. Pilot intervention outcomes of an educational program for biospecimen research participation. J Cancer Educ. 2013;28:52–9. doi: 10.1007/s13187-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque JS, Quinn GP, Montel-Ishino FA, Arevalo M, Bynum SA, Noel-Thomas S, et al. Formative research on perceptions of biobanking: what community members think. J Cancer Educ. 2012;27:91–9. doi: 10.1007/s13187-011-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]