Opinion statement
Cardiac magnetic resonance (CMR) has emerged as a versatile noninvasive tool for the comprehensive evaluation of patients with suspected or established coronary artery disease (CAD). In a single imaging session, CMR can assess left ventricular anatomy and function, myocardial perfusion, viability, and coronary luminal stenosis. Using specific pulse sequences, left ventricular global and regional function can be assessed by cine CMR at rest and in response to inotropic stress; first-pass perfusion quantified by vasodilator stress; myocardial viability evaluated by delayed enhancement imaging and also by functional reserve; and coronary artery stenosis assessed by angiography. All these modalities can be achieved with high spatial resolution and image contrast, without exposure to ionizing radiation, and within a reasonable time frame of about 1 hour of scan time. Also, the imaging planes can be programmed to provide identical views of the heart for each type of image, thereby facilitating intermodality comparisons. There is early but accumulating evidence that the accuracy and prognostic values of many of these modalities are comparable or superior to radionuclide scintigraphy and echocardiography in head-to-head studies. Current limitations unique to CMR include the inability to perform exercise stress testing inside the CMR suite and exclusion of patients with indwelling metallic devices such as defibrillators and pacemakers. Despite these limitations, CMR is unique in its multifaceted approach that can be specifically tailored to the clinical question at hand, making it arguably the best tool for the diagnosis and management of CAD. With the rapid pace of advancement in CMR hardware and pulse sequence technologies, the clinical use of this powerful technique is likely to grow even greater in this area.
Introduction
Although cardiac magnetic resonance (CMR) has been established as the gold standard cardiac imaging technique for assessing ventricular dimensions and function, only in the past decade has this technique emerged to challenge radionuclide scintigraphy and echocardiography in the evaluation of patients with suspected or established coronary artery disease (CAD). This advance is the result of the availability of rapid high-performance gradient systems and parallel imaging techniques that significantly shorten imaging times, allowing cine imaging of the heart at rest and under stress conditions at high heart rates. Moreover, the development of steady-state free precession techniques for cine imaging further improves signal-to-noise ratio and myocardium–blood contrast [1]. These recent advances in CMR, combined with its superior spatial resolution (1–2 mm) and its tomographic three-dimensional (3D) imaging capability, yield diagnostic images of unrivaled quality. Also, because CMR does not use ionizing radiation or iodine-based contrast agent, it is an ideal noninvasive tool for the serial assessment of patients with CAD. Moreover, CMR allows for a comprehensive evaluation for CAD that is unmatched by other imaging techniques. Besides evaluating ventricular anatomy and function, it can assess functional reserve in response to inotropic stress, perfusion in response to vasodilator stress, viability by delayed enhancement imaging and functional reserve, and coronary artery morphology by CMR angiography. A standard examination incorporating most of these elements can be achieved in a single visit lasting about 1 hour. This article summarizes the current state of CMR methods used for evaluating myocardial ischemia and viability and ends with speculations on emerging techniques that may further enhance the utility of CMR in this area.
Assessment of myocardial ischemia
CMR can be used to detect CAD by eliciting myocardial ischemia in response to pharmacologic stress agents. Two such pharmacologic strategies are in common use: one relies on the detection of wall motion abnormalities developed during inotropic stress with dobutamine; the other involves evaluation of perfusion defects in response to vasodilator stress, most commonly with adenosine.
Exercise stress testing is currently not feasible within the narrow confines of the MRI scanner and because of the lack of an intracellular myocardial contrast agent.
These two main diagnostic strategies have been validated against coronary angiography and compared with other competing technologies. In general, the choice of inotropic or vasodilator stress should be based not only on the testing characteristics of each method, but on the individual case. For example, dobutamine stress is preferred when there is a question about viability and adenosine stress may be favored in patients with a history of ventricular tachyarrhythmias.
Dobutamine CMR stress
The dobutamine CMR stress protocol follows the standard high-dose dobutamine/atropine regimen used in stress echocardiography. Because of its superior spatial resolution and endocardial border definition, this CMR method has been shown to yield higher diagnostic accuracy (86%; 89% sensitivity, 86% specificity) in detecting angiographic CAD (luminal stenosis > 50%) than dobutamine echocardiography (accuracy of 79%) [2]. CMR is particularly effective in patients not suited for echocardiography because of poor acoustic windows and suboptimal images despite second harmonic imaging [3]. Recently, myocardial ischemia detected by dobutamine as well as by adenosine stress CMR has been shown to provide useful prognostic information over clinical risk factors and resting wall motion abnormalities by identifying patients at high risk for subsequent cardiac death or nonfatal myocardial infarction (MI) [4••]. In patients with normal CMR stress, the 3-year event-free survival was reported to be 99.2%. In addition, dobutamine CMR has been used to assess preoperative cardiac risk in patients undergoing noncardiac surgery [5].
The widespread adoption of dobutamine CMR for ischemia evaluation has been hampered by concerns about monitoring of patients’ clinical status within the scanner during the stress period. These concerns stem from the fact that electrocardiographic signals such as ST segment changes are rendered nondiagnostic by magneto-hydrodynamic effects related to the use of magnetic fields for imaging. However, CMR can monitor ischemia by frequent real-time cine imaging. Because wall motion abnormalities precede ST segment changes during ischemia, this monitoring method is comparable to detecting ischemic electrocardiographic changes. Safety data from a study of high-dose CMR dobutamine stress in 1000 consecutive patients show a safety profile almost identical to that of dobutamine stress echocardiography: only one patient suffered sustained ventricular tachycardia with successful defibrillation, and no cases of death or MI occurred [6].
Adenosine CMR stress
Adenosine CMR is performed similarly to vasodilator stress with radionuclide imaging but uses gadolinium as a first-pass perfusion agent. By this technique, areas of infarct and ischemia are detected on the basis of decreased blood flow resulting in slower rates of contrast uptake during its transit through the myocardial circulation. In general, vasodilator stress has a theoretic advantage over inotropic stress as perfusion defects develop earlier in the ischemic cascade than do wall motion abnormalities. Moreover, the CMR perfusion protocol is significantly shorter than the dobutamine protocol, with a 3- to 6-minute infusion of adenosine and an imaging time of about 1 minute.
There are more than 20 published clinical studies supporting vasodilating stress CMR perfusion in diagnosing CAD (Table 1). Overall, recent CMR perfusion techniques across different imaging vendors have demonstrated encouraging results, including very high to excellent sensitivity (85% to 95%) and moderate to high specificity in detecting angiographically significant coronary stenosis. This somewhat lower specificity for the detection of epicardial coronary stenosis has been reported for vasodilator versus dobutamine stress CMR imaging [7]. This lower test specificity may be attributed in part to transient hypointense artifacts frequently observed along the myocardial–blood pool interface, mimicking subendocardial perfusion defects. However, this may be improved with the advent of high-field CMR imaging with a 3-Tesla magnet and lower contrast agent requirement [8]. There is hope that the accuracy of perfusion testing can be improved by quantitative rather than qualitative analysis of myocardial perfusion. In a single-center study, this quantitative approach was compared with positron emission tomography (PET) [9], with the sensitivity, specificity, and overall accuracy as high as 88%, 90%, and 89% [10]. However, in a more recent multicenter study, the specificity was not as high (75%), although stress imaging without rest imaging was used in this study [11]. The drawback of a quantitative approach at present is that it is quite time consuming and not feasible for routine clinical use.
Table 1.
Sensitivity and specificity of vasodilator stress perfusion studies for detecting coronary artery disease
| Year | Study group | Patients, n | Stress | Gd dose, mmol/kg | Technique | Reference | Analysis | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|---|---|---|---|
| 2006 | Klem et al. | 92 | Adenosine | 0.065 | Hybrid-EPI | Cath > 70% | Qualitative | 89 | 87 |
| 2006 | Ingkanisorn et al. | 135 | Adenosine | 0.1 | Hybrid-EPI | Prognosis | Qualitative | 100 | 93 |
| 2005 | Okuda et al. | 33 | Dipyridamole | 0.05 | Hybrid-EPI | Cath > 75% | Qualitative | 84 | 87 |
| 2005 | Sakuma et al. | 40 | Dipyridamole | 0.03 | GRE | Cath > 70% | Qualitative | 81 | 68 |
| 2005 | Plein et al. | 92 | Adenosine | 0.05 | GRE | Cath > 70% | SLP | 88 | 82 |
| 2004 | Takase et al. | 102 | Dipyridamole | 0.1 | Hybrid-EPI | Cath > 50% | Qualitative | 93 | 85 |
| 2004 | Paetsch et al. | 53 | Adenosine | 0.05, 0.1, 0.15 | Hybrid-EPI | Cath > 75% | Qualitative | 79 | 75 |
| 2004 | Paetsch et al. | 79 | Adenosine | 0.05 | Hybrid-EPI | QCA > 50% | Qualitative | 91 | 62 |
| 2004 | Wolff et al. | 99 | Adenosine | 0.05, 0.1, 0.15 | Hybrid-EPI | QCA > 70% | Qualitative | 93 | 75 |
| 2004 | Thiele et al. | 32 | Adenosine | 0.05 | GRE | Cath > 70% | SLP | 75 | 97 |
| SPECT | 80 | 91 | |||||||
| 2004 | Plein et al. | 72 | Adenosine | 0.05 | GRE | Cath > 70% | Qualitative | 88 | 83 |
| 2004 | Bunce et al. | 35 | Adenosine | 0.05 | GRE | Cath > 50% | SLP | 74 | 71 |
| 2003 | Nagel el al. | 84 | Adenosine | 0.025 | Hybrid-EPI | Cath > 75% | SLP | 88 | 90 |
| 2003 | Ishida et al. | 104 | Dipyridamole | 0.075 | Hybrid-EPI | Cath > 70% | Qualitative | 84 | 82 |
| 2003 | Doyle et al. | 184 | Dipyridamole | 0.04 | GRE | QCA > 70% | SLP | 57 | 78 |
| SPECT | 52 | 82 | |||||||
| 2003 | Kinoshita et al. | 27 | Dipyridamole | 0.1 | GRE | Cath 75%–90% | Qualitative | 55 | 77 |
| Cath > 90% | 77 | 81 | |||||||
| 2002 | Ibrahim et al. | 25 | Adenosine | 0.05 | Hybrid-EPI | QCA > 75% | SLP | 69 | 89 |
| 2001 | Schwitter et al. | 48 | Dipyridamole | 0.1 | Hybrid-EPI | QCA > 50% | SLP | 87 | 85 |
| PET | 91 | 94 | |||||||
| 2001 | Panting et al. | 26 | Adenosine | 0.05 | SE-EPI | Cath > 50% | SLP, CE, TPK | 79, 72, 60 | 83, 83, 43 |
| Qualitative | 77 | 83 | |||||||
| 2000 | Al-Saadi et al | 34 | Dipyridamole | NA | NA | Cath > 75% | SLP | 90 | 83 |
Cath—coronary angiography; CE—contrast enhancement; EPI—echo planar imaging; Gd—gadolinium; GRE—gradient-recalled echo; NA—information not available; PET—positron emission tomography; QCA—quantitative coronary angiography; SE—spin-echo; SLP—signal intensity upslope; SPECT—single-photon emission CT; TPK—time to peak contrast enhancement. (From Arai and Hsu [41]; with permission.)
Assessment of myocardial viability
CMR can assess myocardial viability by two different methods: 1) a special technique of “late gadolinium enhancement” (LGE) imaging or 2) evaluation of functional reserve using low-dose dobutamine.
LGE imaging is unique to CMR and is based on differential uptake of gadolinium-based contrast agents between normal and infarcted myocardium. When administered intravenously, gadolinium accumulates within the expanded extracellular space of infarcted regions, providing signal enhancement of necrotic areas on T1-weighted images. Because maximal signal gain is at 10 to 20 minutes after administration, imaging at this delayed time point provides the greatest tissue contrast by using an inversion pulse sequence.
The second method of evaluating functional reserve is by using a protocol analogous to that employed in dobutamine stress echocardiography in which a biphasic response of myocardial thickening to low- and high-dose dobutamine indicates viability.
Infarction and viability by LGE
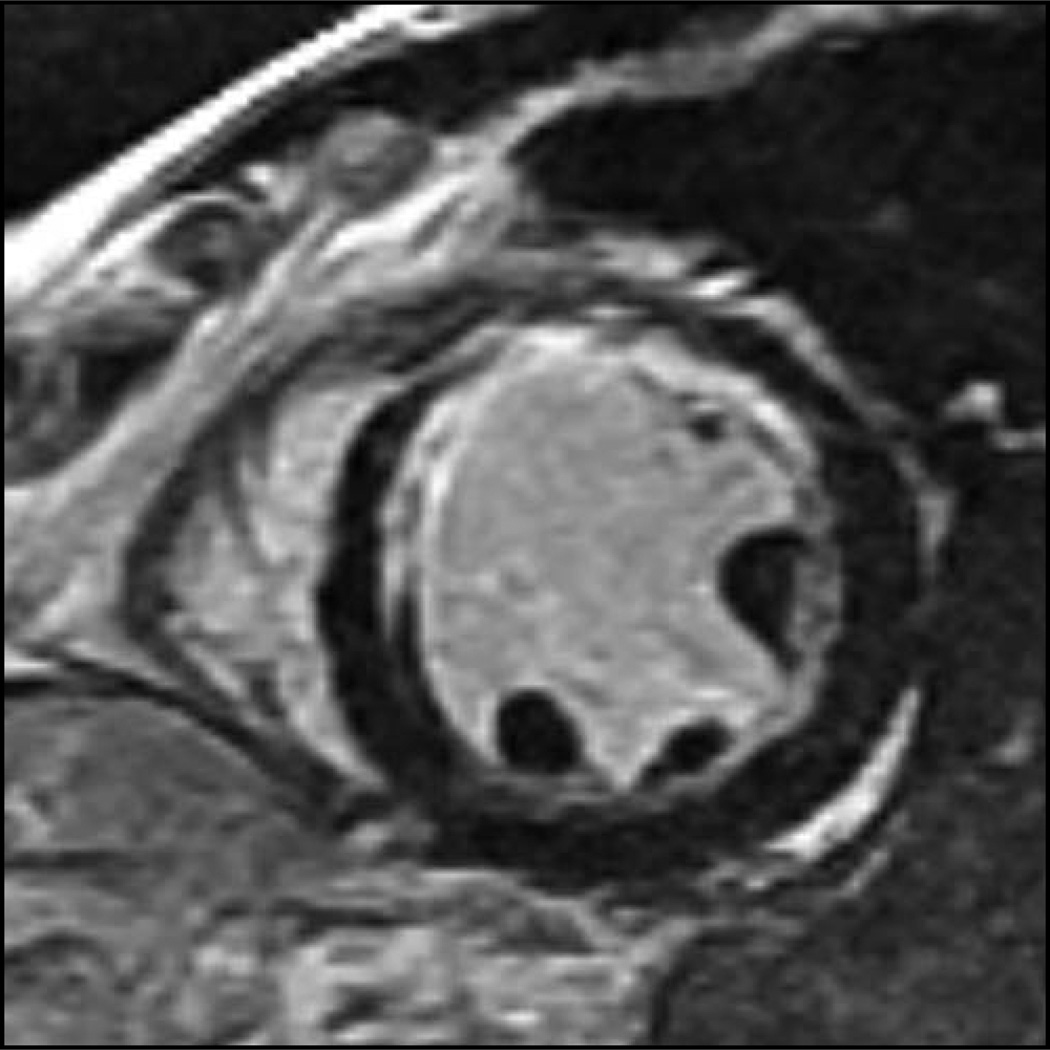
The LGE technique has proved to be highly sensitive in detecting the presence of myocardial scars from CAD. This high sensitivity is mainly attributable to the in-plane resolution of 1 to 2 mm and high contrast–noise ratio of this technique (Fig. 1). LGE was able to detect small regions of infarct that were not visualized by cine wall motion CMR imaging [12]. It has been estimated that the transmural extent of infarct has to be greater than 50% of wall thickness to result in discernable wall motion abnormalities. Moreover, LGE has been shown to detect subendocardial infarcts that were missed by single-photon emission CT (SPECT) [13,14]. It has also been shown to be able to detect microinfarcts in patients who developed mild elevations of serum creatine kinase after percutaneous coronary intervention [15]. Recently, the detection of clinically unrecognized infarcts by LGE has been shown to portend an adverse prognosis [16•].
In addition to the sensitive detection of infarcts, LGE can be used to assess myocardial viability based on the degree of transmural extent of the infarction. Kim et al. [17] were the first to demonstrate the histopathologic correlation of regions of LGE to irreversibly injured myocardium in a canine model of infarction. In a landmark clinical study, this group went on to report the utility of quantifying the degree of transmural involvement of infarction by LGE in predicting functional recovery after revascularization [18]. A progressive, stepwise decrease in the likelihood of function recovery for a given segment was observed as the transmural extent of myocardial scar detected by LGE increased. In this study, if the infarct enhancement spanned more than 75% of the left ventricular wall thickness, it was found to be highly indicative of nonviability, with a less than 1% chance of recovering function. It was further reported that the prediction of segmental functional recovery was even stronger in segments with resting akinesia or dyskinesia. LGE has been compared favorably with PET imaging in patients with severe global left ventricular dysfunction [13,19]. LGE CMR was shown not only to correlate closely with areas of decreased flow and metabolism on PET but also to be more sensitive in detecting endocardial infarction missed by PET. Subsequent studies have further confirmed the utility of LGE in predicting reversible myocardial dysfunction after revascularization [18,20,21]. Because of the extensive validation data available for this technique, many now consider LGE imaging to be the new gold standard technique, superseding the role of PET, for viability assessment.
Figure 1.
Cardiac magnetic resonance late gadolinium enhancement technique, which delineates the myocardial extent of infarction at high spatial resolution and contrast–noise ratio.
Functional reserve with low-dose dobutamine
Improvement in regional wall motion abnormalities in response to low-dose (5–10 µg/kg/min) dobutamine challenge has been well validated for viability assessment from the vast body of evidence from echocardiography as well as from CMR [22–24]. When compared with PET, this technique showed a high sensitivity of 88% and specificity of 87% for detecting viable myocardial segments in patients with mild left ventricular dysfunction [25]. However, this technique has been shown to have limited specificity, in the range of 50% to 70%, with segments exhibiting resting akinesia or dyskinesia [24,26]. This is attributed to the fact that in the presence of severe coronary stenosis and hypoperfusion, viable myocardial segments may fail to demonstrate contractile reserve with low-dose dobutamine because of rapid development of ischemia [27]. These findings suggest that low-dose dobutamine CMR may be inferior to the LGE technique in viability assessment of patients with low left ventricular function. However, several studies showed that this may not be the case. Motoyasu et al. [28] found low-dose dobutamine CMR to have a superior receiver operating characteristic (ROC) over the LGE technique (ROC area under the curve of 0.87 vs 0.78), whereas Wellnhofer et al. [29] further showed that low-dose dobutamine CMR is particularly useful in predicting functional recovery in myocardial segments with transmural involvement by LGE of less than 75%. Similarly, Kaandorp et al. [30] showed that low-dose dobutamine may improve viability assessment in patients with an intermediate degree of transmural involvement by LGE. Taken together, these data suggest that these two techniques are complementary and that the highest sensitivity and specificity in predicting viability may be achieved by combining them.
Assessment of coronary artery stenosis
CMR to detect coronary artery stenosis has great appeal because it may obviate the need for stress testing or invasive coronary angiography. Although considerable progress has been made in this area, reliable detection of luminal narrowing by CMR remains technically demanding because of the small size and tortuous course of the coronary arteries and their complex motion, caused by cardiac contraction and respiration. In addition, high-submillimeter spatial resolution and large-volume coverage of the coronary artery trees are required for this application.
The reported accuracy of earlier studies using two-dimensional coronary CMR angiography for predicting coronary artery stenosis is highly variable, with sensitivity and specificity ranging widely from 50% to 90%. Currently, 3D coronary MR angiography is the most commonly used technique for the assessment of coronary arteries. The overall approach is somewhat analogous to CT angiography, with delineation of the superior and inferior bounds of the heart to define a single imaging volume that includes both coronary arteries.
One recent study showed a per-patient sensitivity of 82% and specificity of 90%, with an overall accuracy of 87% [31]. The segment negative predictive value was 98%. These impressive results are superior to those of a multicenter study [32]. In the latter study, the coronary MR angiography showed high sensitivity (93%) but low specificity (42%) for identifying a patient with significant CAD. However, in the subgroup of patients with left main CAD or three-vessel disease, CMR demonstrated both high sensitivity and high specificity: 100% and 85%, respectively.
This new generation of 3D CMR angiography has been compared with 16-slice CT and found to have comparable diagnostic accuracy [33]. Although there is no published study directly comparing 64-slice CT and 3D CMR angiography, the overall image quality and diagnostic accuracy of the former technique appears to be superior based on clinical experience. Although CT angiography has a shorter scan time and overall better image quality, 3D CMR angiography has advantages in that it does not require ionizing radiation or nephrotoxic contrast and it can assess luminal stenosis despite the presence of significant coronary calcification (which may render a CT coronary angiography uninterpretable). CMR angiography may be used reliably to exclude significant proximal CAD and to delineate an anomalous course of coronary arteries. This technique might have advantages over CT in screening for CAD in subjects with low likelihood of CAD because CMR does not expose subjects to ionizing radiation.
Multifaceted approach of CMR in CAD
CMR is a versatile and powerful diagnostic tool with a full complement of techniques to assess global and regional function, perfusion, viability, and even the coronary arteries. Moreover, an examination can be tailored to address the specific needs of an individual patient. In such an evaluation, LGE imaging likely plays a central role because of its high sensitivity in detecting unrecognized infarction without the need for stress testing. For example, LGE alone or combined with CMR angiography may be used to reliably exclude ischemic heart disease in a patient with dilated cardiomyopathy and heart failure who is deemed too unstable to undergo stress testing.
On the other hand, in a stable patient being considered for coronary revascularization, LGE may be performed in conjunction with dobutamine stress to maximize the accuracy of viability assessment. Recently, a multimodality approach incorporating elements of cine function, LGE, and stress perfusion imaging has been proposed to increase the accuracy of CAD detection [34]. With this approach, perfusion defects that have similar intensity and extent during both stress and rest (“fixed defect”) without exhibiting LGE are considered to be artifacts, thereby improving the specificity of the test.
Other similar strategies have been employed for identifying CAD in settings such as the emergency room [35] or in non–ST segment elevation acute coronary syndromes [36] using cine function, LGE imaging, and CMR coronary angiography [36]. Diagnostic algorithms using the multimodality approach afforded by CMR are becoming increasingly useful. Finally, as a new generation of high-field (3-Tesla) imaging becomes clinically adopted, advances of CMR in the area of CAD evaluation are anticipated in the near future.
Emerging applications
CMR may have a unique ability over other imaging techniques in differentiating between acute and chronic MIs. This differentiation is possible by detecting myocardial edema associated with acute infarct using T2-weighted imaging [37]. T2 relaxation is greatly enhanced by the physical mobility of protons associated with water molecules, thereby increasing signal intensities within edematous regions. Because edema resolves about 1 to 2 months after the occlusive event, its presence represents an acute rather than chronic infarct. In addition, myocardium with abnormal T2 relaxation has been shown to correlate with area at risk as determined by microsphere determinations in a canine model [38]. Combined with LGE, T2-weighted imaging has the potential to determine the amount of myocardium in jeopardy as the difference between area at risk and area of necrosis (LGE).
In addition to infarct sizing, CMR has the ability to characterize tissue properties of the infarcted myocardium. Using the gadolinium-enhanced method of infarct imaging, Yan et al. [39••] reported on the feasibility of characterizing tissue heterogeneity within the infarct zone in humans and demonstrated the adverse impact on mortality of the “peri-infarct zone.” It is well known that infarct tissue heterogeneity is related to ventricular arrhythmia development in animal models, and recently it was shown to be associated with inducibility of ventricular tachycardia in humans [40•]. Together, these findings suggest that tissue characterization by CMR may serve as a novel tool for arrhythmia risk assessment after MI.
Footnotes
Disclosures
Neither author has a possible conflict of interest, financial or otherwise.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Barkhausen J, Ruehm SG, Goyen M, et al. MR Evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219:264–269. doi: 10.1148/radiology.219.1.r01ap12264. [DOI] [PubMed] [Google Scholar]
- 2.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 3.Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 4. Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. This article reports the large clinical experience to date regarding the prognostic impact of stress CMR imaging by either dobutamine or vasodilating stress agents in a cohort with intermediate pretest likelihood of significant CAD. It highlights the excellent negative predictive value of CMR stress imaging in predicting long-term events, including cardiac death and acute MI.
- 5.Rerkpattanapipat P, Morgan TM, Neagle CM, et al. Assessment of preoperative cardiac risk with magnetic resonance imaging. Am J Cardiol. 2002;90:416–419. doi: 10.1016/s0002-9149(02)02501-8. [DOI] [PubMed] [Google Scholar]
- 6.Wahl A, Paetsch I, Roethemeyer S, et al. High-dose dobutamine-atropine stress cardiovascular MR imaging after coronary revascularization in patients with wall motion abnormalities at rest. Radiology. 2004;233:210–216. doi: 10.1148/radiol.2331030463. [DOI] [PubMed] [Google Scholar]
- 7.Paetsch I, Jahnke C, Wahl A, et al. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835–842. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 8.Cheng ASH, Pegg TJ, Karamitsos TD, et al. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440–2449. doi: 10.1016/j.jacc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance : a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 10.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 11.Giang TH, Nanz D, Coulden R, et al. Detection of coronary artery disease by magnetic resonance myocardial perfusion imaging with various contrast medium doses: first European multi-centre experience. Eur Heart J. 2004;25:1657–1665. doi: 10.1016/j.ehj.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Mahrholdt H, Wagner A, Parker M, et al. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol. 2003;42:505–512. doi: 10.1016/s0735-1097(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 13.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim T, Bulow HP, Hackl T, et al. Diagnostic value of contrast-enhanced magnetic resonance imaging and single-photon emission computed tomography for detection of myocardial necrosis early after acute myocardial infarction. J Am Coll Cardiol. 2007;49:208–216. doi: 10.1016/j.jacc.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 16. Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. This study investigated the clinical outcome of 195 patients without a history of clinical MI who were referred for CMR assessment of coronary artery disease. The authors found the association of LGE with adverse cardiovascular outcomes to be stronger than that of clinical markers including ECG evidence of MI and left ventricular function.
- 17.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 18.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 19.Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 20.Knuesel PR, Nanz D, Wyss C, et al. Characterization of dysfunctional myocardium by positron emission tomography and magnetic resonance: relation to functional outcome after revascularization. Circulation. 2003;108:1095–1100. doi: 10.1161/01.CIR.0000085993.93936.BA. [DOI] [PubMed] [Google Scholar]
- 21.Choi KM, Kim RJ, Gubernikoff G, et al. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 22.Baer FM, Theissen P, Schneider CA, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040–1048. doi: 10.1016/s0735-1097(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 23.Dendale PAC, Franken PR, Waldman G-J, et al. Low-dosage dobutamine magnetic resonance imaging as an alternative to echocardiography in the detection of viable myocardium after acute infarction. Am Heart J. 1995;130:134–140. doi: 10.1016/0002-8703(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 24.Sandstede JJW, Bertsch G, Beer M, et al. Detection of myocardial viability by low-dose dobutamine cine MR imaging. Magn Reson Imaging. 1999;17:1437–1443. doi: 10.1016/s0730-725x(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 25.Baer FM, Voth E, Schneider CA, et al. Comparison of low-dose dobutamine–gradient-echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease: a functional and morphological approach to the detection of residual myocardial viability. Circulation. 1995;91:1006–1015. doi: 10.1161/01.cir.91.4.1006. [DOI] [PubMed] [Google Scholar]
- 26.Gunning MG, Anagnostopoulos C, Knight CJ, et al. Comparison of 201Tl, 99mTc-tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation. 1998;98:1869–1874. doi: 10.1161/01.cir.98.18.1869. [DOI] [PubMed] [Google Scholar]
- 27.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 28.Motoyasu M, Sakuma H, Ichikawa Y, et al. Prediction of regional functional recovery after acute myocardial infarction with low dose dobutamine stress cine MR imaging and contrast enhanced MR imaging. J Cardiovasc Magn Reson. 2003;5:563–574. doi: 10.1081/jcmr-120025233. [DOI] [PubMed] [Google Scholar]
- 29.Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation. 2004;109:2172–2174. doi: 10.1161/01.CIR.0000128862.34201.74. [DOI] [PubMed] [Google Scholar]
- 30.Kaandorp TAM, Bax JJ, Schuijf JD, et al. Head-to-head comparison between contrast-enhanced magnetic resonance imaging and dobutamine magnetic resonance imaging in men with ischemic cardiomyopathy. Am J Cardiol. 2004;93:1461–1464. doi: 10.1016/j.amjcard.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma H, Ichikawa Y, Chino S, et al. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48:1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 32.Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 33.Kefer J, Coche E, Legros G, et al. Head-to-head comparison of three-dimensional navigator-gated magnetic resonance imaging and 16-slice computed tomography to detect coronary artery stenosis in patients. J Am Coll Cardiol. 2005;46:92–100. doi: 10.1016/j.jacc.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 35.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 36.Plein S, Greenwood JP, Ridgway JP, et al. Assessment of non–ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2173–2181. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 38.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 39. Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. This single-center study illustrates that because of excellent spatial resolution and contrast-to-noise ratio, CMR has the potential to characterize and even quantify tissue components of the peri-infarct zone, which in this study was found to have marked association with post-MI mortality after adjusting for clinical factors and left ventricular systolic function.
- 40. Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. This study supports the clinical findings of reference [39••] but further reports the arrhythmogenic potentials of the peri-infarct zone characterized by CMR.
- 41.Arai AE, Hsu L-Y. Myocardial perfusion using first-pass gadolinium-enhanced cardiac magnetic resonance. In: Kwong RY, editor. Cardiovascular Magnetic Resonance. Totowa, NJ: Humana Press; 2007. p. 311. [Google Scholar]