Abstract
Cardiovascular MRI has effectively become a reference standard for quantifying ventricular volumes and function and for measuring the myocardial scar burden after myocardial infarction. Imaging of late gadolinium enhancement and microvascular obstruction carries strong prognostic information for identifying patients who would benefit from anti-remodeling therapy. The combination of gadolinium enhancement, perfusion, and cine imaging should make MRI the modality of choice in the assessment of left ventricular dysfunction and remodeling. The use of MRI in clinical trials of heart failure could help reduce sample size requirements because of its accuracy and reproducibility. This review describes the use of MRI in assessing ventricular remodeling and viability and summarizes the few studies that have relied on MRI for image-based markers of ventricular remodeling.
Introduction
MRI has effectively become a reference standard for the assessment of ventricular volumes and ventricular function, thereby providing an accurate tool to monitor ventricular remodeling. Ventricular remodeling, in the general sense of chanages in ventricular function and structure, can have many causes, including adaptations to high-intensity endurance training [1], myocardial injury, and loss of myocardial viability from coronary heart disease [2]. A consensus panel has defined adverse ventricular remodeling as the “genomic expression resulting in molecular, cellular and interstitial changes that are manifested clinically as changes in size, shape and function of the heart after cardiac injury” [3]. The prevention of adverse remodeling after myocardial infarction represents one of the most important challenges in clinical cardiology [2]. An accurate quantitative assessment of ventricular remodeling is therefore of utmost importance for determining prognosis and gauging the effectiveness of therapeutic interventions. Ventricular remodeling is most frequently characterized in terms of the changes in measures relating to ventricular shape and function, but novel MRI approaches can add further histologic markers of structural alterations that initiate or accompany ventricular remodeling. For example, contrast-enhanced MRI has proven to be a highly precise method of determining the location, extent, and transmurality of myocardial scar, which provides important prognostic information in patients at risk for developing heart failure or hard cardiac events [4].
Assessment of ventricular volume and function and myocardial viability can be performed routinely and efficiently with MRI, yielding results that are highly observer- and operator-independent if standard protocols are followed. Should MRI therefore be the modality of choice for assessing ventricular remodeling and viability? This review describes important advantages of MRI for studying ventricular remodeling and summarizes current limitations. Beyond their application as an imaging modality using the signal from the 1H nucleus, magnetic resonance techniques are of considerable benefit for measuring derangements in cardiac high-energy phosphate metabolism by 31P spectroscopy to obtain markers of tissue injury and metabolic indicators of the extent of ventricular remodeling [5–7]. These spectroscopic applications are beyond the scope of this review.
Ventricular Volume, Shape, and Function
Adverse left ventricular (LV) remodeling following myocardial infarction increases the risk of heart failure and death. Changes in ventricular geometry and ventricular function can reflect adverse remodeling but are not the sole markers of adverse remodeling. Tomographic imaging modalities such as MRI and CT provide the ability to obtain complete, three-dimensional representations of the ventricle for a user-defined number of cardiac phases. The data sets have four dimensions: three variables define the spatial location of each voxel in each image, and the time stamp for each data item defines the cardiac phase. Time is generally measured relative to a fiducial event in the cardiac cycle such as the peak of the R wave on the electrocardiogram if electrocardiographic triggering is used to acquire the data. With MRI, stacks of contiguous slices in a short-axis orientation, covering the heart from base to apex, provide complete spatial information for each cardiac phase.
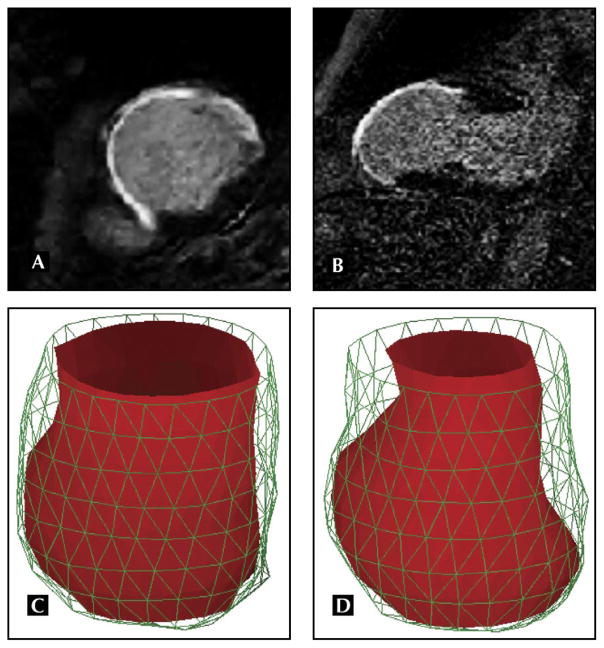
A fully sampled four-dimensional data set covering the entire volume of the heart allows one to calculate ventricular dimensions and volumes in each cardiac phase without reliance on any models of ventricular geometry. This represents a significant advantage for patients with abnormal ventricular geometry and chamber distortion as a result of remodeling. Figure 1 shows an example of a three-dimensional model of the left ventricle built from a four-dimensional MRI cine data set. The extensive remodeling of the left ventricle in the area with scar, reflected in thinning and bulging of the left ventricle, has led in this case to dyskinetic wall motion in the scar area: the thinned wall moves outward during systole, and viable areas still show contractile motion. Extensive dyskinesis can render it difficult to determine the timing of end systole if only a few two-dimensional views are used to assess ventricular volume and function. This problem is circumvented with a complete three-dimensional model, because end systole and end diastole are, for the purpose of quantifying ventricular function, defined as the phases with minimal and maximal ventricular volume, respectively. In the presence of mitral regurgitation, stroke volumes can be accurately estimated by performing phase contrast blood flow velocity measurements above the aortic valve.
Figure 1.
MRI with late gadolinium enhancement in the short-axis (A) and two-chamber (B) views shows precisely the extent of nonviable ventricular wall and the pronounced wall thinning relative to the nonenhancing wall segments. Cine MRI for a stack of slices in the short-axis orientation from base to apex was performed in the same patient to construct a three-dimensional finite-element model of the left ventricle for each cardiac phase, shown for end systole (C) and end diastole (D). The three-dimensional model of this failing heart shows marked bulging of the infarcted ventricular wall relative to the remaining contracting wall segments near the base of the left ventricle. With MRI, images of regional function, viability, and perfusion can be exactly registered to a common coordinate system by matching slice prescriptions.
Although MRI cine imaging is the gold standard for quantifying ventricular size and function, there are continuing efforts to improve the protocols and techniques to avoid some pitfalls. For example, its accuracy can be compromised by poor delineation of the endocardial and epicardial borders due to gating problems or poor cardiac function, registration errors between slices acquired during different breath-holds, partial volume effects related to large slice thickness, and image artifacts from implanted devices. At the basal level of the left ventricle, two-dimensional images in the short-axis view should only be included in the ventricular volume estimate if the image slice lies below the mitral valve plane. Because the slices have a thickness, it is always possible that some left atrial volume is inadvertently included (partial volume effect). The location of the mitral valve plane is not well defined if only short-axis views are used. The location of the mitral valve plane is best ascertained by including long-axis views for the analysis of ventricular volume and function. Partial volume effects can be reduced by imaging thinner slices. Although the need for more slices implies that the examination time is longer, parallel imaging using phased arrays of receiver coils can accelerate the acquisition, and with imaging acceleration multiple slices can be imaged during each breath-hold. Finally, cardiac gating has become more reliable with the vectorcardiographic gating methods.
Ventricular volumes are calculated using cardiac image analysis programs that provide the following features:
Tools for tracing of the endocardial contours at each slice level,
Capabilities for automatic segmentation,
Correction of computer-generated contours, and
Algorithms for calculating the global ventricular volumes from the volume in each slice using, for example, Simpson’s rule to add up the ventricular volume in each slice.
The analysis is predominantly based on the use of images in the short-axis orientation. The curvature of the LV wall perpendicular to the short-axis views is often taken into account to further reduce partial volume effects, in this case near the endocardial border.
The sharpness of the border and the differential contrast between blood pools and myocardium at the endocardial border determine the feasibility of automating the segmentation of the images [8]. Definition of the endocardial border can be improved by choosing magnetic resonance cine techniques with steady-state free precession, which makes blood appear brighter than in conventional cine imaging, particularly in patients with poor ventricular function. Older cine MRI methods based on gradient echo techniques relied more on blood flow into the slice plane for the endocardial border definition and therefore did not work well in patients with poor ventricular function. Other alternatives include intravascular contrast agents [9] or extracellular contrast agents [10] to enhance the signal from blood independently of flow while the signal from myocardium remains relatively low.
The accuracy of the MRI technique (ie, its ability to give volume estimates that are close to the true volume) was ascertained by comparison with water-submerged latex casts of human hearts [11] and comparison of ventricular mass with the mass measured at the time of autopsy [12] and also in heart phantoms [13]. Although the accuracy of MRI in measuring ventricular cavity volumes cannot be directly determined because it is difficult to reproduce the loading conditions of the heart ex vivo, evidence of accuracy from measuring myocardial volume may be an effective surrogate. Reproducibility (ie, the consistency of repeated measurements in the same persons) was assessed in healthy volunteers [14] and patients with compromised LV function [15]. Not surprisingly, interstudy reproducibility was better in healthy volunteers than in patients with heart failure, with coefficients of variability (SD of difference between measurements, divided by the mean of measurements) of 2.9% for end-diastolic LV volume, 6.5% for end-systolic LV volume, 3.9% for stroke volume, and 2.8% for LV mass, all of which were at least 50% lower than the values obtained by two-dimensional echocardiography in the same volunteers [15]. In patients with heart failure, MRI maintained a similar margin of superiority over two-dimensional echocardiography. Bellenger et al. [16] argued that the excellent reproducibility of cardiac cine MRI could be taken advantage of to reduce sample size in clinical trials. In patients with heart failure, they calculated sample sizes of 12 to detect a 10 mL change in end-diastolic LV volume, 10 to detect a 10 mL change in end-systolic volume, 15 to detect a 3% change in ejection fraction, and 9 to detect a 10 g change in mass, all substantially smaller than recently published values for two-dimensional echocardiography (reduction, 81%–97%) [16]. As evidence that these estimates appaear to be realistic, we point out, as an example, a randomized study in 41 patients by Groenning et al. [17] that demonstrated with cine MRI that the β-blocker metoprolol CR/XL has anti-remodeling effects on the left ventricle in patients with chronic heart failure, a result that is consistent with the highly significant decrease in mortality from worsening heart failure found in the Metoprolol Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF), which included nearly 4000 patients.
The most common measures quantifiable by cardiac cine MRI are the ventricular dimensions, volumes, and myocardial mass and derived quantities such as ejection fraction, stroke volume, and cardiac output. Additional measures relate to the shape of the ventricle, such as the ratio of the long axis to the short axis, which plays an important role in the pathogenesis of mitral regurgitation [18]. Another measure of LV shape distortion that was found useful in studies of patients with heart failure is the sphericity index, defined as the ratio of the major axis to the minor axis of the left ventricle [19].
Wall Stress and Strain
Wall stress plays an important role in ventricular remodeling, both as a powerful stimulus for remodeling and as an indicator of the adverse effects of ventricular remodeling, which can result in a significant increase of wall stress [20]. Estimates of regional wall stress require determination of wall thickness and wall curvature, highly accurate measures of which are accessible by cine MRI. Although the use of MRI for structural measurements of the left ventricle is well accepted, approaches diverge considerably for estimating wall stress. An average measure of wall stress [21,22] can be obtained from the modified Laplace relation, using the LV midwall thickness (t) and the lengths of the major (a) and minor (b) semi-axes together with an estimate of the transmural pressure (P): σ = [(P × b)/t] × [1 − (t/2b)] × {1 − [(t × b)/2a2]}.
Regional peak systolic wall stress in the radial direction (σr) can be determined following Grossman et al. [23] by measurement of the inner radius of the left ventricle (R) and wall thickness (t) at end systole: σr = (0.133 × SP × R)/{2t × [1 + (t/2R)]}, where SP is peak systolic ventricular blood pressure in millimeters of mercury, and the final result for wall stress is expressed in 108 N/cm2. Alternatively, finite element models can be loaded with pressure to obtain numerical solutions for the regional stress by treating the myocardium as a linearly elastic and isotropic material [24]. In healthy volunteers, the principal stress component was estimated by finite element analysis to be highest near the base of the heart; it decreases by about 40% toward the apex of the heart, even without accounting for a ventricular pressure gradient from base to apex [24].
Ventricular dilatation increases the radius of curvature and is accompanied by wall thinning, and both of these changes increase wall shear stress. Blom et al. [25•] used MRI to demonstrate the benefits of a ventricular constraint device that provided passive mechanical diastolic support to curb ventricular remodeling and reduce ventricular wall stress in sheep with myocardial infarction.
Myocardial Viability
Ventricular remodeling after myocardial infarction increases in severity with the magnitude of the infarction. The location and extent of nonviable tissue represent important prognostic information. Furthermore, processes such as infarct expansion and infarct resorption [26] require a technique with high spatial resolution and accuracy for monitoring postinfarction remodeling.
Under normal conditions, gadolinium contrast agents used with MRI remain confined to the extracellular space. With myocardial infarction, the distribution volume for gadolinium contrast is significantly expanded and reaches 60% to 70% in myocardial scar tissue. As early as 3 or 4 minutes after administration of gadolinium contrast, one can reliably detect nonviable myocardium by the focal contrast enhancement relative to remote areas. To render the signal from scar as conspicuous as possible, one adjusts the contrast parameters of the pulse sequence so that the signal from normal myocardium is suppressed [27]. The MRI technique for detection of late gadolinium enhancement (LGE) is now well established and allows scanning for LGE over the entire heart in fewer than 10 minutes. It has therefore become feasible with cardiovascular MRI to track early infarct expansion, followed by infarct resorption, scar formation, and wall thinning [28].
Kim et al. [4] demonstrated in a landmark study that the likelihood of improvement in regional contractility over 2 to 3 months after revascularization decreased in inverse proportion to the transmural extent of LGE before revascularization. The transmural extent of LGE was found to be a good predictor of the improvement of LV function caused by β-blocker therapy in patients with heart failure [29]. Orn et al. [30] found that scar size determined by MRI of LGE was the strongest independent predictor of ejection fraction and LV volumes in patients with acute myocardial infarction and signs or symptoms of heart failure. The authors concluded that minimization of scar size is critical to preventing adverse remodeling independent of the location of the scar. Even in patients with suspected coronary artery disease but without a history of myocardial infarction, LGE involving a small amount of myocardium carries a high cardiac risk [31]. This includes patients with normal global LV function and few signs of ventricular remodeling.
LGE MRI was shown to have significantly better sensitivity, specificity, predictive values, and accuracy than resting thallium-201 single-photon emission computed tomography (201Tl SPECT) in the prediction of regional myocardial viability [32]. The interstudy reproducibility of scar size measurements repeated within 20 minutes after a single contrast injection was tested in patients with chronic infarcts using contrast-enhanced MRI and 201Tl SPECT [33]. The coefficient of repeatability (1.95 × SD of difference) for scar size averaged ±2.4% LV with MRI, compared with ±4.0% LV with 201Tl SPECT. The authors concluded that if infarct size is chosen as an end point for a trial, the study cohort for an MRI-based trial needs to reach only 42% of the cohort size of a SPECT-based study. Thiele et al. [34] reported similar results for the limits of agreement (1.95 × SD = ±2.4% LV) between repeated studies (but performed on separate days and with separate contrast injections) in patients with acute or chronic myocardial infarctions.
The prognostic value of LGE can be further enhanced by considering the early phases of contrast enhancement during the first pass of the contrast agent to determine the degree of microvascular obstruction (MO) after revascularization. Wu et al. [35] showed that the presence of MO was a powerful prognostic marker of postinfarction complications, with independent adjustment for infarct size. The degree of regional adverse remodeling after primary angioplasty for acute myocardial infarction was significantly worse in myocardial segments with MO than in infarcted segments detected with LGE but without MO [36•].
Limitations
MRI has some important limitations, including scan time and the resultant cost of the study. However, cost is expected to fall with more widespread use of this clinical technology. Magnetic resonance scanner technology has evolved to the point at which ventricular shape and function can be assessed in fewer than 10 minutes using parallel imaging techniques with phased-array receiver coils. Quantification of scar extends the examination time by no more than another 10 minutes. Therefore, the cost of using an MRI scanner can be considered of secondary importance if imaging protocols are efficiently implemented with the latest generation of MRI scanners.
About 4% of patients suffer from claustrophobia, making MRI in conventional systems with cylindrical bores unsuitable without sedation of the patient. Newer, open systems with magnetic field strengths of up to 1 T can effectively address this problem while still providing sufficient image quality for cine MRI studies. MRI is poorly tolerated by patients with orthopnea, but MRI systems that allow the patient to stand or sit during the examination are not practical or available.
Almost all cardiac MRI protocols require cardiac gating using electrocardiography or pulse oximetry. MRI is difficult in patients who are not in regular sinus rhythm. However, real-time imaging techniques recently have become feasible, and further development of imaging acceleration techniques may make it possible to routinely obtain cine images in patients who are not in a regular sinus rhythm.
Cardiac devices such as pacemakers and implanted defibrillators have precluded MRI at most institutions, although studies suggest that the criteria could be relaxed with modern pacemakers, given proper pre-MRI and post-MRI interrogation of the device [37]. Furthermore, device manufacturers are trying to develop MRI-compatible and MRI-safe cardiac devices.
Recent reports that gadolinium contrast may trigger nephrogenic systemic fibrosis (NSF) in patients with kidney disease have led to restrictions on the use of gadolinium contrast in patients with renal disease. Of the many gadolinium-enhanced MRIs performed worldwide in the past decade, NSF only has been reported primarily in a few patients with preexisting severe renal dysfunction (estimated glomerular filtration rate < 30 mL/min). In addition, although all gadolinium-based contrast agents can cause NSF, registry information indicates that most of the patients who developed NSF received gadodiamide, one of several available gadolinium contrast agents, prior to the development of symptoms. The mechanisms leading to systemic sclerosis after gadodiamide exposure are not well understood, and the number of cases has been too low to observe statistical differences in the incidence of NSF with different contrast agents. It is currently recommended that 1) an individualized decision weighing the risk–benefit ratio be applied in patients with renal conditions who have an absolute indication for gadolinium-enhanced MRI studies, 2) strong consideration be given to eliminating or reducing the amount of gadolinium-based contrast, and 3) dialysis be performed within 24 hours and repeated later in patients with end-stage renal disease who receive gadolinium-based contrast agents.
CT and three-dimensional echocardiography may compete with MRI for the study of ventricular remodeling and viability. CT can be performed on patients with implanted devices, and claustrophobia is generally not a concern for CT examinations. Its drawbacks are significant radiation exposure, particularly with frequent follow-up studies, and the fact that the temporal resolution for cine studies is generally not nearly as high as achievable with MRI. CT has been used to assess myocardial viability, although initial evidence suggests that scar conspicuity is better with the dynamic contrast-enhanced MRI technique [38]. Three-dimensional echocardiography is well suited for model-independent assessment of ventricular shape and function, as with MRI and CT, and therefore for overcoming a limitation of two-dimensional echocardiography. The extent of myocardial scarring cannot be assessed well with echocardiography.
Conclusions
There is substantial evidence to support the use of cardiac MRI as a versatile and accurate imaging modality to assess LV remodeling. In addition, imaging of LGE carries strong prognostic information for identifying patients who would benefit from anti-remodeling therapy. In patients without a hazardous device or contraindications to MRI, the combination of LGE imaging and cine imaging would make MRI the modality of choice in the assessment of LV dysfunction and heart failure.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Pelliccia A. Athlete’s heart and hypertrophic cardiomyopathy. Curr Cardiol Rep. 2000;2:166–171. doi: 10.1007/s11886-000-0015-4. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich J, Apstein CS, Ingwall JS. 31P nuclear magnetic resonance spectroscopic imaging of regions of remodeled myocardium in the infarcted rat heart. Circulation. 1995;92:3527–3538. doi: 10.1161/01.cir.92.12.3527. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S. Cardiac magnetic resonance spectroscopy: potential clinical applications. Herz. 2000;25:452–460. doi: 10.1007/s000590050037. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Wilke N, Wang Y, et al. Functional and bio-energetic consequences of postinfarction left ventricular remodeling in a new porcine model. MRI and 31 P-MRS study. Circulation. 1996;94:1089–1100. doi: 10.1161/01.cir.94.5.1089. [DOI] [PubMed] [Google Scholar]
- 8.Francois CJ, Fieno DS, Shors SM, Fin JP. Left ventricular mass: manual and automatic segmentation of true FISP and FLASH cine MR images in dogs and pigs. Radiology. 2004;230:389–395. doi: 10.1148/radiol.2302020761. [DOI] [PubMed] [Google Scholar]
- 9.Stillman AE, Wilke N, Jerosch-Herold M. Use of an intravascular T1 contrast agent to improve MR cine myocardial-blood pool definition in man. J Magn Reson Imaging. 1997;7:765–767. doi: 10.1002/jmri.1880070425. [DOI] [PubMed] [Google Scholar]
- 10.Pennell DJ, Underwood SR, Longmore DB. Improved cine MR imaging of left ventricular wall motion with gadopentetate dimeglumine. J Magn Reson Imaging. 1993;3:13–19. doi: 10.1002/jmri.1880030104. [DOI] [PubMed] [Google Scholar]
- 11.Rehr RB, Malloy CR, Filipchuk NG, Peshock RM. Left ventricular volumes measured by MR imaging. Radiology. 1985;156:717–719. doi: 10.1148/radiology.156.3.4023232. [DOI] [PubMed] [Google Scholar]
- 12.Shors SM, Cotts WG, Pavlovic-Surjancev B, et al. Heart failure: evaluation of cardiopulmonary transit times with time-resolved MR angiography. Radiology. 2003;229:743–748. doi: 10.1148/radiol.2293021363. [DOI] [PubMed] [Google Scholar]
- 13.Debatin JF, Nadel SN, Paolini JF, et al. Cardiac ejection fraction: phantom study comparing cine MR imaging, radionuclide blood pool imaging, and ventriculography. J Magn Reson Imaging. 1992;2:135–142. doi: 10.1002/jmri.1880020205. [DOI] [PubMed] [Google Scholar]
- 14.Sechtem U, Pflugfelder PW, Gould RG, et al. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697–702. doi: 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- 15.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 16.Bellenger NG, Davies LC, Francis JM, et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 17.Groenning BA, Nilsson JC, Sondergaard L, et al. Antire-modeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36:2072–2080. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 18.Kono T, Sabbah HN, Rosman H, et al. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–1598. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 19.Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. Am J Cardiol. 1989;63:1167–1173. doi: 10.1016/0002-9149(89)90173-2. [DOI] [PubMed] [Google Scholar]
- 20.Mann DL. Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail Rev. 2005;10:95–100. doi: 10.1007/s10741-005-4636-y. [DOI] [PubMed] [Google Scholar]
- 21.Balzer P, Furber A, Delepine S, et al. Regional assessment of wall curvature and wall stress in left ventricle with magnetic resonance imaging. Am J Physiol. 1999;277:H901–H910. doi: 10.1152/ajpheart.1999.277.3.H901. [DOI] [PubMed] [Google Scholar]
- 22.DeAnda A, Jr, Komeda M, Moon MR, et al. Estimation of regional left ventricular wall stresses in intact canine hearts. Am J Physiol. 1998;275:H1879–H1885. doi: 10.1152/ajpheart.1998.275.5.H1879. [DOI] [PubMed] [Google Scholar]
- 23.Grossman W, Braunwald E, Mann T, et al. Contractile state of the left ventricle in man as evaluated from end-systolic pressure-volume relations. Circulation. 1977;56:845–852. doi: 10.1161/01.cir.56.5.845. [DOI] [PubMed] [Google Scholar]
- 24.Wollmuth JR, Bree DR, Cupps BP, et al. Left ventricular wall stress in patients with severe aortic insufficiency with finite element analysis. Ann Thorac Surg. 2006;82:840–846. doi: 10.1016/j.athoracsur.2006.03.100. [DOI] [PubMed] [Google Scholar]
- 25•.Blom AS, Mukherjee R, Pilla JJ, et al. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation. 2005;112:1274–1283. doi: 10.1161/CIRCULATIONAHA.104.499202. One of the first studies using MRI to demonstrate that a reduction of diastolic wall stress with a mechanical constraint device reduces adverse remodeling. [DOI] [PubMed] [Google Scholar]
- 26.Lund GK, Stork A, Muellerleile K, et al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology. 2007;245:95–102. doi: 10.1148/radiol.2451061219. [DOI] [PubMed] [Google Scholar]
- 27.Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–1910. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 28.Fieno DS, Hillenbrand HB, Rehwald WG, et al. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43:2124–2131. doi: 10.1016/j.jacc.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 30.Orn S, Manhenke C, Anand IS, et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99:1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 31.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. Published erratum appears in Circulation 2006, 114:e365. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa K, Sakuma H, Hirano T, et al. Acute myocardial infarction: myocardial viability assessment in patients early thereafter: comparison of contrast-enhanced MR imaging with resting (201)Tl SPECT. Single photon emission computed tomography. Radiology. 2003;226:138–144. doi: 10.1148/radiol.2261012108. [DOI] [PubMed] [Google Scholar]
- 33.Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. doi: 10.1161/01.cir.0000036368.63317.1c. [DOI] [PubMed] [Google Scholar]
- 34.Thiele H, Kappl MJ, Conradi S, et al. Reproducibility of chronic and acute infarct size measurement by delayed enhancement–magnetic resonance imaging. J Am Coll Cardiol. 2006;47:1641–1645. doi: 10.1016/j.jacc.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 35.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 36•.Baks T, van Geuns RJ, Biagini E, et al. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47:40–44. doi: 10.1016/j.jacc.2005.09.008. This study, following earlier work by Wu et al. [35], demonstrated the incremental prognostic value of microvascular obstruction for predicting a recovery of wall motion. [DOI] [PubMed] [Google Scholar]
- 37.Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brodoefel H, Klumpp B, Reimann A, et al. Late myocardial enhancement assessed by 64-MSCT in reperfused porcine myocardial infarction: diagnostic accuracy of low-dose CT protocols in comparison with magnetic resonance imaging. Eur Radiol. 2007;17:475–483. doi: 10.1007/s00330-006-0334-y. [DOI] [PubMed] [Google Scholar]