Abstract
Functional disability is common in older adults. It is often episodic and is associated with a high risk of subsequent health decline. The severity of disability is determined by physical impairments caused by underlying medical conditions, and by external factors such as social support, financial support, and the environment. When multiple health conditions are present, they often result in greater disability than expected because the patient’s ability to compensate for one problem may be affected by comorbid conditions. Evaluation of functional disability is most effective when the physician determines the course of the disability, associated symptoms, effects on specific activities, and coping mechanisms the patient uses to compensate for the functional problem. Underlying health conditions, impairments, and contextual factors (e.g., finances, social support) should be identified using validated screening tools. Interventions should focus on increasing the patient’s capacity to cope with task demands and reducing the demands of the task itself. Interventions for functional decline in older adults are almost always multifactorial because they must address multiple conditions, impairments, and contextual factors.
Professor B. is an 86-year-old woman who presents for a comprehensive geriatric evaluation, stating: “I don’t care whether or not you make me live longer, but can you help me live better?” She was a college professor who spent early retirement traveling, earning a pilot’s license, and maintaining an active lifestyle. She describes a two-year “downward spiral” that began when she lost her vision rapidly as a result of neovascular macular degeneration. She emphasizes that vision loss is not the only problem: “It just seemed like everything fell apart at the same time.” She has had vertebral fractures, frequent falls, lumbago, osteoarthritis, hypertension, urinary incontinence, mild cognitive impairment, insomnia, depression and anxiety, and idiopathic peripheral neuropathy.
Professor B. is unable to drive or read print smaller than newspaper headlines. She no longer cooks, and requires assistance with finances, medication management, and housekeeping. She has difficulty participating in many previously pleasurable activities, such as entertaining, dancing, and working crossword puzzles. She remains independent in eating, toileting, and ambulating, but she requires assistance with bathing and dressing.
Epidemiology of Disability
Disability, which is defined as limitation in the ability to carry out basic functional activities, affects one in seven Americans. It negatively affects quality of life and contributes to unsustainable health care costs.1 Although disability may arise acutely from a catastrophic illness, physicians often encounter older adults who present with subacute functional decline without a clear precipitating event.2–4 This article provides a framework for the assessment and treatment of progressive disability in older adults.
Implications of Disability
Unlike the relatively permanent disability that is often associated with catastrophic injury, disability that results from the accumulation of chronic diseases is dynamic and episodic.4,5 Observational studies have found that most disability episodes are brief (one to two months), but that they increase the risk of recurrent or progressive functional decline.3 The World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) model suggests that physicians focus on the physical impairments that result from health conditions, and on factors that affect the patient’s ability to adapt to such impairments (e.g., environment, socioeconomic resources).6,7
Health Conditions
Common health conditions that may contribute to functional disability include cardiopulmonary diseases, neurologic conditions, diabetes mellitus, cancer, obesity, dementia, affective disorders, ophthalmologic and auditory disorders, and fractures. Some conditions are rare but highly disabling (e.g., stroke), whereas others are less disabling but common (e.g., arthritis).8 The disabling effects vary depending on the task the patient is trying to perform; for example, heart disease is more likely to cause difficulty with tasks that demand high aerobic work (e.g., housework), whereas stroke is more likely to cause difficulty with basic self-care tasks that require limb mobility.9 Impairment refers to a change in body structure or function, often resulting from these chronic health conditions, and may include cognitive impairment, mood disorders, sensory impairment, pain, adverse effects of medications, and gait disorders.7 Both the impairment and the underlying health condition should be addressed to reduce the disability.
Interactions Between Impairments
The coexistence of two or more health conditions often creates more disability than would be expected. As the number of impairments increases from one to four, the percentage of persons reporting functional dependence increases exponentially (7% to 14% to 28% to 60%).10 Such interactions between impairments may occur because of interference with normal physiologic compensatory strategies. For example, a condition that decreases the biomechanical efficiency of muscles and joints (e.g., arthritis, stroke) increases the work of walking, and a patient with concomitant cardiovascular disease may lack the capacity to compensate for this increased demand.11,12 Interactions may also result from an inability to adopt compensatory behaviors; for example, persons with memory problems have difficulty learning new self-care techniques to compensate for poor eyesight.13 Some combinations of conditions have predominant effects on self-care (e.g., arthritis and stroke), whereas others primarily affect mobility (e.g., arthritis and heart disease).14
Contextual Factors
According to the ICF model, successful treatment of disability should take into account the patient’s personality, compensatory skills, and interaction with the environment.7 These contextual factors have especially important roles in the early and late stages of disability development. Mood, self-efficacy, and personal coping strategies affect self-management of disease, adherence to exercise programs, and long-term health outcomes in patients with diabetes.15 After disability related to end-organ damage occurs (e.g., retinopathy, neuropathy), the physical environment, financial resources to pay for adaptive equipment or assistance, and availability of caregiver support determine whether the patient remains in his or her home or requires a higher level of care.
Assessment of New or Progressive Disability
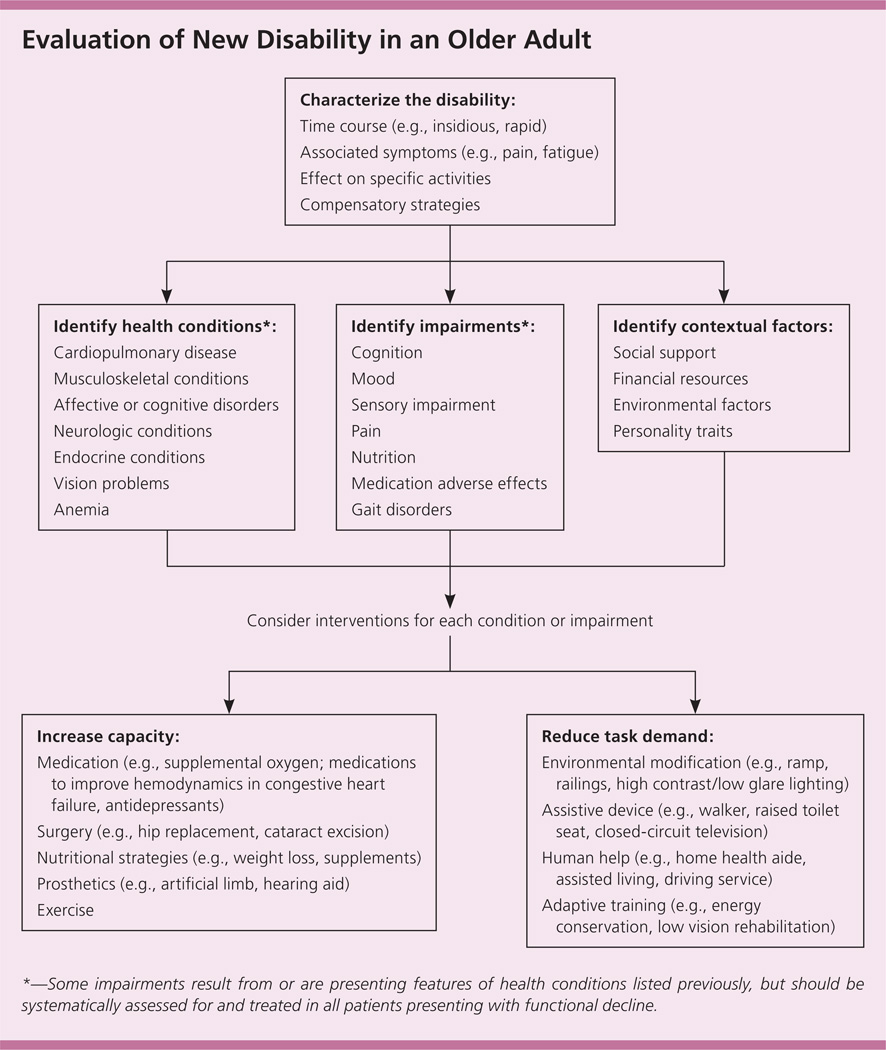
Because of the complex interactions between health conditions, impairments, and contextual factors, a systematic evaluation and intervention plan is important. Figure 1 depicts a stepwise approach to guide physicians. Evaluation of new or progressive disability begins with characterizing the disability itself and focusing on the course of functional decline, associated symptoms, and specific tasks that have been affected by the disability, including basic activities of daily living.16 Asking the patient about compensatory strategies will inform the treatment plan and provide insight into his or her capacity to cope with functional changes.
Figure 1.
Algorithm for evaluating new disability decline in an older adult.
The next step is to identify the underlying health conditions, focusing on those that are potentially modifiable.16 Because many pathologic processes can ultimately cause disability, using an organ system approach is generally the most efficient diagnostic strategy. The focus of the physical examination may be determined by the presenting symptoms; for example, the evaluation of a patient with decreased exercise tolerance and exertional dyspnea would include a thorough cardiovascular and pulmonary examination.
In addition to assessment for underlying health conditions, the examination should include screening for comorbid impairments. Cognitive impairment, mood disorders, pain, poor nutrition, adverse medication reactions, and sensory impairments are modifiable factors that may contribute to the underlying disability and affect the choice of therapy. Determining which condition precipitated the decline is not as useful as understanding the interactions between various health conditions, and targeting treatments to address them. Table 1 lists useful physical examination maneuvers and screening tests to help identify common underlying health conditions and impairments.17–34
Table 1.
Diagnostic Tools and Maneuvers to Identify Specific Impairments and Contextual Factors Contributing to Disability in Older Adults
| Screening tool or maneuver17 | Function or impairment assessed |
Associated functional limitations |
Potential interventions |
|---|---|---|---|
| Neuromusculoskeletal tests17,18 | |||
| Clasp hands behind head and behind back19 | Internal and external rotation and abduction of the shoulder, elbow flexion | Dressing (especially upper body), grooming, bathing, housework | Treat musculoskeletal conditions and associated pain; flexibility exercises; occupational therapy |
| Place ankle on opposite knee19 | External rotation of the hip, hip and knee flexion | Dressing lower body, bathing, toileting | |
| Chair stand test18,20 (stand from a chair without using arms; assess qualitatively or time five consecutive attempts) | Lower extremity strength (especially hip and knee) | Bathing, toileting, falls | Treat reversible neuromuscular and musculoskeletal conditions and associated pain; balance and strength exercises; rolling walker; raised toilet seat; bath chair; physical therapy |
| Rise on toes21 (test single heel rise in healthy adults, bilateral heel rise in frail adults) | Lower extremity strength (especially ankle), balance | Stair climbing, housework, bathing, falls | |
| Gentle nudge to the sternum22 | Ankle, hip, and trunk strength; balance | Housework, bathing, falls | |
| Timed Up and Go test23 (stand from a chair without using arms, walk 10 ft, return, sit down; see http://www.youtube.com/watch?v=s0nqzvt9JSs) | Lower extremity strength (especially hip and knee), balance, gross motor coordination | Mobility, bathing, toileting, housework | |
| Pick up a penny from the floor22 (to isolate hand function from sitting balance and vision, place penny on table or opposite hand) | Pinch strength, sensation, fine motor coordination, sitting balance, vision | Cooking, feeding, grooming, dressing, housework | Vision assessment and treatment; easy-to-manage clothing; occupational therapy |
| Two-minute walk test24 (walk at usual pace for two minutes; measure distance) | Endurance | Community mobility, shopping | Treat reversible cardiovascular, pulmonary, and musculoskeletal conditions; aerobic exercise; scooter or wheelchair |
| Sensory and cognitive tests | |||
| Whisper test25 | High-frequency hearing | Social function, telephone use; patient may mistake hearing problems with memory loss | Amplification devices |
| Read a line of 11- to 12-point type26 | Near vision | Medication management, reading | Treat reversible ophthalmologic conditions; magnification devices; referral to low-vision rehabilitation; pill box |
| Snellen chart testing26 | Distance vision | Driving | |
| Geriatric Depression Scale27 (score of 5 or higher suggests depression) | Mood | Short-term memory, social function | Antidepressants, cognitive behavior therapy |
| Time and Change Test28 (tell the time from a clock face set at 11:10, then make $1 in change from three quarters, seven dimes, and seven nickels; incorrect response on either task suggests possible dementia) | Cognition | Instrumental activities of daily living | Consider cholinesterase inhibitor; driving and home safety evaluation; family education and support |
| Clock Drawing Test29 (draw a clock face with hands at 11:10; errors in number or hand placement suggest need for further evaluation) | Executive function | Medication management, financial management, social function | Consider referral for neuropsychological testing |
| STOPP (screening tool of older persons’ potentially inappropriate prescriptions) criteria30 | Medication use | Short-term memory, balance, falls | Taper and stop medication when possible |
| Contextual factors | |||
| Ask “Do you have trouble with stairs inside or outside of your home?”31 | Physical environment and home safety | Falls, social function | Home safety evaluation; physical or occupational therapy; assistive devices; bathroom equipment |
| Ask “How confident are you that you can take a bath or shower, or get on and off the toilet without falling?”32 | Physical environment and home safety | Falls, hygiene | |
| Ask “Who would be able to help you in case of illness or emergency?”31 | Social support | Weight loss, medication management, financial management, social function | Social worker evaluation, community services or home health agency referral |
| Caregiver Burden Scale33 (scores higher than 40 indicate moderate to severe burden) | Caregiver stress | Decreased social function resulting from decreased caregiver support of community roles | |
| Mini Nutritional Assessment short form34 (score of 8 or less indicates risk of malnutrition) | Nutrition | Weight loss, physical performance | Nutritional supplements, nutrition evaluation |
Finally, the physician should assess contextual factors, including social support, financial resources, and environmental factors. It is important to examine these factors from both the patient’s and caregiver’s perspectives, because high caregiver burden is common and negatively affects the health of both.35
Treatment of New or Progressive Disability
After the underlying conditions, impairments, and contextual factors are identified, a systematic treatment plan can be developed. To reduce disability, physicians should consider interventions that increase the patient’s capacity to respond to environmental challenges and interventions to reduce task demands.36 Medical and surgical interventions typically increase capacity (e.g., cataract surgery, hip replacement, oxygen supplementation, medications to improve cardiac output), as do exercise, nutritional supplements, and prosthetic devices (e.g., hearing aids). Examples of interventions to reduce task demands include providing a wheelchair or a raised toilet seat, installing a ramp, or obtaining a personal aide. Some interventions can enhance capacity and reduce demand simultaneously; for example, a cane improves proprioceptive input and balance for persons with visual or peripheral sensory limitations, but also offsets body weight, thereby reducing demand on arthritic joints.
Role of Interdisciplinary Assessment
Effective treatment of disability in older adults is typically multimodal because of the need to address multiple contributing health conditions and impairments, and the importance of simultaneously taking actions to enhance capacity and reduce task demands.16 This complex, multimodal treatment requires a multidisciplinary approach. Referrals to a physical therapist, occupational therapist, nurse, social worker, pharmacist, or nutritionist may be appropriate. Several guidelines provide information on rehabilitation for specific diseases, and provide algorithms to identify the patient population most likely to benefit from rehabilitation.37–39 Close coordination of the timing and types of interventions is required to optimize rehabilitation outcomes. For example, treating delirium and pain before initiating physical therapy will improve patient participation and subsequent outcomes after a fracture.40 Patients with functional decline who do not respond to initial interventions often benefit from comprehensive geriatric assessment, which has been shown to improve function and quality of life, and decrease caregiver burden.41,42
Case Resolution
The primary health conditions and impairments underlying Professor B.’s functional decline were vision loss from macular degeneration and mobility-limiting pain in her knees and back resulting from osteoarthritis and vertebral fractures. Comorbid impairments included mild cognitive impairment, depression and anxiety, and adverse effects from psychoactive medications. Supportive contextual factors included adequate economic and social support, a resilient personality, and high baseline function. Environmental challenges included stairs in the home and a rural location that necessitated driving for community involvement.
The following interventions occurred: referral for low-vision rehabilitation and to local services for the blind; referral to a physical therapist to address strength, endurance, and pain; treatment of depression and anxiety with a selective serotonin reuptake inhibitor; and consolidation of the patient’s pain and sleep regimens with recommendations for behavioral strategies to reduce anxiety and insomnia, and to decrease the use of psychoactive medications.
Six months later, Professor B. was able to use the stove safely, dress independently, and use magnification devices to read and write. She was listening to recorded books and using a local transportation service for the blind. A physical therapy program improved lower extremity strength and knee range of motion, resulting in reconditioning and significantly fewer falls. Her pain was addressed by a consistent regimen of acetaminophen, heat, and massage. Her depression responded to medication, and her cognition improved with discontinuation of narcotics and reduced use of anxiolytics.
SORT: KEY RECOMMENDATIONS FOR PRACTICE.
| Clinical recommendation | Evidence rating |
References | Comments |
|---|---|---|---|
| New or progressive disability in older adults should be evaluated with careful assessment for underlying health conditions, impairments, and contextual factors. | C | 7 | Extrapolation from randomized trials of comprehensive geriatric evaluation |
| Treatment of disability should include strategies to increase the patient’s capacity to respond to environmental challenges, and to reduce task demand. | C | 36 | Consensus of disability researchers |
| Comprehensive geriatric evaluation and treatment programs conducted by an interdisciplinary team should be considered for patients with unexplained or progressive disability. | A | 41, 42 | Randomized trial and meta-analysis of randomized trials of geriatric evaluation and management |
A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to http://www.aafp.org/afpsort.
Acknowledgments
Dr. Whitson is supported by NIA K23-AG-032867, the Durham VA Medical Center Geriatric Research, Education and Clinical Center, NIA P30-AG-028716, the Brookdale Foundation, the John A. Hartford Foundation, and the American Federation for Aging Research. Dr. Hoenig is supported by the Mobility RERC, which is funded by the National Institute on Disability and Rehabilitation Research of the U.S. Department of Education under grant no. H133E080003. The opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Education.
Footnotes
Author disclosure: No relevant financial affiliations.
Data Sources: Medline, the Cochrane Database of Systematic Reviews, ACP Journal Club, and the National Guideline Clearinghouse were searched using the following MeSH headings and key words, limited to aged or frail elderly: rehabilitation, activities of daily living, geriatric assessment, functional status, functional decline, and disability. Last search date: April 14, 2011.
Contributor Information
Cathleen S. Colón-Emeric, associate director at the Geriatric Research, Education and Clinical Center at the Durham (N.C.) Veterans Affairs (VA) Medical Center, and an associate professor of medicine at Duke University Health System in Durham..
Heather E. Whitson, senior fellow in the Center for the Study of Aging and Human Development, an assistant professor of medicine at Duke University Health System, and a physician in the Geriatric Research, Education and Clinical Center at the Durham VA Medical Center..
Juliessa Pavon, advanced geriatric medicine fellow at Duke University Health System..
Helen Hoenig, associate chief of staff of physical medicine and rehabilitation at Durham VA Medical Center, and an associate professor of medicine at Duke University Health System..
REFERENCES
- 1.Chan L, Beaver S, Maclehose RF, et al. Disability and health care costs in the Medicare population. Arch Phys Med Rehabil. 2002;83(9):1196–1201. doi: 10.1053/apmr.2002.34811. [DOI] [PubMed] [Google Scholar]
- 2.Gill TM, Gahbauer EA, Han L, et al. Functional trajectories in older persons admitted to a nursing home with disability after an acute hospitalization. J Am Geriatr Soc. 2009;57(2):195–201. doi: 10.1111/j.1532-5415.2008.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill TM, Gahbauer EA, Han L, et al. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy SE, Dubin JA, Holford TR, et al. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161(6):575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 5.Gill TM, Allore HG, Hardy SE, et al. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54(2):248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 6.Strawbridge WJ, Shema SJ, Cohen RD, et al. Religious attendance increases survival by improving and maintaining good health behaviors, mental health, and social relationships. Ann Behav Med. 2001;23(1):68–74. doi: 10.1207/s15324796abm2301_10. [DOI] [PubMed] [Google Scholar]
- 7.WORLD HEALTH ORGANIZATION. Geneva, Switzerland: WHO; 2002. Towards a common language for functioning, disability and health: ICF. [Google Scholar]
- 8.Verbrugge LM, Patrick DL. Seven chronic conditions: their impact on US adults’ activity levels and use of medical services. Am J Public Health. 1995;85(2):173–182. doi: 10.2105/ajph.85.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettinger WH, Jr, Fried LP, Harris T, et al. CHS Collaborative Research Group. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 1994;42(10):1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. [PubMed] [Google Scholar]
- 11.Wu YJ, Chen SY, Lin MC, et al. Energy expenditure of wheeling and walking during prosthetic rehabilitation in a woman with bilateral transfemoral amputations. Arch Phys Med Rehabil. 2001;82(2):265–269. doi: 10.1053/apmr.2001.19019. [DOI] [PubMed] [Google Scholar]
- 12.Lapointe R, Lajoie Y, Serresse O, et al. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 2001;39(6):327–335. doi: 10.1038/sj.sc.3101167. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence V, Murray J, Ffytche D, et al. “Out of sight, out of mind”: a qualitative study of visual impairment and dementia from three perspectives [published correction appears in Int Psychogeriatr. 2009;21(3):519] Int Psychogeriatr. 2009;21(3):511–518. doi: 10.1017/S1041610209008424. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Bandeen-Roche K, Kasper JD, et al. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999;52(1):27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 15.Koenig HG, George LK, Peterson BL. Religiosity and remission of depression in medically ill older patients. Am J Psychiatry. 1998;155(4):536–542. doi: 10.1176/ajp.155.4.536. [DOI] [PubMed] [Google Scholar]
- 16.Hoenig H, Nusbaum N, Brummel-Smith K. Geriatric rehabilitation: state of the art. J Am Geriatr Soc. 1997;45(11):1371–1381. doi: 10.1111/j.1532-5415.1997.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed April 17, 2013];Geriatric examination tool kit. http://web.missouri.edu/~proste/tool/
- 18.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 19.Jette AM, Branch LG. Musculoskeletal impairment among the non-institutionalized aged. Int Rehabil Med. 1984;6(4):157–161. doi: 10.3109/03790798409165949. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Lunsford BR, Perry J. The standing heel-rise test for ankle plantar flexion: criterion for normal. Phys Ther. 1995;75(8):694–698. doi: 10.1093/ptj/75.8.694. [DOI] [PubMed] [Google Scholar]
- 22.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38(10):1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Brooks D, Parsons J, Tran D, et al. The two-minute walk test as a measure of functional capacity in cardiac surgery patients. Arch Phys Med Rehabil. 2004;85(9):1525–1530. doi: 10.1016/j.apmr.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Yueh B, Shapiro N, MacLean CH, et al. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003;289(15):1976–1985. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]
- 26.Moore AA, Siu A, Partridge JM, et al. A randomized trial of office-based screening for common problems in older persons. Am J Med. 1997;102(4):371–378. doi: 10.1016/s0002-9343(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 27.Lyness JM, Noel TK, Cox C, et al. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157(4):449–454. [PubMed] [Google Scholar]
- 28.Froehlich TE, Robison JT, Inouye SK. Screening for dementia in the outpatient setting: the time and change test. J Am Geriatr Soc. 1998;46(12):1506–1511. doi: 10.1111/j.1532-5415.1998.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 29.Royall DR, Mulroy AR, Chiodo LK, et al. Clock drawing is sensitive to executive control: a comparison of six methods. J Gerontol B Psychol Sci Soc Sci. 1999;54(5):328–333. doi: 10.1093/geronb/54b.5.p328. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- 31.Lachs MS, Feinstein AR, Cooney LM, Jr, et al. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. 1990;112(9):699–706. doi: 10.7326/0003-4819-112-9-699. [DOI] [PubMed] [Google Scholar]
- 32.Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45(6):P239–P243. doi: 10.1093/geronj/45.6.p239. [DOI] [PubMed] [Google Scholar]
- 33.Elmståhl S, Malmberg B, Annerstedt L. Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Arch Phys Med Rehabil. 1996;77(2):177–182. doi: 10.1016/s0003-9993(96)90164-1. [DOI] [PubMed] [Google Scholar]
- 34.Persson MD, Brismar KE, Katzarski KS, et al. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc. 2002;50(12):1996–2002. doi: 10.1046/j.1532-5415.2002.50611.x. [DOI] [PubMed] [Google Scholar]
- 35.Browning JS, Schwirian PM. Spousal caregivers’ burden: impact of care recipient health problems and mental status. J Gerontol Nurs. 1994;20(3):17–22. doi: 10.3928/0098-9134-19940301-05. [DOI] [PubMed] [Google Scholar]
- 36.Iwarsson S. Functional capacity and physical environmental demand. Exploration of factors influencing everyday activity and health in the elderly population. Scand J Occup Ther. 1996;3(3):139. [Google Scholar]
- 37.Cibulka MT, White DM, Woehrle J, et al. Hip pain and mobility deficits—hip osteoarthritis: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2009;39(4):A1–A25. doi: 10.2519/jospt.2009.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savigny P, Watson P, Underwood M. Guideline Development Group. Early management of persistent nonspecific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805. doi: 10.1136/bmj.b1805. [DOI] [PubMed] [Google Scholar]
- 39.Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36(9):e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 40.Morrison RS, Flanagan S, Fischberg D, et al. A novel interdisciplinary analgesic program reduces pain and improves function in older adults after orthopedic surgery. J Am Geriatr Soc. 2009;57(1):1–10. doi: 10.1111/j.1532-5415.2008.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 42.Ellis G, Whitehead MA, Robinson D, et al. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]